Abstract
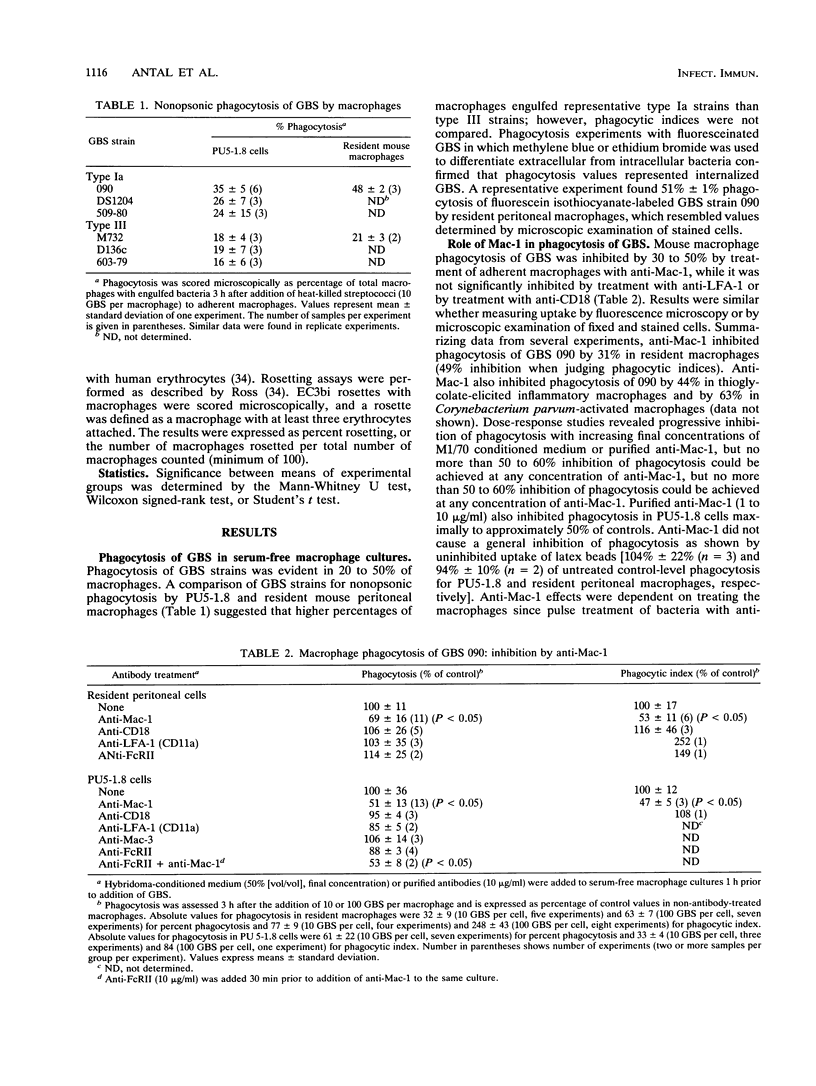
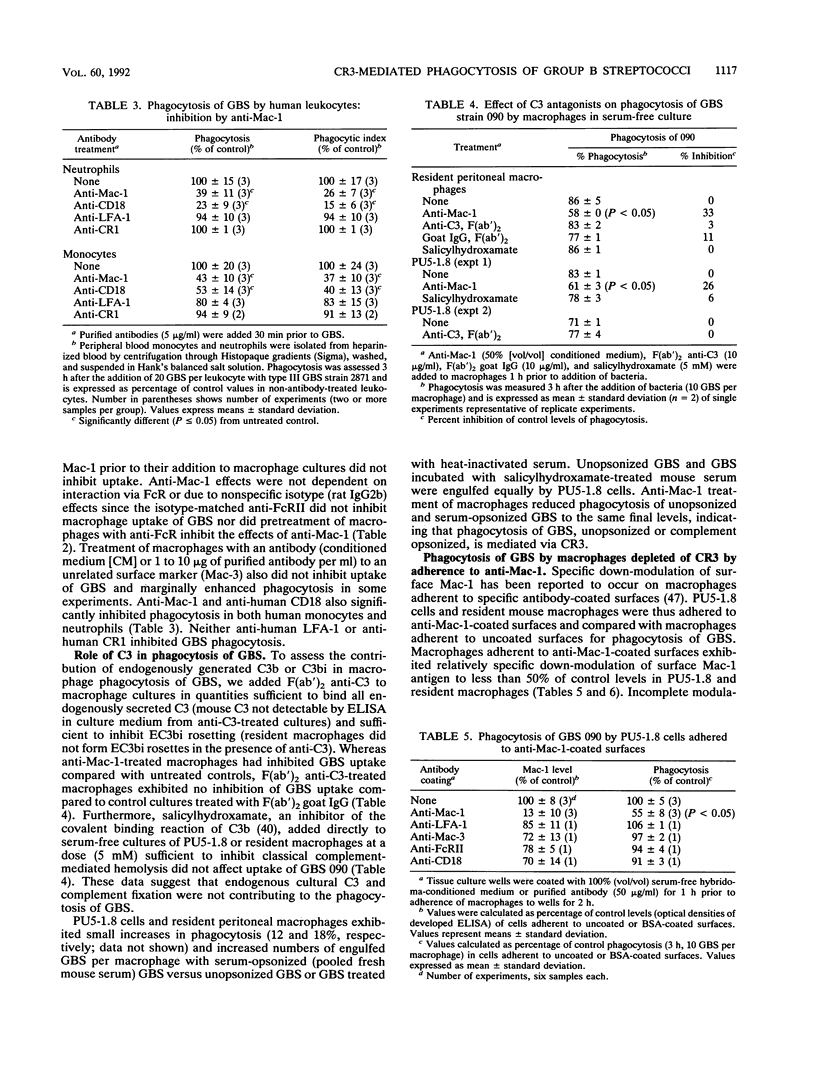
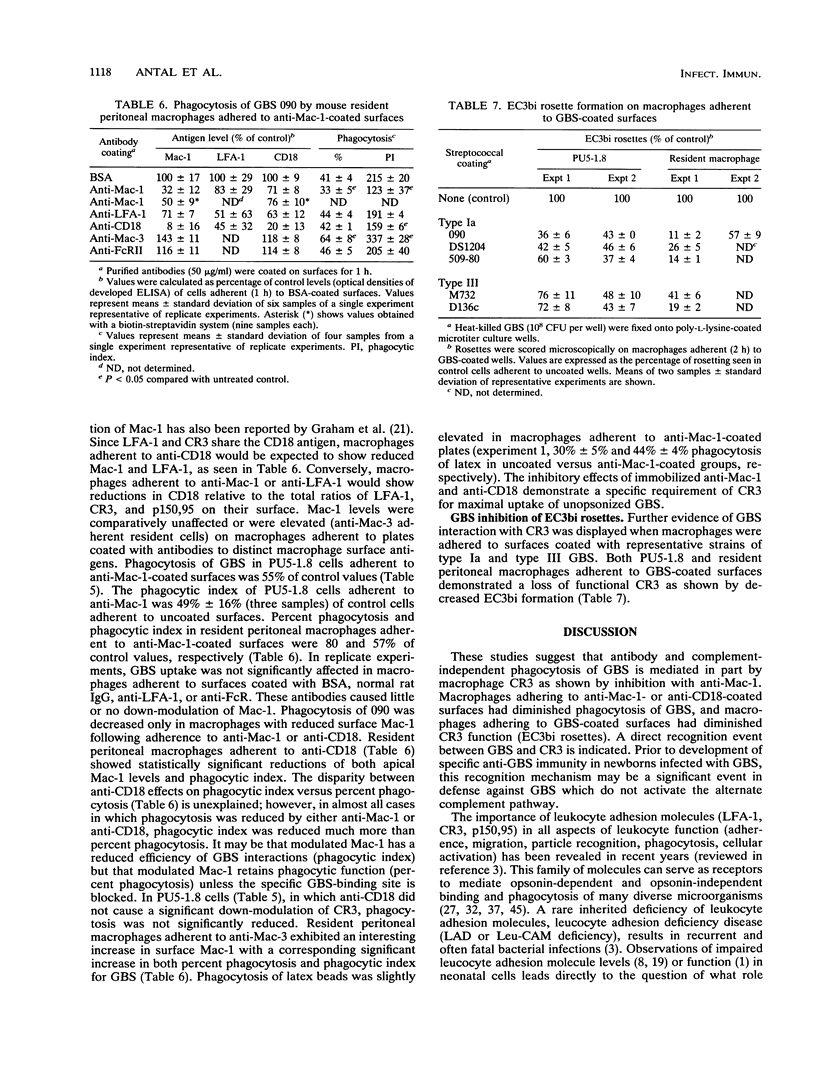
The role of complement receptor type 3 (CR3) in nonopsonic recognition of group B streptococci (GBS) by macrophages was investigated. Monoclonal anti-CR3 (anti-Mac-1) inhibited phagocytosis of GBS strains by as much as 50% in serum-free cultures of both mouse peritoneal macrophages and the macrophage cell line PU5-1.8. GBS uptake was unaffected by the presence of anti-C3 or salicylhydroxamate, an inhibitor of the covalent binding reaction of C3. Soluble antibodies to LFA-1 or to the common beta-chain (CD18) of the LFA-1/CR3/p150,95 family of cell adhesion molecules did not inhibit GBS uptake. Down-modulation of surface Mac-1 on macrophages following adherence to anti-Mac-1- or anti-CD18-coated surfaces also inhibited uptake of GBS. Further evidence for GBS interaction with CR3 was demonstrated by reduction of EC3bi rosette formation in macrophages adherent to GBS-coated plates. These studies suggest that GBS can interact with macrophage CR3, promoting phagocytosis in a C3-independent fashion. In the absence of specific immunity in neonates, this recognition mechanism may be a significant virulence determinant for GBS which poorly activate the alternate complement pathway.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., Freeman K. L., Heerdt B., Hughes B. J., Jack R. M., Smith C. W. Abnormal stimulated adherence of neonatal granulocytes: impaired induction of surface Mac-1 by chemotactic factors or secretagogues. Blood. 1987 Sep;70(3):740–750. [PubMed] [Google Scholar]
- Anthony B. F. Immunity to the group B streptococci: interaction of serum and macrophages with types Ia, Ib, and Ic. J Exp Med. 1976 May 1;143(5):1186–1198. doi: 10.1084/jem.143.5.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaout M. A. Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood. 1990 Mar 1;75(5):1037–1050. [PubMed] [Google Scholar]
- Baker C. J. Immunization to prevent group B streptococcal disease: victories and vexations. J Infect Dis. 1990 May;161(5):917–921. doi: 10.1093/infdis/161.5.917. [DOI] [PubMed] [Google Scholar]
- Baker C. J., Kasper D. L. Microcapsule of type III strains of group B Streptococcus: production and morphology. Infect Immun. 1976 Jan;13(1):189–194. doi: 10.1128/iai.13.1.189-194.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker I. D., Robinson O. M., Bazán T. S., López-Osuna M., Kretschmer R. R. Bactericidal capacity of newborn phagocytes against group B beta-hemolytic streptococci. Infect Immun. 1981 Nov;34(2):535–539. doi: 10.1128/iai.34.2.535-539.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. J., Bohnsack J. F., Gresham H. D. Mechanism of inhibition of immunoglobulin G-mediated phagocytosis by monoclonal antibodies that recognize the Mac-1 antigen. J Clin Invest. 1988 Feb;81(2):365–375. doi: 10.1172/JCI113328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce M. C., Baley J. E., Medvik K. A., Berger M. Impaired surface membrane expression of C3bi but not C3b receptors on neonatal neutrophils. Pediatr Res. 1987 Mar;21(3):306–311. doi: 10.1203/00006450-198703000-00022. [DOI] [PubMed] [Google Scholar]
- Bullock W. E., Wright S. D. Role of the adherence-promoting receptors, CR3, LFA-1, and p150,95, in binding of Histoplasma capsulatum by human macrophages. J Exp Med. 1987 Jan 1;165(1):195–210. doi: 10.1084/jem.165.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain J. A., Newman S. L., Ross G. D. Role of complement receptor type three and serum opsonins in the neutrophil response to yeast. Complement. 1987;4(2):75–86. doi: 10.1159/000463011. [DOI] [PubMed] [Google Scholar]
- Ding A., Wright S. D., Nathan C. Activation of mouse peritoneal macrophages by monoclonal antibodies to Mac-1 (complement receptor type 3). J Exp Med. 1987 Mar 1;165(3):733–749. doi: 10.1084/jem.165.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets D. A., Campbell P. A. Roles of complement and complement receptor type 3 in phagocytosis of Listeria monocytogenes by inflammatory mouse peritoneal macrophages. Infect Immun. 1991 Aug;59(8):2645–2652. doi: 10.1128/iai.59.8.2645-2652.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham D. L., Mattingly S. J., Doran T. I., Milligan T. W., Straus D. C. Correlation between the production of extracellular substances by type III group B streptococcal strains and virulence in a mouse model. Infect Immun. 1981 Nov;34(2):448–454. doi: 10.1128/iai.34.2.448-454.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. S., Baker C. J., Kasper D. L. Opsonic specificity of human antibody to the type III polysaccharide of group B Streptococcus. J Infect Dis. 1979 Dec;140(6):1004–1008. doi: 10.1093/infdis/140.6.1004. [DOI] [PubMed] [Google Scholar]
- Edwards M. S. Complement in neonatal infections: an overview. Pediatr Infect Dis. 1986 May-Jun;5(3 Suppl):S168–S170. [PubMed] [Google Scholar]
- Edwards M. S., Nicholson-Weller A., Baker C. J., Kasper D. L. The role of specific antibody in alternative complement pathway-mediated opsonophagocytosis of type III, group B Streptococcus. J Exp Med. 1980 May 1;151(5):1275–1287. doi: 10.1084/jem.151.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Sim R. B., Hill M., Gordon S. Local opsonization by secreted macrophage complement components. Role of receptors for complement in uptake of zymosan. J Exp Med. 1984 Jan 1;159(1):244–260. doi: 10.1084/jem.159.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrieri P. Neonatal susceptibility and immunity to major bacterial pathogens. Rev Infect Dis. 1990 May-Jun;12 (Suppl 4):S394–S400. doi: 10.1093/clinids/12.supplement_4.s394. [DOI] [PubMed] [Google Scholar]
- Fleit H. B. Fc and complement receptor (CR1 and CR3) expression on neonatal human polymorphonuclear leukocytes. Biol Neonate. 1989;55(3):156–163. doi: 10.1159/000242911. [DOI] [PubMed] [Google Scholar]
- Goodrum K. J. Stimulation of complement component C3 synthesis in macrophagelike cell lines by group B streptococci. Infect Immun. 1987 May;55(5):1101–1105. doi: 10.1128/iai.55.5.1101-1105.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham I. L., Gresham H. D., Brown E. J. An immobile subset of plasma membrane CD11b/CD18 (Mac-1) is involved in phagocytosis of targets recognized by multiple receptors. J Immunol. 1989 Apr 1;142(7):2352–2358. [PubMed] [Google Scholar]
- Krause P. J., Malech H. L., Kristie J., Kosciol C. M., Herson V. C., Eisenfeld L., Pastuszak W. T., Kraus A., Seligmann B. Polymorphonuclear leukocyte heterogeneity in neonates and adults. Blood. 1986 Jul;68(1):200–204. [PubMed] [Google Scholar]
- Levy N. J., Kasper D. L. Antibody-independent and -dependent opsonization of group B Streptococcus requires the first component of complement C1. Infect Immun. 1985 Jul;49(1):19–24. doi: 10.1128/iai.49.1.19-24.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy N. J., Nicholson-Weller A., Baker C. J., Kasper D. L. Potentiation of virulence by group B streptococcal polysaccharides. J Infect Dis. 1984 Jun;149(6):851–860. doi: 10.1093/infdis/149.6.851. [DOI] [PubMed] [Google Scholar]
- Maródi L., Leijh P. C., van Furth R. Characteristics and functional capacities of human cord blood granulocytes and monocytes. Pediatr Res. 1984 Nov;18(11):1127–1131. doi: 10.1203/00006450-198411000-00014. [DOI] [PubMed] [Google Scholar]
- Mosser D. M., Edelson P. J. The mouse macrophage receptor for C3bi (CR3) is a major mechanism in the phagocytosis of Leishmania promastigotes. J Immunol. 1985 Oct;135(4):2785–2789. [PubMed] [Google Scholar]
- Myones B. L., Dalzell J. G., Hogg N., Ross G. D. Neutrophil and monocyte cell surface p150,95 has iC3b-receptor (CR4) activity resembling CR3. J Clin Invest. 1988 Aug;82(2):640–651. doi: 10.1172/JCI113643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polin R. A., Kennett R. Use of monoclonal antibodies in an enzyme immunoassay for rapid identification of group B Streptococcus types II and III. J Clin Microbiol. 1980 Apr;11(4):332–336. doi: 10.1128/jcm.11.4.332-336.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirante J., Ceballos R., Cassady G. Group B beta-hemolytic streptococcal infection in the newborn. I. Early onset infection. Am J Dis Child. 1974 Nov;128(5):659–665. doi: 10.1001/archpedi.1974.02110300069009. [DOI] [PubMed] [Google Scholar]
- Ralph P., Moore M. A., Nilsson K. Lysozyme synthesis by established human and murine histiocytic lymphoma cell lines. J Exp Med. 1976 Jun 1;143(6):1528–1533. doi: 10.1084/jem.143.6.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relman D., Tuomanen E., Falkow S., Golenbock D. T., Saukkonen K., Wright S. D. Recognition of a bacterial adhesion by an integrin: macrophage CR3 (alpha M beta 2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell. 1990 Jun 29;61(7):1375–1382. doi: 10.1016/0092-8674(90)90701-f. [DOI] [PubMed] [Google Scholar]
- Riches D. W., Stanworth D. R. Studies on the possible involvement of complement component C3 in the initiation of acid hydrolase secretion by macrophages. I. Correlation between enzyme-releasing and complement-activating capacities of several secretagogues. Immunology. 1981 Sep;44(1):29–39. [PMC free article] [PubMed] [Google Scholar]
- Ross G. D., Cain J. A., Lachmann P. J. Membrane complement receptor type three (CR3) has lectin-like properties analogous to bovine conglutinin as functions as a receptor for zymosan and rabbit erythrocytes as well as a receptor for iC3b. J Immunol. 1985 May;134(5):3307–3315. [PubMed] [Google Scholar]
- Ross G. D., Cain J. A., Myones B. L., Newman S. L., Lachmann P. J. Specificity of membrane complement receptor type three (CR3) for beta-glucans. Complement. 1987;4(2):61–74. doi: 10.1159/000463010. [DOI] [PubMed] [Google Scholar]
- Ross G. D., Thompson R. A., Walport M. J., Springer T. A., Watson J. V., Ward R. H., Lida J., Newman S. L., Harrison R. A., Lachmann P. J. Characterization of patients with an increased susceptibility to bacterial infections and a genetic deficiency of leukocyte membrane complement receptor type 3 and the related membrane antigen LFA-1. Blood. 1985 Oct;66(4):882–890. [PubMed] [Google Scholar]
- Russell D. G., Talamas-Rohana P., Zelechowski J. Antibodies raised against synthetic peptides from the Arg-Gly-Asp-containing region of the Leishmania surface protein gp63 cross-react with human C3 and interfere with gp63-mediated binding to macrophages. Infect Immun. 1989 Feb;57(2):630–632. doi: 10.1128/iai.57.2.630-632.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman M. P., Lehrer R. I. Oxidative metabolism of neonatal and adult rabbit lung macrophages stimulated with opsonized group B streptococci. Infect Immun. 1985 Jan;47(1):26–30. doi: 10.1128/iai.47.1.26-30.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim R. B., Twose T. M., Paterson D. S., Sim E. The covalent-binding reaction of complement component C3. Biochem J. 1981 Jan 1;193(1):115–127. doi: 10.1042/bj1930115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L., Baker C. J., Anderson D. C., Edwards M. S. Role of complement receptors in opsonophagocytosis of group B streptococci by adult and neonatal neutrophils. J Infect Dis. 1990 Aug;162(2):489–495. doi: 10.1093/infdis/162.2.489. [DOI] [PubMed] [Google Scholar]
- Spitznagel J. K., Goodrum K. J., Warejcka D. J. Rat arthritis due to whole group B streptococci. Clinical and histopathologic features compared with groups A and D. Am J Pathol. 1983 Jul;112(1):37–47. [PMC free article] [PubMed] [Google Scholar]
- Talamás-Rohana P., Wright S. D., Lennartz M. R., Russell D. G. Lipophosphoglycan from Leishmania mexicana promastigotes binds to members of the CR3, p150,95 and LFA-1 family of leukocyte integrins. J Immunol. 1990 Jun 15;144(12):4817–4824. [PubMed] [Google Scholar]
- Wennerstrom D. E., Schutt R. W. Adult mice as a model for early onset group B streptococcal disease. Infect Immun. 1978 Feb;19(2):741–744. doi: 10.1128/iai.19.2.741-744.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Jong M. T. Adhesion-promoting receptors on human macrophages recognize Escherichia coli by binding to lipopolysaccharide. J Exp Med. 1986 Dec 1;164(6):1876–1888. doi: 10.1084/jem.164.6.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Levin S. M., Jong M. T., Chad Z., Kabbash L. G. CR3 (CD11b/CD18) expresses one binding site for Arg-Gly-Asp-containing peptides and a second site for bacterial lipopolysaccharide. J Exp Med. 1989 Jan 1;169(1):175–183. doi: 10.1084/jem.169.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Rao P. E., Van Voorhis W. C., Craigmyle L. S., Iida K., Talle M. A., Westberg E. F., Goldstein G., Silverstein S. C. Identification of the C3bi receptor of human monocytes and macrophages by using monoclonal antibodies. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5699–5703. doi: 10.1073/pnas.80.18.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Silverstein S. C. Tumor-promoting phorbol esters stimulate C3b and C3b' receptor-mediated phagocytosis in cultured human monocytes. J Exp Med. 1982 Oct 1;156(4):1149–1164. doi: 10.1084/jem.156.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]


