Abstract
Truncated escape responses characteristic of the zebrafish shocked mutant result from a defective glial glycine transporter (GlyT1). In homozygous GlyT1 mutants, irrigating brain ventricles with glycine-free solution rescues normal swimming. Conversely, elevating brain glycine levels restores motility defects. These experiments are consistent with previous studies that demonstrate regulation of global glycine levels in the CNS as a primary function of GlyT1. As GlyT1 mutants mature, their ability to mount an escape response naturally recovers. To understand the basis of this recovery, we assay synaptic transmission in primary spinal motor neurons by measuring stimulus-evoked postsynaptic potentials. At the peak of the motility defect, inhibitory synaptic potentials are both significantly larger and more prolonged indicating a prominent role for GlyT1 in shaping fast synaptic transmission. However, as GlyT1 mutants naturally regain their ability to swim, the amplitude of inhibitory potentials decreases to below wild-type levels. In parallel with diminishing synaptic potentials, the glycine concentration required to evoke the mutant motility defect increases 61-fold during behavioral recovery. Behavioral recovery is also mirrored by a reduction in the levels of both glycine receptor protein and transcript. These results suggest that increased CNS glycine tolerance and reduced glycine receptor expression in GlyT1 mutants reflect compensatory mechanisms for functional recovery from excess nervous system inhibition.
INTRODUCTION
The glial glycine transporter (GlyT1) is known to control the glycine concentration at both excitatory and inhibitory synapses of the CNS (Brasnjo and Otis 2003; Eulenburg et al. 2005). GlyT1 is present in glia surrounding excitatory glutamatergic synapses in the brain (Smith et al. 1992) where glycine serves as a co-agonist for the N-methyl-d-aspartate (NMDA) receptor (Johnson and Ascher 1987). Signaling through NMDA receptors is augmented in mice with reduced GlyT1 function due to elevated levels of synaptic glycine (Gabernet et al. 2005; Martina et al. 2005; Tsai et al. 2004). GlyT1 is also present in glia surrounding inhibitory neurons, where the transporter controls levels of tonic inhibition in the hindbrain and spinal cord (Bradaia et al. 2004; Cui et al. 2005; Gomeza et al. 2003). In addition to setting levels of tonic inhibition, GlyT1 plays a modest role in shaping synaptic events: inactivation of GlyT1 either pharmacologically (Bradaia et al. 2004) or genetically (Gomeza et al. 2003), results in a slowing in decay kinetics with little change in peak amplitudes of spontaneous inhibitory synaptic currents. By controlling the concentration of CNS glycine, GlyT1 both modulates the amplitude of glutamatergic synaptic responses through the excitatory NMDA receptor and globally regulates levels of tonic inhibition through the inhibitory glycine receptor.
The zebrafish GlyT1 mutant shocked provides an alternate vertebrate genetic model in which to study the impact of GlyT1 on glycinergic signaling. In contrast to mouse glyT1 knockouts that die at birth, zebrafish GlyT1 mutants exhibit a dramatic behavioral recovery. Because the zebrafish preparation provides unprecedented access to nerve and muscle for recording (Luna and Brehm 2006; Wen and Brehm 2005), we were able to investigate the physiological basis for both the immotile and recovered mutant phenotypes. Mechanisms underlying the mutant phenotype had been the subject of controversy (Cui et al. 2004; Luna et al. 2004) as to whether muscle or CNS functional deficits were causal to the phenotype. Even after identification of the mutated gene (Cui et al. 2005), it was not known to what extent synaptic transmission was affected in zebrafish GlyT1 mutants, or the relationship between the initial motility dysfunction and later recovery of swimming behaviors. Our analyses of shocked mutant fish revealed dual roles for the glial glycine transporter. First, we found that GlyT1 plays a major role in shaping inhibitory, glycinergic synaptic transmission. Second, as shown for the mouse mutant, we found that GlyT1 participates in regulating global levels of glycine and confirmed that the initial behavioral dysfunction was due to an increase in tonic CNS inhibition. In addition, we identify homeostatic changes including increased CNS glycine tolerance, reduced glycinergic synaptic potentials and reduced glycine receptor expression that likely represent compensatory mechanisms linked to functional behavioral recovery.
METHODS
Zebrafish (Danio rerio) heterozygous for the shocked te301 allele were obtained from the Max Planck Institute, Tübingen, Germany. To identify the mutations in glyT1 corresponding to the te301 shocked allele, exons were sequenced from genomic DNA. Then, a derived cleaved amplified polymorphic sequence (dCAPS) protocol was used to track the shocked te301 mutation at position 893 of zebrafish glyT1c (Genbank Accession No. NM_001030073). Primer f-TCACTCTGGACGGAGCCATCAGTGG r-CATGGTGATAAGGCCACCCCATGCA flanks the mutation site. The 3′ end of the reverse primer contains a single mismatch (underlined T) that creates an AVAIII (Fermentas, Hanover, MD) restriction site when it abuts the mutant but not the wild-type allele. Therefore amplification and digestion of this marker from genomic DNA of mutant and wild-type fish yields bands that are distinguishable by size. Individual fish were selected for recordings or analysis based on phenotype, and after the experiment, the genotype was determined using dCAPs.
For physiology, larval fish between the ages of 50 and 106 h post fertilization (hpf) were prepared as detailed elsewhere (Wen and Brehm 2005) with the following differences. To preserve the Mauthner neurons, fish were not decapitated. Following removal of the skin, they were paralyzed by application of 1 mg/ml α-bungarotoxin for 5 min (Sigma, St. Louis MO). Subsequently, fish were treated with 1 mg/ml collagenase (Invitrogen, Carlsbad CA) to facilitate removal of the muscle cells overlaying the spinal cord. Collagenase treatment was followed by extensive washes in bath solution prior to recording. The bath recording solution contained (in mM) 140 NaCl, 10 Na-HEPES, 3 KCl, 2 CaCl2, 1 MgCl2, and 13 sorbitol, pH 7.4 and 293 mosM. Patch recording pipettes were filled with (in mM) 134 K-gluconate, 10 K-HEPES, 10 K-EGTA, and 6 KCl, pH 7.2 plus 0.03% sulforhodamine B (Sigma) for post recording verification of cell type. Extracellular stimulating pipettes were pulled to a long taper with a final tip size of ∼2–3 μm, and filled with 140 mM NaCl. This pipette was placed adjacent to Mauthner ipsi- or contralateral axons in tail segments 12–14, and constant current pulses (200 μs, 2–5 μA) were delivered at 0.5 Hz (AM Systems Model 2100 stimulator).
Fish were prepared for immunohistochemical analysis as previously described (Ono et al. 2004). Anti-GlyRα antibodies, (mAB4a: Synaptic Systems GmbH, Goettingen, Germany), were used at 1:100 dilution. After a 30-min incubation in 100 μg/mL RNase A, prepared fish were mounted in Vectashield with propidium iodide (PPI; Vector Labs, Burlingame, CA). and images were captured on a Zeiss 510 Meta confocal microscope. Image settings were first established using wild-type specimens and then applied to mutant image collection so staining could be directly compared. Zeiss LSM 510 image analysis software was used to quantify fluorescence intensity in each neuronal cell body. Single z-sections in which the nucleus was largest in diameter were selected for analysis. Fluorescence was then quantified in a line below the nucleus and the maximum value recorded for comparison across neurons in mutant and wild-type spinal cord.
Stocks of 10 mM N [3-(4′-fluorophenyl)-3-(4′phenylphenoxy) propyl]sarcosine (NFPS, Sigma) dissolved in DMSO were diluted in 10% Hank's balanced salt solution (HBSS, Invitrogen) just prior to use. Strychnine (Sigma) was freshly made as 100 mM stock in chloroform and diluted for use in 10% HBSS or bath solution. 2-amino-5-phosphovaleric acid (APV; Sigma) was made as 10 mM stock in water, and stored at −20°C. 6-cyano-7-nitroquinoxaline-2, 3-dione (CNQX; Sigma) was made as 10 mM stocks in water and stored in the dark at 4°C.
RESULTS
Three alleles of shocked, ta229g, te301, and ta51e, were isolated in the large-scale Tübingen screen (Granato et al. 1996). All three mutant lines exhibited severely compromised touch avoidance responses to mechanical stimulation during early development. The impact of the te301 mutation was assessed by characterizing touch avoidance of fish either homozygous or heterozygous for the shocked te301 allele at several developmental stages. To elicit an escape response, fish were tapped on the head or tail with a tungsten wire and the subsequent movement was filmed at 1,000 frame/s with a high-speed CCD camera. At 30 hpf, wild-type fish spontaneously generated a quick coiling response when dechorionated (Fig. 1A), whereas, in response to mechanical stimulation, homozygous shocked embryos were motionless (Fig. 1B). shocked embryos also spontaneously coil indicating that mutant muscle is contractile but only from 17 to 21 hpf and at a reduced frequency (Cui et al. 2005). At 56 hpf, wild-type fish generated a robust escape response, initiated by a C-bend and immediately followed by rhythmic swimming responses (Fig. 1A). Homozygous shocked generated a single protracted C-bend followed by an abrupt termination of swimming (Fig. 1B). Remarkably, during the subsequent 48 h of development (104 hpf), homozygote shocked mutants naturally acquired the ability to mount normal stimulus-evoked escape responses (Fig. 1B). Heterozygous shocked were indistinguishable from wild-type fish at all stages (Fig. 1C), so future reference to mutant fish applies exclusively to homozygotes.
FIG. 1.
Escape swimming responses are abrogated by the glial glycine transporter (GlyT1) mutation. Mechanically evoked escape responses are plotted as fish pixel displacement (arbitrary units with all plots on the same scale) per frame as a function of time for wild-type (A), shocked homozygote (B), shocked heterozygote (C), and wild-type raised in 1 μM N [3-(4′-fluorophenyl)-3-(4′phenylphenoxy) propyl]sarcosine ]NFPS, D]. The displacement time lines were constructed using motion analysis software (Videoquant software, Viewpoint LS, Montreal, Ontario, Canada) from sequentially captured 512 × 512 pixel images acquired at 1,000 frame/s (Photron Fastcam PCI camera, San Diego, CA). Representative images below each graph are placed at the appropriate point of displacement. The swimming behaviors are shown for 3 different developmental ages. Calibration bar in A corresponds to 10 ms.
The first shocked allele to be positionally cloned (ta229g) revealed a mutation in the gene encoding GlyT1 (Cui et al. 2005). The te301 shocked allele (Luna et al. 2004) was independently mapped to a region on linkage group 2 which contains the glyT1 gene. Consequently, we sequenced genomic glyT1 from te301 and identified a mutation at position 893 that converted the wild-type cysteine to a mutant tyrosine. This residue is located in the middle of the sixth transmembrane domain of the GlyT1 protein. dCAPS markers, used to track the mutation, segregated with the mutant phenotype 100% of the time (n = 500), indicating that the mutation is responsible for the motility dysfunction. Also, as shown for ta229g, morpholinos directed against GlyT1 phenocopied the shocked motility dysfunction in wild-type fish (Cui et al. 2005). For te301, two distinct morpholinos directed against the third and fourth intron/exon boundaries of the glyT1 gene independently mimicked early aspects of the shocked phenotype when injected into wild-type embryos at the one-to-two-cell stage (data not shown). Finally, all aspects of the shocked te301 phenotype including embryonic immotility at 30 hpf, the aborted escape response at 56 hpf, and subsequent behavioral recovery at 104 hpf were recapitulated in wild-type fish raised in 1 μM NFPS, a specific blocker of GlyT1 function (Fig. 1D) (Aubrey and Vandenberg 2001), indicating that GlyT1 mutations ta229g (Cui et al. 2005) and te301 both severely compromise GlyT1 function.
In a previous study, rhythmic motor drive recorded in muscle could be restored in zebrafish GlyT1 mutants by irrigating brain ventricles and relieving CNS glycine build-up (Cui et al. 2005). We used a variation of this technique to establish the glycine levels required for the behavioral phenotype of shocked te301. At the peak of the motility phenotype (48–72 hpf), mutants exhibit an exaggerated and prolonged C-bend, which abruptly terminates the subsequent rhythmic swimming (Fig. 1B). The wild-type escape behavior could be restored in GlyT1 mutants by removal of the skin over the fourth ventricle of the hindbrain in solution lacking glycine. Under these conditions, improved swimming was evident within 15–30 min after surgical manipulation in 81% of the fish tested (n = 21).
This technique was exploited to compare the sensitivity of the escape response to CNS glycine levels before and after phenotypic recovery in GlyT1 mutants. For this purpose, ventricle-exposed mutant and wild-type fish were allowed to swim in glycine-free solution to ensure normal activity following surgery. Embryos were then transferred to solutions containing 10 mM glycine, a concentration that consistently resulted in an aborted escape response in all fish tested, including wild type. Subsequently, individual GlyT1 mutant fish were exposed to solutions containing successively lower concentrations of glycine and tested for their ability to mount a wild-type escape response. Dose-response curves were constructed reflecting the percentage of fish tested at each glycine concentration that mounted a wild-type escape response. A linear fit of the log values of glycine concentration curves yielded the half-maximal concentration of glycine permissive to wild-type escapes (glycine tolerance). Comparisons at 50–58 hpf indicated a 186-fold difference in glycine tolerance between GlyT1 mutant and wild-type fish (Fig. 2A; sho 5.9 μM; n = 17; WT 1.1 mM; n = 13). However, at later developmental stages, when GlyT1 mutant fish naturally recovered the ability to mount a normal escape response, they exhibit a 61-fold increase in glycine tolerance (Fig. 2B; sho 360 μM; n = 30). Over this same period, wild-type glycine tolerance increased 3.5-fold (Fig. 2B; WT 3.9 mM; n = 15). An increased glycine tolerance was also obtained in wild-type fish that were reared in 1 μM NFPS. Prior to recovery and at the peak of the swimming dysfunction the half-maximal value for NFPS treated wild-type fish was 8.6 μM glycine (Fig. 2A, n = 7), and following behavioral recovery the value rose to 223.6 μM glycine (Fig. 2B, n = 10).
FIG. 2.
CNS glycine tolerance before and after behavioral recovery. Percentage of brain-ventricle-exposed fish that could swim away from a tactile stimulus is plotted as a function of glycine concentration in the bath solution. For each concentration, an individual fish was scored as either positive or negative for ability to swim. •, shocked mutants; ○, wild-type reared in 1 μM NFPS; and ▴, wild-type control. The NFPS was included at all glycine concentrations during behavioral testing. The lines were fitted through 10–90% response curves by linear regression, and the 50% intersection of the line was used as a measure of sensitivity to glycine. At early stages [A, 50–58 h post fertilization (hpf)], GlyT1 mutants (n = 17) and wild-type fish in NFPS (n = 14) are nearly 200 times less glycine tolerant than wild-type fish (n = 13). After they regain normal motility (B, 98–122 hpf), GlyT1 mutants (n = 30) and wild-type fish in NFPS (n = 15) are only 11 times less glycine tolerant than wild-type fish.
To address potential mechanisms that underlie increased glycine tolerance as mutants mature, fast glycinergic synaptic transmission was directly examined at inhibitory synapses onto primary motor neurons in vivo (Fig. 3A). The rapid escape response of larval zebrafish is normally initiated by sensory input to the Mauthner neurons (Korn and Faber 2005). The two Mauthner cell bodies in the hindbrain extend axons that cross in the brain and descend the length of the spinal cord, forming excitatory synapses on ipsilateral primary motor neurons (Fig. 3A). Mauthner axons are also electrically coupled to glycinergic commissural interneurons that cross the spinal cord to inhibit contralateral primary motor neurons (Fetcho 1990). Escape responses were elicited by antidromic stimulation of the Mauthner axon while patch clamping a primary motor neuron. When the contralateral Mauthner axon was stimulated, a rapid hyperpolarization from −40 mV was followed by a slow depolarization in the motor neuron (Fig. 3A, Contra). In contrast, when the ipsilateral axon was stimulated, the response in the motor neuron was purely positive going and sufficiently large to result in the generation of an action potential (Fig. 3A, Ipsi). The ability of the Mauthner neuron to excite or inhibit motor neurons in GlyT1 mutant fish is consistent with the behavioral C-bend that occurs in response to touch (Fig. 1B).
FIG. 3.
Motor neuron postsynaptic potentials elicited by stimulation of Mauthner axons. A: diagram of the circuit that underlies escape behavior. Mauthner neurons, M, in the hindbrain extend their axons the length of the spinal cord. Axons make excitatory synapses (▴) on primary motor neurons, P. Mauthner axons are also electrically coupled (=) to inhibitory interneurons ( ) the axons of which cross the spinal cord to inhibit Ps on the opposite side. Axons of the Ps exit the spinal cord to innervate peripheral fast muscle (hatched parallelograms). SIpsi: stimulation of the ipsilateral Mauthner axon triggers an action potential. SContra: stimulation of the contralateral Mauthner axon triggers an inhibitory postsynaptic potential (IPSP) followed by a small depolarization. B: the relative amplitudes of the individual excitatory and inhibitory components of the synaptic potentials are dependent on membrane potential of the motor neuron. Synaptic potentials were triggered by stimulating the contralateral Mauthner axon in 50–58 hpf wild-type fish. Representative individual sweeps taken between −80 and 10 mV are shown. Near −40 mV the IPSP is negative-going and the excitatory postsynaptic potential (EPSP) is sufficiently positive to generate an action potential. Near −80 mV, both components are positive-going.
) the axons of which cross the spinal cord to inhibit Ps on the opposite side. Axons of the Ps exit the spinal cord to innervate peripheral fast muscle (hatched parallelograms). SIpsi: stimulation of the ipsilateral Mauthner axon triggers an action potential. SContra: stimulation of the contralateral Mauthner axon triggers an inhibitory postsynaptic potential (IPSP) followed by a small depolarization. B: the relative amplitudes of the individual excitatory and inhibitory components of the synaptic potentials are dependent on membrane potential of the motor neuron. Synaptic potentials were triggered by stimulating the contralateral Mauthner axon in 50–58 hpf wild-type fish. Representative individual sweeps taken between −80 and 10 mV are shown. Near −40 mV the IPSP is negative-going and the excitatory postsynaptic potential (EPSP) is sufficiently positive to generate an action potential. Near −80 mV, both components are positive-going.
The excitatory and inhibitory components of the contralaterally driven response in the motor neuron could be examined in isolation by taking advantage of the differences in reversal potentials (Fig. 3B). At potentials between −50 and 0 mV, motor neuron synaptic responses were biphasic with a fast negatively directed inhibitory component, followed by a slower positively directed excitatory component. As the membrane potential was shifted toward 0 mV, the excitatory component disappeared due to proximity to the excitatory reversal potential. Conversely, as the membrane potential was shifted negative to −54 ± 7 mV (n = 6), the inhibitory reversal potential, the early inhibitory component turned positive going (Fig. 3B). Consequently, at potentials negative to the reversal potential of the inhibitory component, there appeared to be two components of positive-going responses: a fast-activating component of depolarization that corresponds to the inhibitory component and a slowly activating, prolonged depolarization corresponding to the excitatory component. The reversal potentials of the excitatory and inhibitory components were similar for wild-type and GlyT1 mutants, so the two components were likewise distinguishable in voltage excursions from GlyT1 mutants (Fig. 4A). Based on the bath and pipette solutions, the predicted reversal potential for the chloride-mediated inhibitory component was −80 mV. The finding that the measured reversal potential for the inhibitory component was considerably more positive than predicted may be due to incomplete dialysis of the neuron or alternatively to accumulation of chloride in the pipette from the silver-chloride wire.
FIG. 4.
Pharmacological dissection of glutamatergic and glycinergic components of the motor neuron postsynaptic response. Representative sweeps of synaptic responses triggered by stimulation of the contralateral Mauthner axon in nontreated GlyT1 mutant fish (A) vs. 2-amino-5-phosphonovaleric acid (APV)/6-cyano-7-nitroquinoxalene-2,3-dione (CNQX; B) treated to block glutamate receptors or strychnine treated to block glycine receptors (C). The drug concentrations correspond to 100 μM APV, 50 μM CNQX, and 100 μM strychnine. The biphasic nature of the synaptic response is particularly apparent between −50 and −30 mV in A, and arbitrary shading distinguishes the slower kinetics of the excitatory component from the nonshaded, faster inhibitory component of the response. The slow component of the response is APV/CNQX sensitive (B) and therefore glutamatergic while the fast component of the response is strychnine sensitive (C) and therefore glycinergic. All traces were derived from GlyT1 homozygous fish at 50–58 hpf.
A second means of isolating the inhibitory and excitatory synaptic components utilized pharmacology (Fig. 4). The excitatory component of the synaptic response was effectively blocked by a combination of 100 μM APV and 50 μM CNQX, blockers of glutamate receptors (Fig. 4B). In the presence of these blockers, the inhibitory synaptic response was examined in isolation. At −80 mV, the voltage excursion was depolarizing with a rapid rising phase (10–90% rise time = 0.95 ± 0.06 (SD) ms; n = 19) and falling phase (10–90% decay time = 52.6 ± 8.4 ms). In two preparations tested, application of 100 μM strychnine, a specific inhibitor of the glycine receptor, blocked the evoked hyperpolarizing component of the synaptic response (Fig. 4C). In the presence of strychnine (Fig. 4C), the remaining excitatory response at −80 mV was slower to rise (10–90% = 14.6 ± 6.7 ms; n = 22 traces) and decay (10–90% = 192.7 ± 41.6 ms) than the inhibitory component seen in the presence of APV/CNQX (Fig. 4B). Only a few traces from each cell could be used to measure kinetics of the excitatory response in the presence of strychnine due to the propensity of the motor neuron to fire action potentials when glycine receptors were blocked. At −55 mV, the excitatory response consistently triggered action potentials, and at 0 mV, there was no observed postsynaptic response in the presence of strychnine, presumably due to the proximity of the reversal potential for the excitatory component (Fig. 4C).
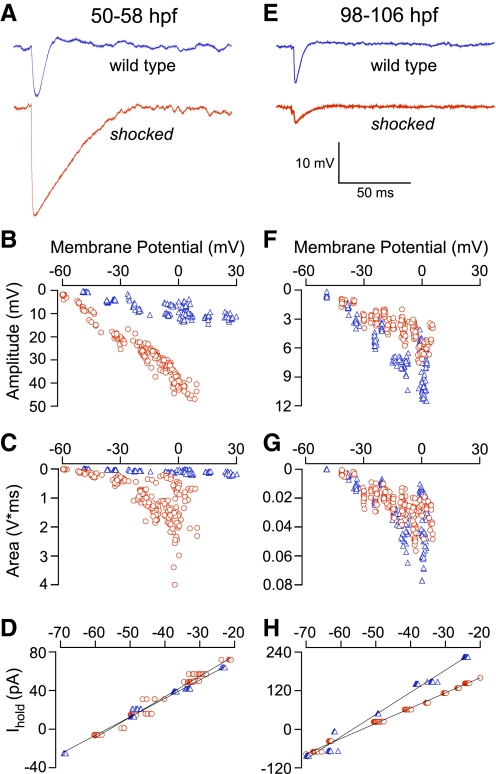
Taking advantage of the ability to isolate the glycinergic response by holding the membrane potential of the motor neuron at 0 mV, recordings of synaptic responses from GlyT1 mutant larvae at 50–58 hpf were compared with those of wild type. Representative synaptic responses recorded at 0 mV illustrated the larger amplitude and prolonged time course in GlyT1 mutant fish when compared with wild-type fish of the same age (Fig. 5A). The increased amplitude of GlyT1 mutant synaptic potentials was consistent at all membrane potentials tested (Fig. 5B). Overall comparisons indicated that the peak amplitude at 0 mV was significantly larger (P < 0.05) in GlyT1 mutants (26.7 ± 6.2 mV; n = 5) compared with wild type (15.0 ± 5.6 mV; n = 4). To quantify the overall response of the evoked inhibitory postsynaptic potentials (IPSPs), the area was integrated and plotted as a function of potential (Fig. 5C). The integral of the IPSP measured 876.7 ± 665.9 mV·ms (n = 5) in GlyT1 mutants compared with 194.8 ± 166.3 mV·ms (n = 4) for wild-type fish (P < 0.07). These differences in IPSP amplitude and kinetics did not reflect differences in motor neuron input resistance. Steady state measurements of input resistance between −80 and −30 mV indicated no significant differences (P = 0.14) between GlyT1 mutant (428.2 ± 81.1 MΩ) and wild-type fish (312.6 ± 107.8 MΩ; Fig. 5D).
FIG. 5.
Comparisons of IPSPs in wild-type and GlyT1 mutants before and after recovery. A: sample sweeps of evoked IPSPs from wild-type (blue) and shocked (red) motor neurons in response to stimulation of the contralateral Mauthner axon. Motor neurons are held at 0 mV to isolate the inhibitory component of the response. At early stages (50–58 hpf) when shocked exhibit motility dysfunction, inhibitory responses are larger and longer-lasting in shocked motor neurons. B: scatter plots of amplitude vs. membrane potential for all evoked IPSPs recorded from representative wild-type (triangles) and shocked (circles) fish. At all membrane potentials, shocked IPSPs are larger in amplitude than stage-matched wild type. C: scatter plots of the IPSP integral vs. membrane potential reflect both the greater amplitude and the slower decay of shocked IPSPs. D: scatter plots of steady-state holding current vs. membrane potential indicate no difference in input resistance between mutant and wild-type motor neurons. E: after shocked mutants have regained normal motility (98–106 hpf), sample sweeps of evoked IPSPs at 0 mV show mutant responses are similar to wild type. F: scatter plots show that recovered GlyT1 mutant inhibitory responses are smaller in amplitude than wild type; G: the integral of response is comparable to wild-type across holding potentials. Linear fit shown in both D and H was used to calculate input resistance.
Synaptic potentials were also compared at 98–106 hpf, an age when the GlyT1 mutant fish have recovered the ability to mount a wild-type-like escape response (Fig. 5E). Representative traces taken at 0 mV illustrated the significant differences (P < 0.02) in the amplitudes of inhibitory synaptic potentials for recovered GlyT1 mutant (4.1 ± 0.6 mV; n = 3) compared with wild-type fish (6.8 ± 0.9 mV; n = 3; Fig. 5, E and F). Following recovery there was no difference (P = 0.33) in the integrated area of the response between GlyT1 mutant (20.2 ± 11.8 mV·ms; n = 3) and wild-type fish (29.7 ± 9.1 mV·ms; n = 3; Fig. 5G). The age-dependent decrease in IPSP amplitudes was due, in part, to reductions in input resistance of the motor neurons. However, reductions in input resistance occurred for both wild-type (146.4 ± 47.8 MΩ) and recovered GlyT1 mutants (204.33 ± 39.5 MΩ; Fig. 5H). Moreover, at the time corresponding to recovery, the input resistance for GlyT1 mutants was higher than wild-type fish.
To identify genes the differential expression of which could explain functional recovery in mutants, transcript levels were compared in wild-type and GlyT1 mutant fish. We performed a developmental time course for GlyT1 mRNA as well as for neuronal glycine transporter (GlyT2), NMDA receptor glycine binding subunit (NR1.1), and the alpha 1 subunit of the glycine receptor (GlyRα1) (Fig. 6, A–D). The developmental profiles of GlyT1 mRNA in mutant and wild-type fish were similar. In contrast, at 50 hpf, GlyT2, NR1.1, and GlyRα1 transcript levels in GlyT1 mutants were all significantly different from wild-type (Fig. 6, B–D). NR1.1 was slightly elevated while GlyRα1 and GlyT2 were reduced in the mutants. At 122 hpf, when GlyT1 mutants had recovered the ability to swim, expression levels of GlyT2 and NR1.1 were similar to those in wild-type but expression levels of GlyRα1 mRNA remained significantly reduced (P < 0.05; Fig. 6D, 122 hpf; WT 4.67 ± 0.43; n = 3; sho 2.33 ± 0.9; n = 3 independent RNA samples, 20 fish each).
FIG. 6.
Glycine receptor transcript and protein expression levels are depressed during maturation of GlyT1 larvae. A: GlyT1 glial glycine transporterl B: GlyT2 neuronal glycine transporter; C: NR1.1 the glutamate/glycine receptor; D: GlyRα1 subunit of the glycine receptor. mRNA expression levels normalized to β-actin were compared for wild-type (dark fill) and shocked mutant fish (light fill) over the developmental time course of 26–122 hpf. Asterisks indicate significant differences between wild-type and shocked mutants (P < 0.05, Student's t-test). GlyRα1 transcript was the only transcript to remain significantly reduced during mutant development. E: immunohistochemical labeling of spinal neurons by mAB4a, an anti-GlyRα antibody. Wild-type (left) and GlyT1 mutants (right) labeled with antibody show comparable signal levels at 50 hpf, but signal is significantly diminished in GlyT1 mutant spinal cord at 122 hpf. Each of the 4 images is a projection of 10 sequential 1-μm-thick sections. The scale bar corresponds to 10 μm.
Glycine receptor expression was also assessed at the level of protein using the mAB4a antibody that recognizes all alpha subunits of the glycine receptor (Pfeiffer et al. 1984). In whole-mount immunohistochemistry of wild-type fish, the lateral spinal cord was heavily labeled as previously observed (McDearmid et al. 2006). To look more specifically at primary motor neurons, the mAB4a fluorescence was quantified in a subset of superficial neurons with large nuclei that reside in the lateral spinal cord (Fig. 6E). This class of cells should include the primary motor neurons. To control for consistency across samples, an independent fluorescent nuclear label, propidium iodide (PPI), was also quantified. At 50 hpf, when GlyT1 mutants are unable to swim, there were no significant differences between mutants and wild-type fish in peak fluorescence staining intensities for either mAB4a (P = 0.66; sho 3831 ± 241, n = 20; WT 3789 ± 353, n = 20) or PPI (P = 0.44; sho 2539 ± 390, n = 20; WT 2575 ± 253, n = 20). However, at 122 hpf following behavioral recovery, while PPI fluorescence remained unchanged (PPI: P = 0.23; sho 2835 ± 504, n = 20; WT 2528 ± 860, n = 20), there was significantly less GlyRα protein in GlyT1 mutants (mAB4a: P < 0.05; sho 3140 ± 778, n = 20; WT 4064.35 ± 80, n = 20).
DISCUSSION
Three distinct shocked alleles have been isolated (Granato et al. 1996) of which ta229g (Cui et al. 2005) and te301 (present study) are known to represent point mutations in the glyT1 gene. GlyT1, a member of the SLC6 family of amino acid transporters, is responsible for glycine reuptake into glial cells (Guastella et al. 1992; Liu et al. 1992; Smith et al. 1992). In the te301 allele, a point mutation in the sixth transmembrane region (TM6) converts a cysteine to a tyrosine (C298Y). While the GlyT1 cysteine 298 is conserved across taxa, it is not conserved in other members of the SLC6 transporter family. In the bacterial SLC6 transporter LeuT, where crystal structure is known (Yamashita et al. 2005), TM6 has been implicated in substrate binding. Although the extent to which glycine transport is compromised in te301 is not known, the ta229g allele lacks the ability to transport glycine altogether (Cui et al. 2005), and both mutant alleles share the same motility defects. Moreover, shocked motility defects are phenocopied in wild-type fish by injecting morpholinos directed against GlyT1 transcript or by treatment with an inhibitor of GlyT1 function. These data support that the te301 GlyT1 mutation severely compromises glycine transporter function.
Our direct recordings of a CNS glycinergic synapse in shocked suggest that GlyT1 normally functions to shape fast glycinergic synaptic responses. At 50–58 hpf, inhibitory synaptic potentials in GlyT1 mutants were ∼50% larger in amplitude and 450% larger in area than wild-type. A role for glial transporters in synaptic function is well established at glutamatergic nerve terminals (Tzingounis and Wadiche 2007). Given the intimate relationship between nerve and glia required for involvement of a glial transporter in synaptic transmission, it would be expected to find variability in the extent to which the transporter regulates kinetics at different glycinergic synapses. Indeed the synapses examined on goldfish mauthner neurons (Titmus et al. 1996), mouse hypoglossal neurons (Gomeza et al. 2003), and rat lamina X spinal neurons (Bradaia et al. 2004) did not reveal a prominent role for GlyT1 in fast inhibitory transmission. Nonetheless, fast synaptic transmission was profoundly affected by the GlyT1 mutation at the glycinergic synapse onto the zebrafish primary motor neuron.
Our experimental findings provide further support for the role of GlyT1 in global glycinergic inhibition. The idea that glial glycine transporters regulate global glycine concentrations originated from mouse knockout models of the glyT1 gene (Gomeza et al. 2003). Recordings of respiratory circuit activity from the brain slices of GlyT1−/− newborn mice showed a significant reduction in the frequency of neuronal firing. Hypoglossal motor neurons exhibited increased membrane noise and standing current, both of which were strychnine-sensitive (Gomeza et al. 2003). Elegant studies in the zebrafish GlyT1 mutant ta229g also emphasize the importance of GlyT1 in setting global levels of glycine (Cui et al. 2005). Muscle recordings demonstrate that rhythmic motor output could be restored in GlyT1 mutants by irrigating the fourth ventricle of the brain with glycine-free solution (Cui et al. 2005). We adapted this technique by irrigating mutant brains with glycine free solution and monitoring recovery at the level of behavior. Reintroduction of glycine to the bath restored the GlyT1 mutant phenotype provided that the concentration was sufficiently high. These studies confirm that GlyT1 regulation of global CNS glycine levels is crucial for normal function of the motor circuit.
Exposing GlyT1 mutant and wild-type brains to known glycine concentrations allowed us both to titrate glycine concentrations permissive for normal behavior and to provide insight into the behavioral recovery that occurs in GlyT1 mutant fish during development. At 50–58 hpf, wild-type glycine tolerance was nearly 200-fold greater than in GlyT1 mutants. However, between 96 and 120 hpf, corresponding to the time that GlyT1 mutant fish acquired the ability to swim, glycine tolerance in mutants increased >60-fold. Over the same time period, the glycine tolerance in wild-type increased only threefold. Consequently, following the developmental acquisition of swimming by GlyT1 mutants, CNS glycine tolerance in mutants approaches that of wild-type fish.
What might account for the altered sensitivity to global glycine during development? Insights to possible mechanisms came from our recordings of inhibitory synaptic potentials from primary motor neurons. During the period of behavioral recovery in GlyT1 mutant fish, motor neuron inhibitory synaptic potentials decreased significantly, adopting the kinetics and amplitude of wild-type responses. The decreased response was not a secondary consequence of disproportionate changes in input resistance. Although the synaptic changes at this motor neuron synapse cannot account for the recovery, we suggest that this synapse serves to reflect synaptic changes that are taking place globally and that underlie increased glycine tolerance in recovered GlyT1 mutants.
We propose that the developmental decrease in synaptic potential amplitude results, in part, from a reduction in the number of postsynaptic glycine receptors. This is based on quantitative measurements of RNA coding for the alpha subunit of the glycine receptor as well as immunohistochemical labeling of motor neurons by anti-GlyRα subunit antibodies. Because we detect a reduction in GlyRα1 transcript levels in RNA samples isolated from whole animals, this reduction must have occurred throughout the nervous system. Indeed GlyRα immunoreactivity is reduced in neurons viewed throughout the spinal cord. The largest decreases in glycine receptor expression measured in the motor neurons are only apparent after decreases in synaptic potentials. This could reflect the greater sensitivity of the physiological assays or that receptors are functionally inactivated prior to their transcriptional down-regulation. At early stages of excess inhibition, bath application of strychnine to block glycine receptors rescues rhythmic swimming (Supplemental Movie).1 (Cui et al. 2005). These findings support that reducing glycine receptor function is a viable mechanism for restoring swim circuit function.
By analogy to cholinergic synapses, a reduction in glycine receptor number would be expected to both reduce amplitude and accelerate kinetics of inhibitory responses in the absence of glycine uptake. At neuromuscular synapses, inhibition of acetylcholine hydrolysis increases the amplitude and time course of synaptic currents, much like that seen at inhibitory synapses of GlyT1 mutant fish before recovery. Experimental reduction of receptor density by application of either curare or alpha-bungarotoxin decreased the amplitude and greatly accelerated the kinetics of synaptic current (Katz and Miledi 1973). The proposed mechanism responsible for the altered kinetics is reduced probability of rebinding to receptors due to lowered density, thereby facilitating clearance from the synaptic cleft. Should similar mechanisms occur in the hindbrain, the altered density of glycine receptors would explain the observed homeostatic decrease in sensitivity to glycine.
Homeostatic changes in receptor expression have been described in other SLC6 transporter mutants including serotonin, norepinephrine, GABA, and dopamine transporter knockout mice (Bengel et al. 1997; Jensen et al. 2003; Jones et al. 1998; Xu et al. 2000). However, GlyT1−/− mice lack the compensatory changes in inhibitory synaptic machinery, including the mouse GlyRα protein (Gomeza et al. 2003). This difference is likely due to the fact that GlyT1−/− mutant mice die at birth, whereas all other transporter mutant lines are viable and fertile, often studied days to weeks after birth (Bengel et al. 1998; Jensen et al. 2003; Jones et al. 1998; Xu et al. 2000). The compensatory downregulation of inhibitory glycine receptors in GlyT1 mutant zebrafish is likely to represent general homeostatic mechanisms that compensate for excessive levels of neurotransmitter when transporters are inactivated.
Compensatory receptor expression in response to changes in circuit-wide activity has been demonstrated at other vertebrate CNS synapses (reviewed in Turrigiano 2007); however, the signals that trigger the compensatory response are unknown. Because glycine levels produce tonic inhibition, it is likely that activity-dependent mechanisms are involved. This idea is supported by the fact that fast skeletal muscle exhibits electrical coupling in GlyT1 mutant larvae at a stage when wild-type fast muscle has already lost gap junctional coupling (Luna et al. 2004). Loss of electrical coupling in skeletal muscle also occurs in Xenopus and in this preparation has been shown to depend on CNS electrical activity (Armstrong et al. 1983). The mechanisms that trigger these dramatic homeostatic modifications in the GlyT1 mutant will be the focus of future studies.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants NS-18205 to P. Brehm, NS-25639 to J. R. Fetcho, NS-44758 to M. A. Masino, and NS-048200 to J. E. Dallman. G. Mandel is an Investigator of the Howard Hughes Medical Institute.
Acknowledgments
We thank Drs. Hua Wen and Meng Wang for technical assistance with recordings and Dr. James Baker for his critical reading of the manuscript.
Present addresses: G. Mandel, P. Brehm, and R. Mongeon, Vollum Institute, Oregon Health Science University, Portland, OR 97239; M. R. Gleason, Laboratory of Sensory Neuroscience, The Rockefeller University, 1230 York Ave., Box 314, New York, NY 10021; M. A. Masino, Dept. of Neuroscience, University of Minnesota, 321 Church St., 6-145 Jackson Hall, Minneapolis, MN 55455.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Armstrong et al. 1983.Armstrong DL, Turin L, Warner AE. Muscle activity and the loss of electrical coupling between striated muscle cells in Xenopus embryos. J Neurosci 3: 1414–1421, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubrey and Vandenberg 2001.Aubrey KR, Vandenberg RJ. N[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)propyl]sarcosine (NFPS) is a selective persistent inhibitor of glycine transport. Br J Pharmacol 134: 1429–1436, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengel et al. 1998.Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, Lesch KP. Altered brain serotonin homeostasis and locomotor insensitivity to 3,4-methylenedioxymethamphetamine (“ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol 53: 649–655, 1998. [DOI] [PubMed] [Google Scholar]
- Bradaia et al. 2004.Bradaia A, Schlichter R, Trouslard J. Role of glial and neuronal glycine transporters in the control of glycinergic and glutamatergic synaptic transmission in lamina X of the rat spinal cord. J Physiol 559: 169–186, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasnjo and Otis 2003.Brasnjo G, Otis TS. Glycine transporters not only take out the garbage, they recycle. Neuron 40: 667–669, 2003. [DOI] [PubMed] [Google Scholar]
- Cox et al. 2005.Cox JA, Kucenas S, Voigt MM. Molecular characterization and embryonic expression of the family of N-methyl-d-aspartate receptor subunit genes in the zebrafish. Dev Dyn 234: 756–766, 2005. [DOI] [PubMed] [Google Scholar]
- Cui et al. 2005.Cui WW, Low SE, Hirata H, Saint-Amant L, Geisler R, Hume RI, Kuwada JY. The zebrafish shocked gene encodes a glycine transporter and is essential for the function of early neural circuits in the CNS. J Neurosci 25: 6610–6620, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui et al. 2004.Cui WW, Saint-Amant L, Kuwada JY. shocked gene is required for the function of a premotor network in the zebrafish CNS. J Neurophysiol 92: 2898–2908, 2004. [DOI] [PubMed] [Google Scholar]
- David-Watine et al. 1999.David-Watine B, Goblet C, de Saint Jan D, Fucile S, Devignot V, Bregestovski P, Korn H. Cloning, expression and electrophysiological characterization of glycine receptor alpha subunit from zebrafish. Neuroscience 90: 303–317, 1999. [DOI] [PubMed] [Google Scholar]
- Eulenburg et al. 2005.Eulenburg V, Armsen W, Betz H, Gomeza J. Glycine transporters: essential regulators of neurotransmission. Trends Biochem Sci 30: 325–333, 2005. [DOI] [PubMed] [Google Scholar]
- Fetcho 1990.Fetcho JR Morphological variability, segmental relationships, and functional role of a class of commissural interneurons in the spinal cord of goldfish. J Comp Neurol 299: 283–298, 1990. [DOI] [PubMed] [Google Scholar]
- Gabernet et al. 2005.Gabernet L, Pauly-Evers M, Schwerdel C, Lentz M, Bluethmann H, Vogt K, Alberati D, Mohler H, Boison D. Enhancement of the NMDA receptor function by reduction of glycine transporter-1 expression. Neurosci Lett 373: 79–84, 2005. [DOI] [PubMed] [Google Scholar]
- Gomeza et al. 2003.Gomeza J, Hulsmann S, Ohno K, Eulenburg V, Szoke K, Richter D, Betz H. Inactivation of the glycine transporter 1 gene discloses vital role of glial glycine uptake in glycinergic inhibition. Neuron 40: 785–796, 2003. [DOI] [PubMed] [Google Scholar]
- Granato et al. 1996.Granato M, van Eeden FJ, Schach U, Trowe T, Brand M, Furutani-Seiki M, Haffter P, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Odenthal J, Nusslein-Volhard C. Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development 123: 399–413, 1996. [DOI] [PubMed] [Google Scholar]
- Guastella et al. 1992.Guastella J, Brecha N, Weigmann C, Lester HA, Davidson N. Cloning, expression, and localization of a rat brain high-affinity glycine transporter. Proc Natl Acad Sci USA 89: 7189–7193, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen et al. 2003.Jensen K, Chiu CS, Sokolova I, Lester HA, Mody I. GABA transporter-1 (GAT1)-deficient mice: differential tonic activation of GABAA versus GABAB receptors in the hippocampus. J Neurophysiol 90: 2690–2701, 2003. [DOI] [PubMed] [Google Scholar]
- Johnson and Ascher 1987.Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature 325: 529–531, 1987. [DOI] [PubMed] [Google Scholar]
- Jones et al. 1998.Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci USA 95: 4029–4034, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz and Miledi 1973.Katz B, Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol 231: 549–574, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn and Faber 2005.Korn H, Faber DS. The Mauthner cell half a century later: a neurobiological model for decision-making? Neuron 47: 13–28, 2005. [DOI] [PubMed] [Google Scholar]
- Liu et al. 1992.Liu QR, Nelson H, Mandiyan S, Lopez-Corcuera B, Nelson N. Cloning and expression of a glycine transporter from mouse brain. FEBS Lett 305: 110–114, 1992. [DOI] [PubMed] [Google Scholar]
- Luna and Brehm 2006.Luna VM, Brehm P. An electrically coupled network of skeletal muscle in zebrafish distributes synaptic current. J Gen Physiol 128: 89–102, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna et al. 2004.Luna VM, Wang M, Ono F, Gleason MR, Dallman JE, Mandel G, Brehm P. Persistent electrical coupling and locomotory dysfunction in the zebrafish mutant shocked. J Neurophysiol 92: 2003–2009, 2004. [DOI] [PubMed] [Google Scholar]
- Martina et al. 2005.Martina M, ME BT, Halman S, Tsai G, Tiberi M, Coyle JT, Bergeron R. Reduced glycine transporter type 1 expression leads to major changes in glutamatergic neurotransmission of CA1 hippocampal neurons in mice. J Physiol 563: 777–793, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDearmid et al. 2006.McDearmid JR, Liao M, Drapeau P. Glycine receptors regulate interneuron differentiation during spinal network development. Proc Natl Acad Sci USA 103: 9679–9684, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono et al. 2004.Ono F, Mandel G, Brehm P. Acetylcholine receptors direct rapsyn clusters to the neuromuscular synapse in zebrafish. J Neurosci 24: 5475–5481, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer et al. 1984.Pfeiffer F, Simler R, Grenningloh G, Betz H. Monoclonal antibodies and peptide mapping reveal structural similarities between the subunits of the glycine receptor of rat spinal cord. Proc Natl Acad Sci USA 81: 7224–7227, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith et al. 1992.Smith KE, Borden LA, Hartig PR, Branchek T, Weinshank RL. Cloning and expression of a glycine transporter reveal colocalization with NMDA receptors. Neuron 8: 927–935, 1992. [DOI] [PubMed] [Google Scholar]
- Titmus et al. 1996.Titmus MJ, Korn H, Faber DS. Diffusion, not uptake, limits glycine concentration in the synaptic cleft. J Neurophysiol 75: 1738–1752, 1996. [DOI] [PubMed] [Google Scholar]
- Tsai et al. 2004.Tsai G, Ralph-Williams RJ, Martina M, Bergeron R, Berger-Sweeney J, Dunham KS, Jiang Z, Caine SB, Coyle JT. Gene knockout of glycine transporter 1: characterization of the behavioral phenotype. Proc Natl Acad Sci USA 101: 8485–8490, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano 2007.Turrigiano G Homeostatic signaling: the positive side of negative feedback. Curr Opin Neurobiol 17: 318–324, 2007. [DOI] [PubMed] [Google Scholar]
- Tzingounis and Wadiche 2007.Tzingounis AV, Wadiche JI. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci 8: 935–947, 2007. [DOI] [PubMed] [Google Scholar]
- Wen and Brehm 2005.Wen H, Brehm P. Paired motor neuron-muscle recordings in zebrafish test the receptor blockade model for shaping synaptic current. J Neurosci 25: 8104–8111, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita et al. 2005.Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl–dependent neurotransmitter transporters. Nature 437: 215–223, 2005. [DOI] [PubMed] [Google Scholar]
- Xu et al. 2000.Xu F, Gainetdinov RR, Wetsel WC, Jones SR, Bohn LM, Miller GW, Wang YM, Caron MG. Mice lacking the norepinephrine transporter are supersensitive to psychostimulants. Nat Neurosci 3: 465–471, 2000. [DOI] [PubMed] [Google Scholar]