Abstract
Understanding the performance of the left ventricle (LV) requires not only examining the properties of the LV itself, but also investigating the modulating effects of the arterial system on left ventricular performance. The interaction of the LV with the arterial system, termed arterial-ventricular coupling (EA/ELV), is a central determinant of cardiovascular performance and cardiac energetics. EA/ELV can be indexed by the ratio of effective arterial elastance (EA; a measure of the net arterial load exerted on the left ventricle) to left ventricular end-systolic elastance (ELV; a load-independent measure of left ventricular chamber performance). At rest, in healthy individuals, EA/ELV is maintained within a narrow range, which allows the cardiovascular system to optimize energetic efficiency at the expense of mechanical efficacy. During exercise, an acute mismatch between the arterial and ventricular systems occurs, due to a disproportionate increase in ELV (from an average of 4.3 to 13.2, and 4.7 to 15.5 mmHg·ml−1·m−2 in men and women, respectively) vs. EA (from an average of 2.3 to 3.2, and 2.3 to 2.9 mmHg·ml−1·m−2 in men and women, respectively), to ensure that sufficient cardiac performance is achieved to meet the increased energetic requirements of the body. As a result EA/ELV decreases from an average of 0.58 to 0.34, and 0.52 to 0.27 in men and women, respectively. In this review, we provide an overview of the concept of EA/ELV, and examine the effects of age, hypertension, and heart failure on EA/ELV and its components (EA and ELV) in men and women. We discuss these effects both at rest and during exercise and highlight the mechanistic insights that can be derived from studying EA/ELV.
Keywords: left ventricular function, arterial system, exercise, aging, disease
aging and cardiovascular (CV) diseases, such as hypertension and congestive heart failure (HF), modify the structure and function of both the central arteries and the left ventricle (LV). Left ventricular performance is influenced by the arterial load (33), and arterial properties are, in turn, influenced by left ventricular performance (33, 77). Indeed, the interaction between the LV and the arterial system, known as arterial-ventricular coupling (EA/ELV), is an important determinant of net CV performance (33) and cardiac energetics (74). In this paper, we will review the concept of EA/ELV and its components, effective arterial elastance (EA; a measure of the net arterial load) and left ventricular end-systolic elastance (ELV; a measure of left ventricular performance), and we will discuss the novel mechanistic insights EA/ELV provides into the effects of aging, hypertension, and HF on CV performance at rest and during exercise.
ARTERIAL-VENTRICULAR COUPLING AND ITS COMPONENTS
Traditionally, arterial load was characterized in the frequency domain as impedance spectra (49, 54), whereas left ventricular performance was characterized in the time domain by indexes of pressure and volume (77). This difference hindered the ability to examine the cross talk, or the interactions, between the LV and the central arteries. Studying these interactions required that novel methods be developed, which assess left ventricular and arterial properties in the same domain.
Effective Arterial Elastance
Sunagawa's pioneering work (77), using a three-element windkessel model in the isolated canine heart, showed that the arterial load could globally be characterized in the time domain as EA (elastance is the change in pressure for a change in volume). EA is not a measure of a specific arterial property; rather, it is an integrative index that incorporates the principal elements of arterial load, including peripheral vascular resistance (PVR), total arterial compliance, characteristic impedance, and systolic and diastolic time intervals (77). EA can, therefore, be considered a measure of the net arterial load that is imposed on the LV. EA is determined from pressure-volume (P-V) loops as the negative slope of the line joining the end-diastolic volume (EDV) and end-systolic pressure (ESP) points (Fig. 1) and can be approximated by the ratio of ESP to stroke volume (SV) (77). ESP can be estimated from the formula [2 × (systolic BP + diastolic BP)]/3 (37), where BP is blood pressure, or from the formula ESP = 0.9 × systolic BP (37). Kelly et al. (37) showed that EA measured invasively as ESP/SV closely approximated the arterial load obtained from aortic input impedance and arterial compliance data based on a three-element windkessel model [EA = 1.0 × EA(windkessel) − 0.11, SE of estimate = 0.12; coefficient of variation = 0.97, P < 0.001] (37). One limitation of the three-element windkessel model is that it does not include the effects of the reflected pressure waves, which originate from areas of major impedance mismatches or major bifurcations. These reflected waves arrive earlier in the cardiac cycle when the velocity of the pulse wave is increased, as occurs with aging or with hypertension, and they can substantially augment the systolic load on the heart. However, the net effects of the reflected waves are functionally accounted for in P-V loops (37). Thus EA can be considered as a surrogate measure of aortic input impedance whose advantage is that it can be related to measures of ELV, thus allowing the study of arterial and ventricular interactions (see below).
Fig. 1.

Ventricular pressure-volume diagram from which effective arterial elastance (EA) and left ventricular (LV) end-systolic elastance (ELV) are derived. EA represents the negative slope of the line joining the end-diastolic volume (EDV) and the end-systolic pressure (ESP) points. ELV represents the slope of the end-systolic pressure-volume relationship passing through the volume intercept (V0). The shaded area represents the cardiac stroke work (SW), and the hatched area represents the potential energy (PE). LV ESP is the LV pressure at the end of systole. EDV is the LV volume at the end of diastole. End-systolic volume (ESV) is the LV volume at the end of systole. Stroke volume (SV) is the volume of blood ejected by the LV with each beat and is obtained from subtracting ESV from EDV. BP, blood pressure; EF, ejection fraction; PVA, pressure-volume area.
Left Ventricular End-Systolic Elastance
Sagawa et al. (67), in an isolated canine heart model, showed that left ventricular contractility (or end-systolic stiffness) could be indexed by ELV. ELV is determined from the slope of the end-systolic P-V relationship (Fig. 1), which can be obtained from a series of P-V loops recorded while the preload of the heart is altered. An increase in contractility is depicted by an increase in the slope and a shift in the end-systolic P-V relationship to the left, which allows the ventricle to generate more pressure for a given LV volume. ELV can be calculated as ESP/[end-systolic volume (ESV) − V0], where V0 is the x-axis volume intercept of the end-systolic P-V relationship (obtained from the linear extrapolation of the end-systolic P-V relationship). The calculation of ELV assumes that the end-systolic P-V relationship is independent of load, that its slope is linear, and that V0 is insensitive to inotropic influences. Under physiological loading conditions, these assumptions are reasonable approximations (12, 34).
Although ELV is widely regarded as a load-independent index of left ventricular contractility (66, 67), it is also influenced by the geometric and biochemical properties that underlie left ventricular end-systolic stiffness (9). Thus caution should be exercised in interpreting the significance of an elevated ELV, particularly when other measures of left ventricular systolic function are normal (32). It is likely that acute changes in ELV (e.g., with inotropic agents or exercise) reflect acute alterations in left ventricular contractility, whereas baseline values of ELV represent an index that integrates intrinsic left ventricular contractility as well as the modulating effects of the geometric, structural, and functional properties of the LV (32). ELV should, therefore, be considered an integrated measure of left ventricular chamber performance that can be related to an integrated measure of arterial load (i.e., EA).
Arterial-Ventricular Coupling
Importantly, EA shares common units with ELV, and their ratio EA/ELV is a measure of the interaction between the LV and the arterial system (77). EA/ELV is an important determinant of net cardiac performance (33) and cardiac energetics (74). Appropriate matching between the LV and the arterial system at rest results in an optimal transfer of blood from the LV to the periphery without excessive changes in pressure; an optimal or near-optimal stroke work (SW); and energetic efficiency, i.e., the energy consumed by the heart to achieve the required SW (9). In healthy men and women, in the resting state, mean ± SD values of EA/ELV, EA, and ELV measured invasively are ≈1.0 ± 0.36, 2.2 ± 0.8 mmHg/ml, and 2.3 ± 1.0 mmHg/ml, respectively (18).
Noninvasive Measures of Arterial-Ventricular Coupling and Its Components
The initial studies from Sagawa's group (67, 77–79) were performed in isolated canine hearts, which were instrumented to obtain P-V loops. Subsequently, EA and ELV were measured in humans from P-V loops acquired in the cardiac catheterization laboratory (11, 36). However, the invasive nature of these techniques limited their use in humans to a few skilled research groups. Fortunately, noninvasive estimates of EA and ELV were subsequently developed, which are discussed below (19, 48, 60, 61, 64).
As noted above, EA can be calculated as ESP/SV. SV can be readily measured noninvasively (e.g., by echocardiography or gated blood pool scans) (25, 73). Chen et al. (17) found that the calculation of ESP from 0.9 × brachial systolic BP reasonably approximated ESP measured invasively: the correlation coefficient between the two variables was 0.75, and the regression line had a slope of 1.01 (P < 0.0001).
Importantly, the equation EA = ESP/SV can be algebraically rearranged to show that EA is proportional to the sum of HR (heart rate) × PVR and [(ESP − mean arterial pressure)/SV], suggesting that the main determinants of EA include HR, a resistive component (PVR), and a stiffness component (change in pressure/change in volume) (20).
For the noninvasive assessment of ELV, two approaches have been developed. The first is based on the equation noted above (ELV = ESP/ESV − V0), and assumes that V0 is negligible compared with ESV. Thus, by measuring ESV noninvasively (e.g., by echocardiography or by gated blood pool scans) and by calculating ESP as 0.9 × systolic BP, ELV can be noninvasively estimated.
The second noninvasive approach to assess ELV attempts to derive the slope of the end-systolic P-V relationship without altering the loading conditions of the heart. This approach takes advantage of the finding that, under physiological loading conditions, the time-varying elastance curves, from which the end-systolic P-V relationships are derived, are fairly independent of loading conditions, left ventricular contractile state, and HR, particularly when they are normalized to peak amplitude (end-systolic stiffness) and time to peak amplitude (70). As a result, the end-systolic P-V relationship can be estimated from a single heartbeat (70). This method requires the measurement of systolic and diastolic BPs, ejection fraction (EF), and SV, preejection period, and total systolic ejection period on Doppler echocardiography. This single-beat elastance approach has been validated against invasively measured ELV with a correlation coefficient of 0.81 (P < 0.001) [single-beat ELV = 0.78 × ELV(invasive) + 0.55, SE of estimate = 0.6] (17).
From these noninvasive determinations of EA and ELV, the EA/ELV ratio can be calculated. The noninvasively obtained values of EA/ELV closely approximate those obtained invasively (21, 65). EA/ELV is inversely related to EF [EA/ELV ≈ (1/EF) − 1] (20). The advantage of EA/ELV over EF is that examining the components of EA/ELV allows us to evaluate whether alterations in EA/ELV are due to alterations in arterial properties, left ventricular properties, or both. Importantly, the ability to noninvasively assess EA/ELV has expanded the range of clinical trials and the scope of conditions in which EA/ELV could be investigated. For example, EA/ELV has been examined in epidemiological studies (61) and in studies evaluating the effects of exercise (50).
Scaling of Effective Arterial Elastance and Left Ventricular End-Systolic Elastance
Because body size is an important determinant of CV structure and function, adjusting for body size (or for left ventricular mass) is often needed when CV variables are being characterized or compared among groups (26). However, there is no consensus as to the best strategy for normalizing EA or ELV. Both SV and ESV vary directly with body size. Thus some investigators have scaled SV to body surface area (BSA) (5, 50, 65), whereas others have scaled EA (i.e., ESP/SV) to BSA (61). For ELV, a broader spectrum of scaling variables has been utilized, including BSA (29, 47), BSA to the power 1.19 (24), body mass (28), left ventricular mass (7), left ventricular EDV (30), and left ventricular mass/EDV (42). For the most part, the scaling technique has consisted of dividing ELV by the variable of interest. However, this approach does not necessarily provide adequate adjustment for the variable of interest, because ELV may not necessarily be linearly related to the variable. Instead, a preferred approach might be the allometric scaling method (6, 26, 52), whereby ELV is divided by the variable of interest raised to a scalar exponent, which is derived from an equation that linearly relates the two. In the following sections, CV variables that are normalized to body size will be depicted by the suffix “I” (e.g., EAI and ELVI).
Arterial-Ventricular Coupling and Its Components During Exercise
Exercise provides a powerful tool to examine the response of the CV system to stress and to assess its functional reserve. During exercise, the goal of the CV system is to prioritize cardiac efficacy over energetic efficiency (50). It uses a complex combination of alterations in HR, LV contractility, preload (EDV and SV), and afterload to ensure adequate blood supply to the tissues.
Few studies have examined the changes in EA/ELV and its components during exercise. In adult dogs, Little and Cheng (43) reported that EA/ELV decreased by ∼25% from rest to submaximal exercise. In healthy human subjects undergoing supine cycle ergometry, Asanoi et al. (3) observed that EA/ELV decreased by 35 and 54% at workloads corresponding to 30% below and 30% above the anaerobic threshold, respectively; and Chantler et al. (15) found that EAI/ELVI decreased by ≈65% (from an average of 0.58 to 0.34, and 0.52 to 0.27 in men and women, respectively) from rest to peak exercise, which was attributed to a larger increase in ELVI (from an average of 4.3 to 13.2, and 4.7 to 15.5 mmHg·ml−1·m−2 in men and women, respectively) vs. EAI (from an average of 2.3 to 3.2, and 2.3 to 2.9 mmHg·ml−1·m−2 in men and women, respectively) during exercise.
The change in EA during exercise results from a complex interplay among the changes in BP, PVR, arterial stiffness, and HR. At rest, the sensitivity of EA to a change in PVR/HR is approximately three times higher than to a similar change in arterial stiffness (16, 69). In contrast, during exercise, arterial stiffness has a progressively and intensity-dependent greater impact on EA than PVR (57, 58), such that EA (on average) increases during exercise, paralleling the increase in arterial stiffness, despite a reduction in PVR (57).
Some of the methodological issues pertaining to the noninvasive assessment of EA/ELV and its components during exercise should be highlighted. At rest, the single-beat elastance approach is regarded as the preferred noninvasive method to measure ELV (17). However, the single-beat elastance approach may be technically challenging during exercise, because of the difficulties in measuring cardiac volumes and systolic time intervals with echocardiography during exercise (63). If imaging modalities other than echocardiography are available for measuring ESV and SV during exercise (e.g., gated blood pool scans), then estimating ELV from the formula ELV = ESP/ESV may be more feasible. However, the noninvasive measurement of ELV from ESP/ESV has, in turn, its own limitations. 1) It assumes that V0 is negligible compared with ESV. V0 has not been well characterized in humans, particularly during exercise. In healthy adult dogs, Little and Cheng (43) found that, although the absolute values of V0 did not significantly change during exercise, V0 as a percentage of ESV increased by 9%. In contrast, in healthy subjects, Starling (74) found that V0, both in absolute values and as a percentage of ESV, did not appreciably change during dobutamine infusion. 2) The formula used to noninvasively estimate ESP (ESP = 0.9 × systolic BP) has not been validated during exercise. In this regard, methodologies that use radial applanation tonometry may be of help as they allow noninvasive and accurate estimations of central SBP at rest and during exercise, at least in the supine position and at low intensities of exercise (71, 72).
In the subsequent sections, we will examine the effects of age, hypertension, and HF on EA/ELV and its components, both at rest and during exercise, and we will highlight important sex differences when appropriate. Advancing age, hypertension, and HF all result in alterations in EA/ELV and its components, both at rest and during exercise. These changes are summarized in Table 1.
Table 1.
Summary of the alterations in arterial-ventricular coupling and its components with age, hypertension, and heart failure at rest and during exercise
| At Rest |
At Peak Exercise |
Reserve |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EA/ELV | EA | ELV | EA/ELV | EA | ELV | EA/ELV | EA | ELV | |||||||
| Old vs. young | |||||||||||||||
| Men | ↔ | ↑ | ↑ | ↑ | ↔ | ⇊ | ↓ | ↔ | ↓ | ||||||
| Women | ↓ | ↑ | ⇈ | ↑ | ↑ | ↓ | ↓ | ↔ | ↓ | ||||||
| HTN vs. NT | |||||||||||||||
| Men | ↔ | ↑ | ↑ | ↔ | ↑ | ↑ | ↔ | ↔ | ↔ | ||||||
| Women | ↓ | ↑ | ⇈ | ↔ | ↔ | ↔ | ↓ | ↓ | ↔ | ||||||
| SHF vs. healthy controls | |||||||||||||||
| Men and Women | ↑ | ↑ | ⇊ | ↑ | ⇊ | ⇊ | ⇊ | ⇊ | ⇊ | ||||||
| HFpEF vs. healthy controls | |||||||||||||||
| Men and Women | ↔ | ↑ | ↑ | ? | ? | ⇊ | ? | ? | ⇊ | ||||||
Arrows indicate directionality of the comparison. EA, effective arterial elastance; ELV, LV end-systolic elastance; EA/ELV, arterial-ventricular coupling; HTN, hypertensive; NT, normotensive; SHF, systolic heart failure; HFpEF, heart failure with a preserved ejection fraction.
AGE
Arterial-Ventricular Coupling and Its Components at Rest
Aging influences the structural and functional properties of both the arterial system and the LV. With advancing age, the central arteries dilate, and their walls become thicker and stiffer (51). EA increases with advancing age in individuals without known CV diseases (18, 21, 65). For example, in 2,042 individuals from Olmsted County, Minnesota, Redfield et al. (61) observed an age-associated increase in EAI in both men (r = 0.28, P < 0.001) and women (r = 0.28, P < 0.001) (Fig. 2). Similar results were obtained in a subset of the population (n = 623) that was free from CV diseases (61). The age-associated increase in EAI was principally attributed to the age-associated increase in arterial stiffness, because pulse pressure increased with age, whereas PVR and HR did not change.
Fig. 2.
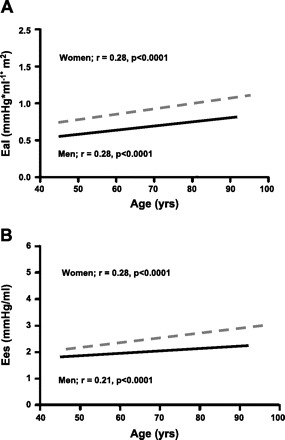
The association between age and EA indexed to body surface area (EAI) (A) and ELV (Ees = ELV) (B) in men (solid line) and women (dashed line) at rest. Pearson correlation coefficients, and probability values for each association are shown. With advancing age, both EaI and Ees increase. However, the increase in Ees with age is significantly greater in women than men. Furthermore, EaI and Ees are higher in women vs. men at all ages. [Modified from Redfield et al. (61).]
Advancing age is also associated with alterations in left ventricular structure and function. Most notably, there is a reduction in myocyte number in men, but not in women, and there is an increase in left ventricular wall thickness and collagen deposition (40, 55). These alterations are accompanied by an increase in resting ELV with advancing age (18, 21, 61). For example, healthy 75-yr-old men and women had a ≈10% (P < 0.01) and ≈15% (P < 0.01) higher resting ELV, respectively, compared with their 55-yr-old counterparts (Fig. 2) (61). These findings are unlikely to be due to differences in left ventricular chamber size, as similar results were obtained when ELV was normalized to left ventricular EDV (61). Sex-related differences were also noted, whereby ELV was higher in women than in men at all ages and increased more steeply with advancing age (61). Other markers of left ventricular systolic function, such as stress-corrected fractional shortening (61), circumferential end-systolic stress/ESV index (8), and preload-recruitable SW (30), have also been shown to be higher in women than in men. In addition, female rats have an enhanced systolic function compared with male rats (14, 81), suggesting that the higher ELV in women may reflect a higher left ventricular systolic function.
Because a higher ELV represents a steeper slope of the end-systolic P-V relationship, it results in an increased sensitivity of systolic pressure to changes in volume (36). Kass et al. (35) reported, in an isolated canine heart model, larger reductions in ELV after a myocardial infarction in hearts with a higher resting ELV. This greater mechanical vulnerability to an ischemic insult may help to explain why older individuals, who have an increased ELV [particularly older women and hypertensive (HTN) subjects, see below] experience worse outcomes following a myocardial infarction.
Even though EA and ELV both increase with advancing age, their ratio, EA/ELV, remains relatively unchanged across the age spectrum in men (18, 21, 50), suggesting that the increases in EA and ELV are matched. In contrast, EA/ELV declines slightly with advancing age in healthy women (61), reflecting a disproportionate increase in ELV compared with EAI. This suggests a greater impact of aging on ventricular vs. arterial properties in women compared with men. Furthermore, women have a higher resting EA, and an even higher resting ELV than men (50, 61), but a lower resting EA/ELV, suggesting that the sex differences in EA/ELV are predominantly related to sex differences in ventricular properties. These sex differences in EA and ELV persist even after adjusting for age, body size, and HR (61).
Arterial-Ventricular Coupling and Its Components During Exercise
It is well known that there is an age-associated deficit in CV performance at peak exercise that is evident from age-associated reductions in HR, EF, and oxygen consumption at peak exercise (41). Najjar et al. (50) reported that the decline in EAI/ELVI from rest to peak upright bicycle exercise is blunted in older (>60 yr) compared with younger (<40 yr) healthy men and women (Fig. 3A). This blunted EAI/ELVI response was attributed to a blunted increase in ELVI during exercise in older compared with younger individuals (Fig. 3C), as the increase in EAI during exercise did not differ between young and older subjects (Fig. 3B) (50). Consequently, advancing age is associated with a smaller EAI/ELVI reserve (EAI/ELVI peak − EAI/ELVI rest) and ELVI reserve (ELVI peak − ELVI rest) (50). Thus examining EA/ELV and its components during exercise provides additional insights into the age-associated deficits in EF reserve and suggests that it is due to age-associated deficits in left ventricular contractile reserve, more so than to age-associated differences in arterial properties.
Fig. 3.
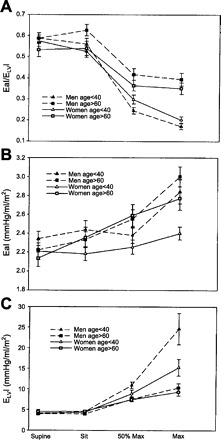
Arterial ventricular coupling (EaI/ELVI) (A), EaI (B), and ELV indexed to body surface area (ELVI) (C) in men (dashed lines) and women (solid lines) <40 yr of age (triangles) and >60 yr of age (squares) in the supine and seated positions, at 50% of maximal workload, and at peak exercise. EaI/ELVI decreases during exercise in both young and older men and women (P < 0.0001). However, older men and women have a blunted decline in EaI/ELVI (P < 0.001). EAI increases during exercise in both young and older men and women (P < 0.0001). At maximal exercise, EAI is greater in older vs. younger women (P < 0.002). In contrast, EAI does not differ between young and older men. ELVI increases during exercise in both young and older men and women (P < 0.0001). At maximal exercise, ELVI is greater in younger vs. older men (P < 0.001) and tended to be greater in younger than older women (P = 0.07). [From Najjar et al. (50).]
Najjar et al. (50) also observed age by sex interactions in EAI/ELVI in their normotensive (NT) cohort. In younger (<40 yr) individuals, peak EAI/ELVI was lower in men than in women, whereas in older (>60 yr) individuals, peak EAI/ELVI was higher in men than in women (Fig. 3A). This was attributed to an age by sex interaction in ELVI, whereby, at peak exercise, ELVI was higher in younger men vs. younger women, but did not differ between older men and women (Fig. 3C). As for EAI, it was higher in both young and old men compared with women (Fig. 3B).
Only one study has examined the effects of exercise training on EA/ELV and its components during exercise. In 26 patients with coronary artery disease, Rinder et al. (62) found that 12 mo of aerobic endurance exercise training lead to a 37% increase in ELV and a 23% decrease in EA/ELV during handgrip exercise performed at 30% of maximal voluntary contraction. However, the change in EA during handgrip exercise remained unaltered after the exercise training.
HYPERTENSION
Arterial-Ventricular Coupling and Its Components at Rest
The prevalence of hypertension markedly increases with advancing age, such that it affects 66% of individuals over 60 yr of age (56). Hypertension is an important risk factor for mortality, stroke, and HF (23, 31). The age-associated changes in arterial and left ventricular structure and function are accelerated in the presence of hypertension. HTN patients exhibit greater carotid wall thickness (2), central arterial wall stiffness (1), and reflected waves (53) than NT subjects, even after adjusting for age. Furthermore, hypertension is associated with LV remodeling and fibrosis (46).
Few studies have examined the impact of hypertension on EA/ELV and its components. Cohen-Solal et al. (20) showed that HTN individuals have a 60 and 95% higher EA and ELV, respectively, compared with NT controls. Saba et al. (64) found that HTN individuals have a 23 and 29% higher EA and ELV, respectively, compared with NT controls. However, EA/ELV did not differ between HTN and NT men (20, 64), suggesting that the increases in EA and ELV in HTN men was matched. In contrast, EAI/ELVI was ≈23% lower in systolic HTN compared with NT women (15), a finding that persisted even after adjusting for age (Fig. 4A). The lower EAI/ELVI in systolic HTN women was due to a disproportionate increase in ELVI (Fig. 4B) compared with EAI (45 vs. 16%) (Fig. 4C), suggesting an adaptation by these women to limit the impact of systolic hypertension on the vasculature or, alternatively, a more pronounced impact of systolic hypertension on ventricular vs. arterial elastance.
Fig. 4.
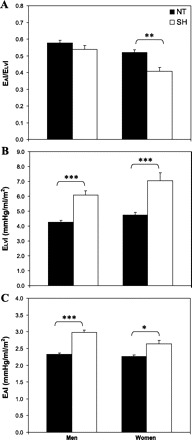
EAI/ELVI (A), ELVI (B), and EAI (C), measured at rest in normotensive (NT) and systolic hypertensive (SH) men and women. EAI/ELVI does not differ at rest between NT and SH men due to tandem increases in EAI and ELVI in SH vs. NT men. In contrast, resting EAI/ELVI is lower in SH women vs. NT women, due to a disproportionate increase in ELVI vs. EAI. *P < 0.05, **P < 0.01, ***P < 0.001, comparing NT to SH after adjusting for age. [From Chantler et al. (15).]
Arterial-Ventricular Coupling and Its Components During Exercise
Only one study has examined the effects of systolic hypertension on the changes in EA/ELV and its components during exercise. EAI/ELVI did not differ between NT and HTN men and women at 50% of maximal workload or at peak upright bicycle exercise (Fig. 5) (15). In men, this was because EAI and ELVI were proportionally higher at peak exercise in HTN compared with NT, whereas, in women, this was because EAI and ELVI did not differ at peak exercise between HTN and NT. Thus EAI/ELVI reserve did not differ between HTN and NT men, but it was 61% lower in HTN compared with NT women, because HTN women have a lower EAI/ELVI at rest. This was accompanied by a 60% lower EAI reserve in HTN compared with NT women (15).
Fig. 5.
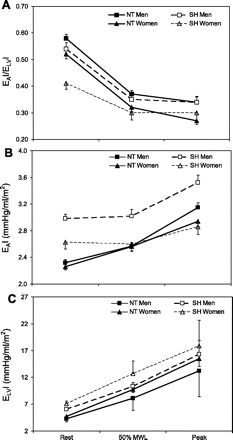
The change in EAI/ELVI (A), EAI (B), and ELVI (C) in NT (solid symbols) and SH (open symbols) men (solid lines) and women (dashed lines). At rest, EAI/ELVI is similar between NT and SH men and is lower in SH vs. NT women (P < 0.01). EaI/ELVI decreases during exercise in both NT and SH men and women (P < 0.01). There are no differences between NT and SH men and women at 50% maximal workload (MWL) or at peak exercise. At rest, EAI is higher in SH vs. NT men and women (P < 0.001). EAI increases during exercise in both NT and SH men and women (P < 0.05). However, only SH men have a higher EAI at 50% MWL and at peak exercise vs. NT men (P < 0.001), as no differences are found between NT and SH women at 50% MWL or peak exercise. At rest, ELVI is higher in SH vs. NT men (P < 0.05). ELVI increases during exercise in both NT and SH men and women. However, only SH men have a higher ELVI at 50% MWL or at peak exercise vs. NT men (P < 0.001), as no differences are found between NT and SH women at 50% MWL or at peak exercise. [Modified from Chantler et al. (15).]
HEART FAILURE
Arterial-Ventricular Coupling and Its Components at Rest
HF is a syndrome that is characterized by an inability of the heart to pump a sufficient amount of blood to meet the demands of the metabolizing tissues, or can do so only at the expense of elevated filling pressures (30a). We will only provide a brief summary of the alterations in EA/ELV and its components in HF, as insights they provide into the pathophysiology of HF and response to treatments have recently been reviewed (9, 27).
Systolic HF.
HF patients with systolic dysfunction are characterized by a diminished resting EF and left ventricular contractility (5). Systolic HF patients have a downward and rightward shift of the end-systolic P-V relationship (Fig. 6) and thus a reduced ELVI (range 0.6–2.6 mmHg·ml−1·m−2) (5). Systolic HF patients have an augmented EAI (range 1.7–3.7 mmHg·ml−1·m−2) due to a decrease in SVI and to increases in HR and PVR (5). The increase in EAI and decrease in ELVI result in marked, up to three- to fourfold, increases in EA/ELV (range 1.3–4.3) (5, 68). This suboptimal coupling reflects diminished CV performance and efficiency of the failing heart.
Fig. 6.
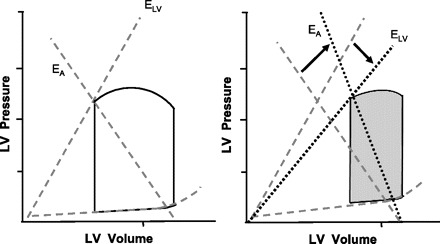
Pressure-volume relationships comparing the slope of the end-systolic pressure-volume relationship (ELV) and the slope of the line joining the ESP and EDV points (EA) between healthy controls (left) and systolic heart failure (HF) patients (right). Systolic HF patients have a downward and rightward shift of the end-systolic pressure-volume relationship, reflecting a reduced LV contractility. In addition, systolic HF patients have a higher EA than healthy controls. Thus, systolic HF patients have a higher resting arterial-ventricular coupling ratio than healthy controls.
HF with a preserved EF.
Approximately 40% of patients with HF have an EF ≥ 50% (59). These individuals are classified as HF patients with a preserved EF (HFpEF). HFpEF is more prevalent in older women than men (39), and systolic HTN is the most common risk factor for this syndrome (39). HFpEF patients have an 18 and 20% higher resting EA and ELV, respectively, compared with healthy controls (42). One study found that patients with HFpEF had a disproportionately higher ELV than EA compared with young and old NT controls, and thus a lower EA/ELV (36). However, in a larger sample population, Lam et al. (42) noted that patients with HFpEF had matched increases in EA and ELV; thus their EA/ELV did not differ from EA/ELV of NT controls or individuals with HTN but without HF. These conflicting findings may be due to differences in the characteristics of the control groups with which the HFpEF patients were compared, or to the heterogeneity among patients classified as having HFpEF. For example, Maurer et al. (45) noted that some patients with HFpEF who were NT had values of EA and ELV that did not differ from those of healthy NT controls.
Arterial-Ventricular Coupling and Its Components During Exercise
Systolic HF.
The baseline alterations in EA/ELV and its components in patients with systolic HF are also evident during exercise (22). Indeed, EA, ELV, and EA/ELV do not appreciably change during exercise in systolic HF patients, whereas they markedly change in healthy subjects (22). Thus the limited capacity of systolic HF patients to augment their CV function during times of stress (such as exercise) involve marked deficits in both LV and arterial elastance reserves.
HF with a preserved EF.
To date, there are no studies that have examined the change in EA/ELV or EA during exercise in HFpEF. Borlaug et al. (10) examined the change in ELV and EF in HFpEF during upright bicycle exercise. Compared with HTN controls with LV hypertrophy, HFpEF patients had a threefold smaller increase in ELV and a reduced ability to lower their PVR and increase their HR during exercise (10). They also had a 50% smaller increase in EF during exercise. As EF is inversely related to EA/ELV (20), this suggests that the change in EA/ELV during exercise in HFpEF may also be severely blunted. Since female sex and systolic hypertension are risk factors for HFpEF (39), and as the pathophysiology of HFpEF involves a limited CV reserve (38), the diminished EA/ELV reserve observed in systolic HTN women (15) suggests that they may be exhibiting signs of subclinical (Stage B) HF. This raises the possibility that they may be on a trajectory to progressive exercise intolerance and perhaps functional limitations.
CARDIAC ENERGETICS
EA/ELV is an important determinant of cardiac energetics (13, 74). From P-V loops, total mechanical energy can be quantified as the pressure-volume area (PVA), which is composed of SW (shaded area in Fig. 1) and potential energy (hatched area in Fig. 1) (76). PVA and myocardial oxygen demand are linearly related (75); therefore, PVA has been used as an index of the oxygen cost of performing a given SW (74). Thus the amount of SW performed by the LV and the amount of oxygen the LV consumes while performing this SW vary with the loading conditions imposed on the LV and the contractile state of the LV (76).
EA/ELV in the range of 0.6–1.2 in the resting state (9) is thought to represent the optimal interaction between the arterial and LV systems (74) and confers the near-optimal balance between mechanical efficacy and energetic efficiency (74). Interestingly, this range is also observed across species (44, 80), suggesting that it has been conserved through mammalian evolution.
During exercise, energetic efficiency is sacrificed in favor of cardiac efficacy to augment CV performance. To date, very few studies in humans have examined the impact of age, hypertension, and HF on cardiac energetics during exercise. Chantler et al. (15) found that HTN women, but not men, had a higher SWI and PVA at peak exercise compared with NT controls. Furthermore, an exaggerated increase in EA during exercise, as noted in older women (50) and in women with hypertension (60), increases the pulsatile load on the LV, which may increase the cardiac energy costs to provide blood flow by raising myocardial oxygen consumption.
CONCLUSIONS
Examination of the alterations in EA/ELV and its components, EA and ELV with aging, hypertension, and HF at rest and during exercise is often overlooked, yet it can yield mechanistic insights into the pathophysiology of these conditions and how they differ between men and women. Future studies should examine the effects of lifestyle changes (e.g., physical conditioning), other medical conditions (e.g., diabetes), and the various classes of medications used to treat hypertension and HF on EA/ELV and its components, EA and ELV. Furthermore, longitudinal studies are needed to evaluate whether alterations in EA/ELV, EA, and ELV provide any prognostic information for adverse outcomes, such as HF.
GRANTS
This work was supported (in part) by the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
REFERENCES
- 1.Amar J, Ruidavets JB, Chamontin B, Drouet L, Ferrieres J. Arterial stiffness and cardiovascular risk factors in a population-based study. J Hypertens 19: 381–387, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Arnett DK, Tyroler HA, Burke G, Hutchinson R, Howard G, Heiss G. Hypertension and subclinical carotid artery atherosclerosis in blacks and whites. The Atherosclerosis Risk in Communities Study. ARIC Investigators. Arch Intern Med 156: 1983–1989, 1996 [PubMed] [Google Scholar]
- 3.Asanoi H, Kameyama T, Ishizaka S, Miyagi K, Sasayama S. Ventriculoarterial coupling during exercise in normal human subjects. Int J Cardiol 36: 177–186, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Asanoi H, Sasayama S, Kameyama T. Ventriculoarterial coupling in normal and failing heart in humans. Circ Res 65: 483–493, 1989 [DOI] [PubMed] [Google Scholar]
- 6.Batterham AM, George KP, Whyte G, Sharma S, McKenna W. Scaling cardiac structural data by body dimensions: a review of theory, practice, and problems. Int J Sports Med 20: 495–502, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Belcher P, Boerboom LE, Olinger GN. Standardization of end-systolic pressure-volume relation in the dog. Am J Physiol Heart Circ Physiol 249: H547–H553, 1985 [DOI] [PubMed] [Google Scholar]
- 8.Bella JN, Palmieri V, Roman MJ, Paranicas MF, Welty TK, Lee ET, Fabsitz RR, Howard BV, Devereux RB. Gender differences in left ventricular systolic function in American Indians (from the Strong Heart Study). Am J Cardiol 98: 834–837, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Borlaug BA, Kass DA. Ventricular-vascular interaction in heart failure. Heart Fail Clin 4: 23–36, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation 114: 2138–2147, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Burkhoff D, de Tombe PP, Hunter WC, Kass DA. Contractile strength and mechanical efficiency of left ventricle are enhanced by physiological afterload. Am J Physiol Heart Circ Physiol 260: H569–H578, 1991 [DOI] [PubMed] [Google Scholar]
- 12.Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol 289: H501–H512, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Burkhoff D, Sagawa K. Ventricular efficiency predicted by an analytical model. Am J Physiol Regul Integr Comp Physiol 250: R1021–R1027, 1986 [DOI] [PubMed] [Google Scholar]
- 14.Capasso JM, Remily RM, Smith RH, Sonnenblick EH. Sex differences in myocardial contractility in the rat. Basic Res Cardiol 78: 156–171, 1983 [DOI] [PubMed] [Google Scholar]
- 15.Chantler PD MV, Schulman SP, Gerstenblith G, Becker LC, Ferrucci L, Fleg JL, Lakatta EG, Najjar SS. The sex-specific impact of systolic hypertension, and systolic blood pressure on arterial-ventricular coupling at rest and during exercise. Am J Physiol Heart Circ Physiol 295: H145–H153, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chemla D, Antony I, Lecarpentier Y, Nitenberg A. Contribution of systemic vascular resistance and total arterial compliance to effective arterial elastance in humans. Am J Physiol Heart Circ Physiol 285: H614–H620, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA, Kawaguchi M, Kass DA. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol 38: 2028–2034, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Chen CH, Nakayama M, Nevo E, Fetics BJ, Maughan WL, Kass DA. Coupled systolic-ventricular and vascular stiffening with age: implications for pressure regulation and cardiac reserve in the elderly. J Am Coll Cardiol 32: 1221–1227, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Chen CH, Nakayama M, Talbot M, Nevo E, Fetics B, Gerstenblith G, Becker LC, Kass DA. Verapamil acutely reduces ventricular-vascular stiffening and improves aerobic exercise performance in elderly individuals. J Am Coll Cardiol 33: 1602–1609, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Cohen-Solal A, Caviezel B, Himbert D, Gourgon R. Left ventricular-arterial coupling in systemic hypertension: analysis by means of arterial effective and left ventricular elastances. J Hypertens 12: 591–600, 1994 [PubMed] [Google Scholar]
- 21.Cohen-Solal A, Caviezel B, Laperche T, Gourgon R. Effects of aging on left ventricular-arterial coupling in man: assessment by means of arterial effective and left ventricular elastances. J Hum Hypertens 10: 111–116, 1996 [PubMed] [Google Scholar]
- 22.Cohen-Solal A, Faraggi M, Czitrom D, Le Guludec D, Delahaye N, Gourgon R. Left ventricular-arterial system coupling at peak exercise in dilated nonischemic cardiomyopathy. Chest 113: 870–877, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Collins R, Peto R, MacMahon S, Godwin J, Qizilbash N, Collins R, MacMahon S, Hebert P, Eberlein KA, Taylor JO, Hennekens CH, Fiebach NH, Qizilbash N, Hennekens CH. Blood pressure, stroke, and coronary heart disease. Part 2. Short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet 335: 827–838, 1990 [DOI] [PubMed] [Google Scholar]
- 24.de Simone G, Devereux RB, Daniels SR, Mureddu G, Roman MJ, Kimball TR, Greco R, Witt S, Contaldo F. Stroke volume and cardiac output in normotensive children and adults. Assessment of relations with body size and impact of overweight. Circulation 95: 1837–1843, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Dehmer GJ, Firth BG, Lewis SE, Willerson JT, Hillis LD. Direct measurement of cardiac output by gated equilibrium blood pool scintigraphy: validation of scintigraphic volume measurements by a nongeometric technique. Am J Cardiol 47: 1061–1067, 1981 [DOI] [PubMed] [Google Scholar]
- 26.Dewey FE, Rosenthal D, Murphy DJ Jr, Froelicher VF, Ashley EA. Does size matter? Clinical applications of scaling cardiac size and function for body size. Circulation 117: 2279–2287, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Fox JM, Maurer MS. Ventriculovascular coupling in systolic and diastolic heart failure. Curr Heart Fail Rep 2: 204–211, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Georgakopoulos D, Mitzner WA, Chen CH, Byrne BJ, Millar HD, Hare JM, Kass DA. In vivo murine left ventricular pressure-volume relations by miniaturized conductance micromanometry. Am J Physiol Heart Circ Physiol 274: H1416–H1422, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Grossman W, Braunwald E, Mann T, McLaurin LP, Green LH. Contractile state of the left ventricle in man as evaluated from end-systolic pressure-volume relations. Circulation 56: 845–852, 1977 [DOI] [PubMed] [Google Scholar]
- 30.Hayward CS, Kalnins WV, Kelly RP. Gender-related differences in left ventricular chamber function. Cardiovasc Res 49: 340–350, 2001 [DOI] [PubMed] [Google Scholar]
- 30a.Heart Failure Society of America. Executive Summary: HFSA 2006 Comprehensive Heart Failure Practice Guideline. J Card Fail 12: 10–38, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Kannel WB. Blood pressure as a cardiovascular risk factor: prevention and treatment. JAMA 275: 1571–1576, 1996 [PubMed] [Google Scholar]
- 32.Kass DA. Age-related changes in venticular-arterial coupling: pathophysiologic implications. Heart Fail Rev 7: 51–62, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Kass DA. Ventricular arterial stiffening: integrating the pathophysiology. Hypertension 46: 185–193, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Kass DA, Kelly RP. Ventriculo-arterial coupling: concepts, assumptions, and applications. Ann Biomed Eng 20: 41–62, 1992 [DOI] [PubMed] [Google Scholar]
- 35.Kass DA, Marino P, Maughan WL, Sagawa K. Determinants of end-systolic pressure-volume relations during acute regional ischemia in situ. Circulation 80: 1783–1794, 1989 [DOI] [PubMed] [Google Scholar]
- 36.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation 107: 714–720, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS, Kass DA. Effective arterial elastance as index of arterial vascular load in humans. Circulation 86: 513–521, 1992 [DOI] [PubMed] [Google Scholar]
- 38.Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan MJ. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol 17: 1065–1072, 1991 [DOI] [PubMed] [Google Scholar]
- 39.Klapholz M, Maurer M, Lowe AM, Messineo F, Meisner JS, Mitchell J, Kalman J, Phillips RA, Steingart R. Hospitalization for heart failure in the presence of a normal left ventricular ejection fraction: results of the New York heart failure registry. J Am Coll Cardiol 43: 1432–1438, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. III. Cellular and molecular clues to heart and arterial aging. Circulation 107: 490–497, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Lakatta EG. Cardiovascular regulatory mechanisms in advanced age. Physiol Rev 73: 413–467, 1993 [DOI] [PubMed] [Google Scholar]
- 42.Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation 115: 1982–1990, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Little WC, Cheng CP. Effect of exercise on left ventricular-arterial coupling assessed in the pressure-volume plane. Am J Physiol Heart Circ Physiol 264: H1629–H1633, 1993 [DOI] [PubMed] [Google Scholar]
- 44.Little WC, Cheng CP. Left ventricular-arterial coupling in conscious dogs. Am J Physiol Heart Circ Physiol 261: H70–H76, 1991 [DOI] [PubMed] [Google Scholar]
- 45.Maurer MS, King DL, El-Khoury Rumbarger L, Packer M, Burkhoff D. Left heart failure with a normal ejection fraction: identification of different pathophysiologic mechanisms. J Card Fail 11: 177–187, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Mayet J, Hughes A. Cardiac and vascular pathophysiology in hypertension. Heart 89: 1104–1109, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mehmel HC, Stockins B, Ruffmann K, von Olshausen K, Schuler G, Kubler W. The linearity of the end-systolic pressure-volume relationship in man and its sensitivity for assessment of left ventricular function. Circulation 63: 1216–1222, 1981 [DOI] [PubMed] [Google Scholar]
- 48.Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, Lakatta EG, Najjar SS, Kass DA. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol 49: 198–207, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Milnor WR. Arterial impedance as ventricular afterload. Circ Res 36: 565–570, 1975 [DOI] [PubMed] [Google Scholar]
- 50.Najjar SS, Schulman SP, Gerstenblith G, Fleg JL, Kass DA, O'Connor F, Becker LC, Lakatta EG. Age and gender affect ventricular-vascular coupling during aerobic exercise. J Am Coll Cardiol 44: 611–617, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension 46: 454–462, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Nevill AM, Holder RL. Scaling, normalizing, and per ratio standards: an allometric modeling approach. J Appl Physiol 79: 1027–1031, 1995 [DOI] [PubMed] [Google Scholar]
- 53.Nichols WW, Nicolini FA, Pepine CJ. Determinants of isolated systolic hypertension in the elderly. J Hypertens Suppl 10: S73–S77, 1992 [PubMed] [Google Scholar]
- 54.O'Rourke MF. Pressure and flow waves in systemic arteries and the anatomical design of the arterial system. J Appl Physiol 23: 139–149, 1967 [DOI] [PubMed] [Google Scholar]
- 55.Olivetti G, Giordano G, Corradi D, Melissari M, Lagrasta C, Gambert SR, Anversa P. Gender differences and aging: effects on the human heart. J Am Coll Cardiol 26: 1068–1079, 1995 [DOI] [PubMed] [Google Scholar]
- 56.Ong KL, Cheung BMY, Man YB, Lau CP, Lam KSL. Prevalence, awareness, treatment, and control of hypertension among United States Adults 1999–2004. Hypertension 49: 69–75, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Otsuki T, Maeda S, Iemitsu M, Saito Y, Tanimura Y, Ajisaka R, Miyauchi T. Contribution of systemic arterial compliance and systemic vascular resistance to effective arterial elastance changes during exercise in humans. Acta Physiol (Oxf) 188: 15–20, 2006 [DOI] [PubMed] [Google Scholar]
- 58.Otsuki T, Maeda S, Iemitsu M, Saito Y, Tanimura Y, Ajisaka R, Miyauchi T. Systemic arterial compliance, systemic vascular resistance, and effective arterial elastance during exercise in endurance-trained men. Am J Physiol Regulatory Integrative Comp Physiol 295: R228–R235, 2008 [DOI] [PubMed] [Google Scholar]
- 59.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. New Engl J Med 355: 251–259, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Park S, Ha JW, Shim CY, Choi EY, Kim JM, Ahn JA, Lee SW, Rim SJ, Chung N. Gender-related difference in arterial elastance during exercise in patients with hypertension. Hypertension 51: 1163–1169, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation 112: 2254–2262, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Rinder MR, Miller TR, Ehsani AA. Effects of endurance exercise training on left ventricular systolic performance and ventriculoarterial coupling in patients with coronary artery disease. Am Heart J 138: 169–174, 1999 [DOI] [PubMed] [Google Scholar]
- 63.Rowland T, Obert P. Doppler echocardiography for the estimation of cardiac output with exercise. Sports Med 32: 973–986, 2002 [DOI] [PubMed] [Google Scholar]
- 64.Saba PS, Ganau A, Devereux RB, Pini R, Pickering TG, Roman MJ. Impact of arterial elastance as a measure of vascular load on left ventricular geometry in hypertension. J Hypertens 17: 1007–1015, 1999 [DOI] [PubMed] [Google Scholar]
- 65.Saba PS, Roman MJ, Ganau A, Pini R, Jones EC, Pickering TG, Devereux RB. Relationship of effective arterial elastance to demographic and arterial characteristics in normotensive and hypertensive adults. J Hypertens 13: 971–977, 1995 [DOI] [PubMed] [Google Scholar]
- 66.Sagawa K. The ventricular pressure-volume diagram revisited. Circ Res 43: 677–687, 1978 [DOI] [PubMed] [Google Scholar]
- 67.Sagawa K, Suga H, Shoukas AA, Bakalar KM. End-systolic pressure/volume ratio: a new index of ventricular contractility. Am J Cardiol 40: 748–753, 1977 [DOI] [PubMed] [Google Scholar]
- 68.Sasayama S, Asanoi H. Coupling between the heart and arterial system in heart failure. Am J Med 90: 14S–18S, 1991 [DOI] [PubMed] [Google Scholar]
- 69.Segers P, Stergiopulos N, Westerhof N. Relation of effective arterial elastance to arterial system properties. Am J Physiol Heart Circ Physiol 282: H1041–H1046, 2002 [DOI] [PubMed] [Google Scholar]
- 70.Senzaki H, Chen CH, Kass DA. Single-beat estimation of end-systolic pressure-volume relation in humans. A new method with the potential for noninvasive application. Circulation 94: 2497–2506, 1996 [DOI] [PubMed] [Google Scholar]
- 71.Sharman JE, Lim R, Qasem AM, Coombes JS, Burgess MI, Franco J, Garrahy P, Wilkinson IB, Marwick TH. Validation of a generalized transfer function to noninvasively derive central blood pressure during exercise. Hypertension 47: 1203–1208, 2006 [DOI] [PubMed] [Google Scholar]
- 72.Sharman JE, McEniery CM, Campbell RI, Coombes JS, Wilkinson IB, Cockcroft JR. The effect of exercise on large artery haemodynamics in healthy young men. Eur J Clin Invest 35: 738–744, 2005 [DOI] [PubMed] [Google Scholar]
- 73.Sorensen SG, Ritchie JL, Caldwell JH, Hamilton GW, Kennedy JW. Serial exercise radionuclide angiography. Validation of count-derived changes in cardiac output and quantitation of maximal exercise ventricular volume change after nitroglycerin and propranolol in normal men. Circulation 61: 600–609, 1980 [DOI] [PubMed] [Google Scholar]
- 74.Starling MR. Left ventricular-arterial coupling relations in the normal human heart. Am Heart J 125: 1659–1666, 1993 [DOI] [PubMed] [Google Scholar]
- 75.Suga H. Global cardiac function: mechano-energetico-informatics. J Biomech 36: 713–720, 2003 [DOI] [PubMed] [Google Scholar]
- 76.Suga H. Ventricular energetics. Physiol Rev 70: 247–277, 1990 [DOI] [PubMed] [Google Scholar]
- 77.Sunagawa K, Maughan WL, Burkhoff D, Sagawa K. Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol Heart Circ Physiol 245: H773–H780, 1983 [DOI] [PubMed] [Google Scholar]
- 78.Sunagawa K, Sagawa K, Maughan WL. Ventricular interaction with the loading system. Ann Biomed Eng 12: 163–189, 1984 [DOI] [PubMed] [Google Scholar]
- 79.Sunagawa K, Sugimachi M, Todaka K, Kobota T, Hayashida K, Itaya R, Chishaki A, Takeshita A. Optimal coupling of the left ventricle with the arterial system. Basic Res Cardiol 88, Suppl 2: 75–90, 1993 [PubMed] [Google Scholar]
- 80.van den Horn GJ, Westerhof N, Elzinga G. Optimal power generation by the left ventricle. A study in the anesthetized open thorax cat. Circ Res 56: 252–261, 1985 [DOI] [PubMed] [Google Scholar]
- 81.Wang SN, Wyeth RP, Kennedy RH. Effects of gender on the sensitivity of rat cardiac muscle to extracellular Ca2+ Eur J Pharmacol 361: 73–77, 1998 [DOI] [PubMed] [Google Scholar]


