Abstract
Myogenic cell differentiation is induced by Arg8-vasopressin, whereas high cAMP levels and protein kinase A (PKA) activity inhibit myogenesis. We investigated the role of type 4 phosphodiesterase (PDE4) during L6-C5 myoblast differentiation. Selective PDE4 inhibition resulted in suppression of differentiation induced by vasopressin. PDE4 inhibition prevented vasopressin-induced nuclear translocation of the muscle-specific transcription factor myogenin without affecting its overall expression level. The effects of PDE4 inhibition could be attributed to an increase of cAMP levels and PKA activity. RNase protection, reverse transcriptase PCR, immunoprecipitation, Western blot, and enzyme activity assays demonstrated that the PDE4D3 isoform is the major PDE4 expressed in L6-C5 myoblasts and myotubes, accounting for 75% of total cAMP-hydrolyzing activity. Vasopressin cell stimulation caused a biphasic increase of PDE4 activity, which peaked at 2 and 15 min and remained elevated for 48 h. In the continuous presence of vasopressin, cAMP levels and PKA activity were lowered. PDE4D3 overexpression increased spontaneous and vasopressin-dependent differentiation of L6-C5 cells. These results show that PDE4D3 plays a key role in the control of cAMP levels and differentiation of L6-C5 cells. Through the modulation of PDE4 activity, vasopressin inhibits the cAMP signal transduction pathway, which regulates myogenesis possibly by controlling the subcellular localization of myogenin.
INTRODUCTION
During skeletal muscle development, cells of mesodermal origin become committed to the myogenic lineage, migrate toward their final destination, and become postmitotic (Cossu et al., 1996). Myoblasts fuse into multinucleated myotubes and begin accumulating muscle-specific products (e.g., M-creatine kinase, myosin and other sarcomeric proteins, and acetylcholine receptor subunits; O’Neill and Stockdale 1972; Nadal-Ginard 1978). This process is marked by the sequential expression of specific genes and requires an adequate level of functional muscle regulatory factors (myogenin, myoD, myf-5, and mrf-4) whose temporal expression pattern and relative role may vary among organisms and experimental models of myogenic differentiation (Braun et al., 1989; Ludolph and Konieczny 1995).
Several cultured cell models allow the study of at least portions of the myogenic developmental process. Both in vivo and in culture, myogenic differentiation is under the influence of both inhibitory and stimulatory extracellular signals (Olson et al., 1986; Clegg et al., 1987; Florini et al., 1991). Until recently, insulin-like growth factors (IGFs) were considered as the main myogenic differentiation factors (Florini 1987; Engert et al., 1996). We reported that Arg8-vasopressin (AVP) also acts as a positive effector in several types of skeletal myogenic cells, including rat L6-C5 myoblasts (Nervi et al., 1995; Minotti et al., 1998). Unlike IGFs, which require the presence of other factors supplied by serum to express their full myogenic potential, AVP promotes myogenic differentiation in the absence of additional exogenous factors (Minotti et al., 1998). AVP induces the activation of both phospholipase C and phospholipase D in L6-C5 myogenic cells. However, the concentrations of AVP required to induce myogenesis are compatible only with the activation of phospholipase D, whereas 100-fold higher AVP doses are required to trigger phospholipase C stimulation (Teti et al., 1993; Naro et al., 1997).
It is well established that elevation of intracellular levels of the second messenger cAMP is sufficient to silence the myogenic program (Wahrman et al., 1973; Winter et al., 1993). Elevated cAMP levels inhibit both the expression of endogenous myogenin and the transcriptional activity of a transfected myogenin promoter (Salminen et al., 1991). Furthermore, overexpression of the catalytic subunit of protein kinase A (PKA) inhibits myogenic differentiation (Winter et al., 1993). PKA has also been shown to phosphorylate overexpressed myogenin in COS-1 cells, although this phosphorylation did not affect the ability of myogenin to bind to DNA (Li et al., 1992). Thus, although the exact mechanism is still unknown, cAMP and PKA exert a negative effect on myogenic cell differentiation.
The intracellular cAMP concentration is regulated at the synthesis level by adenylyl cyclase and at the hydrolysis level by phosphodiesterase (PDE) activity. Among the 10 families of PDEs described in mammalian tissues, the PDE4 family specifically hydrolyzes cAMP with high affinity (Conti et al., 1995; Soderling et al., 1998). This family includes a number of isoforms deriving from the expression of four genes in rat (Colicelli et al., 1989; Davis et al., 1989; Swinnen et al., 1989) and in human (Bolger et al., 1993). These isoforms share the cAMP specificity and the sensitivity to inhibition by rolipram (Beavo, 1995; Conti et al., 1995). In L6, as well as in other cell types, the expression of the PDE4D gene, one of the four genes encoding type 4 PDEs, is up-regulated by cAMP (Kovala et al., 1994; Conti et al., 1995; Vicini and Conti, 1997). In addition to transcriptional regulation, it has been shown that one of the isoforms deriving from PDE4D gene expression, PDE4D3, is regulated by PKA-dependent phosphorylation (Sette et al., 1994a,c; Sette and Conti 1996). The PDE4D3 isoform contains an amino-terminal regulatory region, which is absent in other isoforms derived from the same gene, and phosphorylation of serine 54 in this region relieves an inhibitory constraint and activates the enzyme (Sette and Conti 1996). Activation of PDE4D3 is also obtained by interaction of this region of the enzyme with negatively charged phospholipids, such as phosphatidic acid (PA) (Némoz et al., 1997; Grange et al., 1998). The transcriptional and posttranslational regulation of PDE4 activity has been proposed to play a role in short- and long-term desensitization to hormonal stimulation of target cells (Sette et al., 1994b; Vicini and Conti 1997) (reviewed by Conti et al., 1995).
Although cAMP plays a negative role during myogenic differentiation, scarce information is available on the role of PDE activity in this process and its regulation by extracellular factors. To address this point, in the present study, we first investigated the effect of PDE4-specific inhibitors on L6-C5 cell differentiation and observed that inhibition of PDE4 strongly suppresses myogenesis. We characterized the PDE4 isoforms expressed in L6-C5 cells and showed that AVP stimulation modulates PDE4 activity. The observation that AVP markedly stimulates PDE4 and decreases cAMP levels and PKA activity in differentiating L6-C5 cells led us to hypothesize that PDE4 modulation plays a physiological role in myogenic differentiation.
MATERIALS AND METHODS
Materials
Synthetic AVP, snake venom from Crotalus atrox, Kemptide, PKA peptide inhibitor (PKI), a creatine kinase (CK) assay kit, and Tri reagent were purchased from Sigma (St. Louis, MO). Rolipram [(4,3-butoxy-4-methoxybenzyl)-2-imidazolidone], milrinone, and zaprinast were obtained from Calbiochem (La Jolla, CA). IGF1 was purchased from Chemicon (Temecula, CA). RS 23544 was a kind gift from Dr. R. Alvarez (Syntex, Palo Alto, CA). Fatty acid-free BSA, Fugene 6, and PCR reagents were from Boehringer Mannheim (Indianapolis, IN). The anti-myogenin mAb F5D developed by Dr. W.E. Wright (University of Texas, Dallas, TX) was obtained from the Developmental Studies Hybridoma Bank maintained by the Department of Biological Sciences, University of Iowa (Iowa City, IA); the mAb to sarcomeric myosin MF20 was a kind gift from Dr. D. Fischman (Cornell University Medical College, New York, NY); anti-PKA catalytic and regulatory subunit (Iα and IIα) antibodies were from Transduction Laboratories (Lexington, KY). [γ-32P]ATP (3000 Ci/mmol) and a cAMP-125I radioimmunoassay kit were from DuPont NEN (Boston, MA). [3H]cAMP and an ECL Western blot detection kit were from Amersham Pharmacia Biotech (Uppsala, Sweden). AG1-X2 resin was from Bio-Rad (Hercules, CA).
Cell Culture
Subcloning and characterization of L6 (Yaffe 1968) rat myogenic cell clones were previously reported (Teti et al., 1993). Cells of the subclone C5 (L6-C5), a clone that had shown significant differentiation ability (Nervi et al., 1995; Minotti et al., 1998), were used throughout this study. The cells were routinely seeded at the density of 10,000/cm2 in Dulbecco’s modified Eagle’s (DME) medium supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% heat-inactivated FBS. Twenty-four hours after plating, cultures were extensively washed with DME medium and shifted to serum-free medium consisting of DME medium supplemented with 1% (wt/vol) fatty acid-free BSA with or without other additions. Full terminal myogenic differentiation was morphologically evaluated after 6 d by assessing the presence of multinucleated myotubes in May Grunwald-Giemsa–stained cultures.
CK Assay
After 7 d of culture, cells were washed with PBS and homogenized in 30 mM HEPES and 1 mM EDTA, pH 7.2. The 20,000 × g supernatant was used to measure CK activity as previously described (Minotti et al., 1998), and the pellet was used to measure total DNA content as previously reported (Nervi et al., 1995).
Plasmid Construction
The pMYO184-luciferase plasmid was derived from the pMYO184CAT plasmid (Edmondson et al., 1992). PCR-based strategy was used because of the absence of compatible restriction sites in pMYO184CAT and pGl2-Basic (Promega, Madison, WI) polylinkers. Briefly, the myogenin 184-bp fragment was obtained by PCR from the original plasmid using oligonucleotides containing the appropriate restriction sites. The PCR product was purified, digested, and subcloned in the pG12-Basic vector. The construct was analyzed by sequencing to avoid PCR-introduced mutations.
Transfections and Gene Reporter Assays
Transient cotransfections were performed by using Fugene 6 following the manufacturer’s instructions, using 1 μg of reporter construct DNA/plate and 0.3 μg of cytomegalovirus plasmid (pCMV)-β-galactosidase (β-gal) to allow normalization. Seventy-two hours after transfection individual dishes were washed twice with PBS and then scraped in 1× reporter lysis buffer (Promega). The cell lysates were centrifuged (16,000 × g for 2 min) at 4°C, and the supernatants were assayed. Luciferase activity (Brasier et al., 1989) was assayed in duplicate by mixing 20 μl of cell extract with 100 μl of luciferase assay reagent (Promega). The produced light was measured and expressed as relative light units. β-Gal assay was performed in duplicate as previously described (Vicini and Conti 1997).
Myogenin Expression and Translocation
For myogenin localization, monolayers of L6-C5 cells were fixed in 4% paraformaldehyde in PBS for 30 min at 4°C and permeabilized in 0.2% Triton X-100 in PBS for 30 min. Cells were washed with 1% BSA in PBS and incubated overnight at room temperature with the undiluted supernatant of F5D hybridoma cells. After extensive washing with 1% BSA in PBS, the cells were incubated for 1 h at room temperature with fluorescein-conjugated goat anti-mouse immunoglobulin G (Cappel, West Chester, PA; dilution, 1:50) (Cusella De Angelis et al., 1992). The amount of myogenin expressed in L6-C5 cells was evaluated using F5D monoclonal antibody as primary antibody in Western blotting analysis performed as described below.
Myosin Expression and Quantification
A monoclonal antibody to the myosin heavy chain (MF20 antibody), which recognizes all sarcomeric myosin, was used (Bader et al., 1982). Cells were fixed and treated as described above and incubated overnight with MF20 at 4°C. Secondary antibody conjugated to HRP (Bio-Rad) was added (final dilution 1:100), and the reaction was visualized using the diaminobenzidine substrate as previously reported (Minotti et al., 1998). Myosin was quantified by indirect ELISA. Briefly, cells were solubilized in radioimmunoprecipitation assay buffer and centrifuged at 10,000 × g for 10 min, and the supernatant was collected. Microtitration plates (96 wells; Falcon) were coated overnight at 37°C with either 50 μl/well of different known amounts of bovine myosin dissolved in radioimmunoprecipitation assay buffer or 50 μl of cell extract. The assay was carried out as previously described (Naro et al., 1991) using MF20 as the primary antibody (1:50 in PBS), and the peroxidase reaction was visualized by the Peroxidase Substrate System kit (Kirkegaard & Perry, Gaithersburg, MD) according to the manufacturer’s procedure. Optical absorbance was read in a Benchmark microplate reader (Bio-Rad). Each determination was performed in triplicate.
RNase Protection Assay (RPA)
Run-off transcripts were synthesized from each linearized template as previously described (Vicini and Conti 1997), using a Transcription In vitro System kit (Promega) and either T3 or T7 polymerase. The full-length single-stranded RNA probes were purified by PAGE. Poly(A)+ RNA was purified from L6-C5 cells using a Quick Prep mRNA purification kit (Amersham Pharmacia Biotech) according to the supplier’s protocol. RPA was performed with RPA II kit (Ambion, Austin, TX) using 5 μg of extracted mRNA and 1.5–2 × 105 cpm of labeled probe for each reaction. Nuclease-resistant probes were visualized by gel electrophoresis (5% acrylamide, 8 M urea, 90 mM Tris-borate, and 2 mM EDTA) and autoradiography.
Reverse Transcriptase (RT)-PCR
RNA was prepared, using the Tri Reagent procedure as indicated by the manufacturer, from rat brain from L6-C5 myoblasts (cultured for 2 d in serum-free medium) and from L6-C5 myotubes (cultured for 6 d in serum-free medium and 0.1 μM AVP). Five micrograms of RNA from each sample were reverse transcribed using Moloney murine leukemia virus RT and oligo-DT. The PCR reaction was carried out in a final volume of 50 μl in buffer containing 1 μl of RT reaction (equivalent to 1 μg of total RNA), 200 μM dNTPs, 1.5 mM MgCl2, a 0.5 μM concentration of each primer, and 1 U of Taq-DNA polymerase. PCR conditions were 30 cycles, 94°C (45 s), 50°C (45 s), and 72°C (45 s). The following primers from the four different PDE4 genes were used to amplify cDNA fragments of rat brain or L6-C5 cDNA:
PDE4A gene: oligo A (5′-tcaacaccaattcggagctgg-3′), sense on rat PDE4A cDNA position 464–484; and oligo B (5′-gtcttcaggtcagccaggagg-3′), antisense on rat PDE4A cDNA position 660–680 (GenBank accession number M28411), expected amplified fragment size, 216 bp.
PDE4B gene: oligo C (5′-aggattctgaaggaccgg-3′), sense on rat PDE4B cDNA position 2085–2102; and oligo D (5′-agattatgtgtcgatcag-3′) antisense on rat PDE4B cDNA positon 2222–2239 (GenBank accession number L27058), expected amplified fragment size, 154 bp.
PDE4C gene: oligo E (5′-tcaacaccaattcggagctgg-3′), sense on rat PDE4C cDNA position 464–484; and oligo F (5′-cagagtagttgtccaagagc-3′) antisense on rat PDE4C cDNA position 720–739 (GenBank accession number M25347), expected amplified fragment size, 275 bp.
PDE4D gene: oligo G (5′-ggcttcatagactacattg-3′) sense on rat PDE4D cDNA position 1495–1513; and oligo H (5′-ttacactgttacgtgtcagg-3′) antisense on rat PDE4D cDNA position 1894–1913 (GenBank accession number U09455), expected amplified fragment size, 418 bp.
Rat brain cDNA amplification was used as a positive control for the detection of PDE4A, PDE4B, and PDE4D transcripts. Controls performed with brain and L6-C5 RNA processed in the absence of RT did not give rise to any amplification. The integrity of mRNA and equal cDNA loading in the PCR reactions was checked by quantification of β-actin mRNA levels in the samples. PCR products were analyzed on 2% agarose gels.
Immunoprecipitation of PDE4 from L6-C5 Cells
Subconfluent L6-C5 cell monolayers in 90-mm dishes were cultured for 48 h in serum-free medium. Cells were rinsed twice with ice-cold PBS, harvested in hypotonic homogenization buffer (20 mM Tris-Cl, pH 8.0, 10 mM NaF, 1 mM EDTA, 0.2 mM EGTA, 0.7 M 2-mercaptoethanol, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 10 μg/ml pepstatin), and homogenized on ice. Soluble cell extracts were separated by centrifugation (15,000 × g, 10 min at 4°C). For immunoprecipitation experiments, the anti-PDE4 antibodies used were K116, a rabbit polyclonal antibody raised against a peptide sequence conserved among the PDE4A, PDE4B, and PDE4D isoforms; Ac-55, a rabbit polyclonal antibody raised against a GST-PDE4A fusion protein; K118, a rabbit polyclonal antibody raised against a GST-PDE4B fusion protein; and M3S1, a monoclonal antibody raised against a GST-PDE4D fusion protein. The characterization of these antibodies was reported elsewhere (Sette et al., 1994a; Naro et al., 1996; Iona et al., 1998). K116, Ac-55, and K118 (1:100 dilution) were preincubated for 60 min with protein A-Sepharose beads (Sigma), whereas M3S1 was preincubated for 60 min with protein G-Sepharose beads (Amersham Pharmacia Biotech). At the end of the incubation, the beads were washed once with 20 mM Tris-Cl, pH 7.8, containing 0.5 M NaCl, and twice with 20 mM Tris-Cl, pH 7.8, and then incubated for 90 min at 4°C with soluble L6-C5 cell extracts (1 mg of protein) under constant shaking. Protein A- or protein G-Sepharose–bound immunocomplexes were rinsed three times with PBS containing 0.05% BSA, and aliquots of the immunoprecipitates were assayed for PDE activity as described below. After two additional washes with PBS, immunocomplexes were eluted in SDS-PAGE sample buffer (62.5 mM Tris-Cl, pH 6.8, 10% glycerol, 2% [wt/vol] SDS, 0.7 M 2-mercaptoethanol, and 0.0025% [wt/vol] bromphenol blue) for Western blot analysis.
Western Blot Analysis
Immunoprecipitated proteins and/or cell extracts were separated on 10% SDS-PAGE, transferred onto a nitrocellulose membrane (Amersham Pharmacia Biotech), and subjected to Western blot analysis with different antibodies as previously described (Iona et al., 1998). Briefly, for the analysis of PDE4, the first antibody incubation (90 min at room temperature) was carried out with a 1:500 dilution of the rabbit polyclonal K116 antiserum; for myogenin determination, F5D hybridoma supernatant was used at a 1:50 dilution, for PKAc, PKARIα, and PKARIIα; antibodies were diluted at 1:250. Second antibody incubation was carried out with a 1:10,000 dilution of either anti-rabbit or anti-mouse immunoglobulin G antibody conjugated to HRP (Amersham Pharmacia Biotech). Immunostained bands were detected by the ECL method.
PKA Assay
Cells were washed twice with cold PBS, scraped in PBS, and pelleted by centrifugation for 5 min at 1000 rpm. Cell pellets from 60-mm plates were resuspended in 60 μl of hypotonic buffer (20 mM Tris-Cl, pH 7.5, 2 mM EGTA, 0.5 μg/ml leupeptin, 0.7 μg/ml pepstatin, and 4 μg/ml aprotinin) for 10 min at 4°C and centrifuged for 10 min at 15,000 × g. Protein content of the supernatant was measured according to the method of Bradford (1976). PKA activity was evaluated by measuring the incorporation of labeled phosphate from [γ-32P]ATP into the synthetic peptide substrate Kemptide. Reactions were carried out for 10 min at 30°C in 25 μl of 100 mM Tris-Cl containing 20 mM MgCl2, 0.4 mM ATP, 10 μCi [γ-32P]ATP, and 0.2 mM Kemptide, with or without either 5 μg/ml PKI or 1 μM cAMP, using 2–5 μg of cell extracts. To stop the reaction, 20 μl of reaction mixture were spotted onto 1-cm2 phosphocellulose paper squares and immediately immersed in 0.1% (vol/vol) phosphoric acid. Paper squares were washed five times in the same solution, dried, and counted in a liquid scintillation counter. PKA activity was evaluated as the fraction of cAMP-dependent activity that was specifically inhibited by PKI.
cAMP PDE Assay
At the end of the treatment period, the cells were washed with cold PBS and scraped into 300 μl of homogenization buffer (20 mM Tris-Cl, pH 8, 1 mM EDTA, 0.2 mM EGTA, 1.25 mM 2-mercaptoethanol, 50 mM benzamidine, 0.5 μg/ml leupeptin, 0.7 μg/ml pepstatin, 4 μg/ml aprotinin, and 2 mM PMSF). Cells were homogenized and immediately assayed for PDE activity using 1 μM cAMP as substrate, according to the method of Thompson et al. (1974), as previously described. Samples were assayed in a final volume of 200 μl of a solution composed of 40 mM Tris-Cl, pH 8, 1 mM MgCl2, 1.25 mM 2-mercaptoethanol, 1 μM cAMP, 0.2 mg gelatin, and 0.1 μCi [3H]cAMP. When appropriate, rolipram, a specific inhibitor of the cAMP-PDEs (Schwabe et al., 1976), was added to the incubation mixture at a final concentration of 10 μM. The samples were incubated at 34°C for 10 min, and the reaction was stopped by addition of 200 μl of a solution containing 40 mM Tris-Cl, pH 7.5, and 10 mM EDTA, followed by heat denaturation for 50 s at 100°C. To convert AMP to adenosine, 50 μg of C. atrox snake venom were added to each sample. The reaction was allowed to proceed for 20 min at 34°C. The reaction products were separated by anion exchange chromatography performed on 1 ml of AG1-X2 resin (as a 1:4 slurry in water), and the amount of unbound [3H]adenosine was quantitated by scintillation counting.
cAMP Assay
Before harvesting, cells were washed twice with cold PBS, and 0.5 ml of ice-cold 10% trichloroacetic acid were added. Cells extracts were collected and centrifuged at 10,000 × g for 15 min. Supernatants were extracted five times with diethyl ether to eliminate trichloroacetic acid. cAMP was assayed by RIA, according to the manufacturer’s recommendations, using the acetylation procedure.
Statistical Analysis
Data are presented as average ± SE or as otherwise indicated. Statistical analysis was performed by ANOVA.
RESULTS
PDE4 Inhibitors Suppress Myogenic Differentiation of L6-C5 Cells
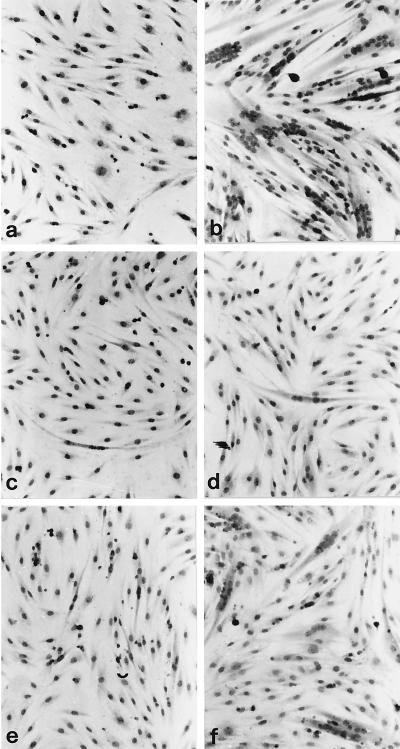
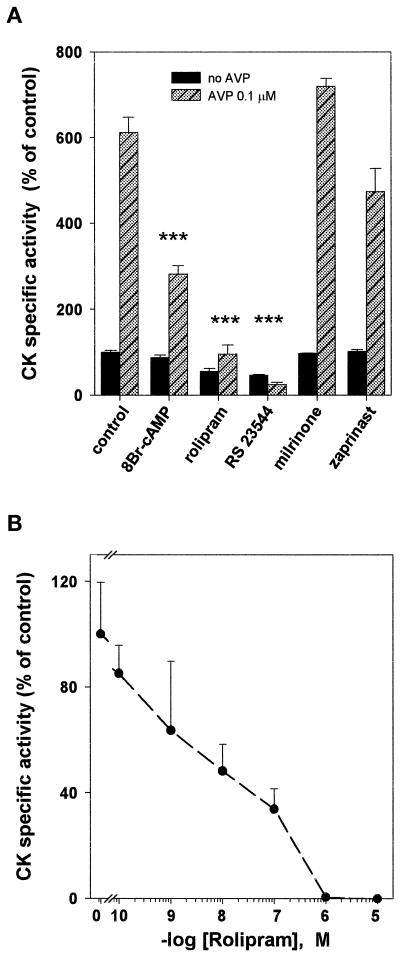
Incubation of L6-C5 cells with AVP induced myogenic differentiation, as indicated morphologically by the formation of multinucleated myotubes (Figure 1, a and b) and biochemically by an increase in the activity of the myogenic marker enzyme CK (Figure 2A). Both AVP effects were completely suppressed by incubation of the cells with the PDE4-specific inhibitor rolipram (10 μM) (Figures 1, c and d, and 2, A and B). The PDE5-specific inhibitor zaprinast (100 μM) and the PDE3-specific inhibitor milrinone (1 μM) had no significant effect on AVP-induced CK activity level (Figure 2A). To rule out the possibility that the effect of rolipram is nonspecific, we used a structurally unrelated PDE4-specific inhibitor, RS 23544 (1 μM) (Alvarez et al., 1995). As shown for rolipram, RS 23544 completely suppressed the AVP-induced increase in CK activity (Figure 2A) and changes in cell morphology (our unpublished results). No toxic effects were evident with any of the treatments, and there was no significant difference in DNA and protein content between cells treated with the inhibitors and the respective controls. A dose–response study of the effect of rolipram showed that half-maximal inhibition of differentiation was achieved at 10 nM rolipram (Figure 2B), a concentration compatible with that necessary to inhibit the PDE4 activity. The ability of PDE4 inhibitors to suppress AVP-induced differentiation is due to an increase in intracellular cAMP levels, because incubation of L6-C5 cells with 8-bromo-cAMP (8-Br-cAMP), a cell-permeable cAMP analogue that is slowly hydrolyzed by PDE, almost suppressed morphological changes and strongly reduced the increase in CK after AVP treatment (Figures 1, e and f, and 2A). Furthermore, incubation of the cells with 10 μM rolipram induced a sixfold increase in cAMP (control cells, 3.8 ± 0.18 pmol of cAMP/mg of protein; rolipram-treated cells, 23.6 ± 3.2 pmol of cAMP/mg of protein; n = 6; p < 0.001) and a significant increase in PKA activity (expressed as −cAMP:+cAMP specific activity ratio: control cells, 0.128 ± 0.012; rolipram-treated cells, 0.22 ± 0.019; n = 6; p < 0.01) after 48 h of treatment.
Figure 1.
Effects of rolipram and 8-Br-cAMP on morphologic changes induced by AVP in L6-C5 cells. L6-C5 cells were grown in complete medium and after 24 h shifted to serum-free medium without (a, c, and e), or with (b, d, and f) 0.1 μM AVP. Ten micromolar rolipram (c and d) or 100 μM 8-Br-cAMP (e and f) was simultaneously added. After 6 d of culture, cells were stained by May Grunwald-Giemsa. Myogenic differentiation was evidenced by the formation of multinucleated myotubes.
Figure 2.
Effect of 8-Br-cAMP and PDE inhibitors on L6-C5 cell differentiation (CK specific activity). (A) L6-C5 myoblasts were allowed to proliferate in complete medium and shifted to serum-free medium in the absence (filled bars) or presence (hatched bars) of 0.1 μM AVP, with the concomitant addition of 100 μM 8-Br-cAMP (a cell-permeable cAMP analogue), 10 μM rolipram, 1 μM RS 23544 (PDE4-specific inhibitors), 1 μM milrinone (a PDE3-specific inhibitor), or 100 μM zaprinast (a PDE5-specific inhibitor). After 6 d, CK specific activity (milli-optical density units per minute per microgram of protein) was measured as described in MATERIALS AND METHODS. Each bar represents the mean ± SEM of data obtained from at least two independent experiments performed in triplicate. ***, Significance at the level of p < 0.01 (assay and AVP vs. control and AVP). (B) Rolipram concentration dependency of the inhibition of AVP-induced L6-C5 cell differentiation. The cultures were treated with 0.1 μM AVP and increasing concentrations of rolipram for 6 d after shifting to serum-free medium; CK activity was then assayed. Means ± SD of three independent measurements are shown.
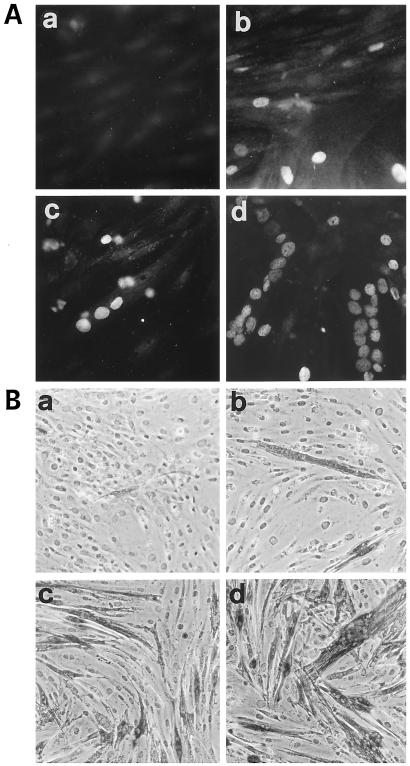
Rolipram Blocks AVP-induced Nuclear Translocation of Myogenin
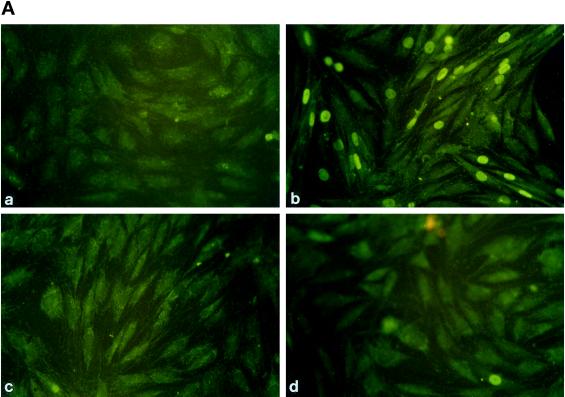
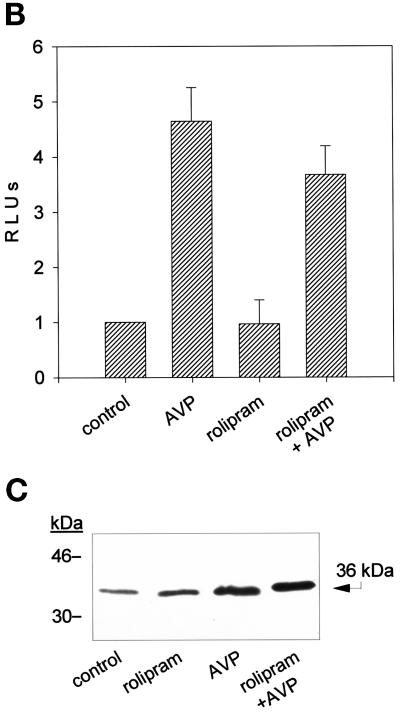
An early event accompanying L6-C5 cell differentiation is the expression of the transcription factor myogenin and its nuclear accumulation. Immunofluorescence analysis of myogenin indicated that 48 h AVP treatment of L6-C5 cells induced nuclear accumulation of the protein (Figure 3A), as previously reported (Minotti et al., 1998). Incubation of the cells with the PDE4 inhibitor rolipram completely prevented AVP-induced nuclear accumulation of myogenin (Figure 3A). To determine whether the lack of fluorescent staining of myogenin in the nuclei of rolipram-treated cells resulted from a decrease in myogenin expression or, rather, reflected an inhibition of the translocation process, myogenin expression was evaluated. L6-C5 cells were transfected with pMYO184-luciferase, in which the reporter gene expression is driven by the myogenin promoter, and induced to differentiate for 48 h with AVP in the absence or presence of 10 μM rolipram. As shown in Figure 3B, rolipram did not significantly modify AVP-stimulated luciferase activity. This result was confirmed at the level of protein expression by Western blot analysis: the amount of myogenin was increased by 48 h of AVP stimulation, but it was not modified by rolipram treatment of the cells (Figure 3C). These data indicate that PDE4 inhibition does not influence the level of expression of myogenin but, rather, affects the nuclear translocation of the transcription factor.
Figure 3.
Rolipram inhibits the AVP-dependent nuclear translocation of myogenin but not its expression. (A) Immunofluorescence analysis of the expression of myogenin in L6-C5 cells. The cells, cultured as described in MATERIALS AND METHODS, were left untreated for 48 h (a) or were treated with 0.1 μM AVP (b), 10 μM rolipram (c), or both 10 μM rolipram and 0.1 μM AVP (d) for 48 h. Myogenin was detected by using the anti-myogenin antibody F5D. (B) Lack of effect of rolipram on the AVP inducibility of the myogenin promoter. Cells were transfected with pMYO184-luciferase plasmid containing the myogenin promoter linked to the luciferase gene, as described in MATERIALS AND METHODS. Twenty-four hours after transfection, the cells were shifted to serum-free medium and incubated with 0.1 μM AVP for 48 h, either in the presence or absence of 10 μM rolipram. Luciferase activity was normalized for β-gal activity. Data are the means ± SD of three different experiments performed in duplicate. (C) Western blot analysis of myogenin expression in L6-C5 cells. The cells, cultured as described above, were left untreated for 48 h (control) or were treated with 10 μM rolipram, 0.1 μM AVP, or both 10 μM rolipram and 0.1 μM AVP. Total protein concentrations were determined by Bradford (1976) protein assay, and well loading was normalized. Myogenin was detected by using the anti-myogenin antibody F5D. The experiment shown is representative of four experiments giving similar results.
Type 4 PDE Expression in L6-C5 Cells
To investigate which PDE4 isoforms are present in L6-C5 myogenic cells, we used different approaches. First, by using the specific PDE4 inhibitor rolipram, it was assessed that 76 ± 4% (n = 3) of the total cAMP-PDE activity was attributable to type 4 enzymes. The cytosolic fraction obtained after homogenization of L6-C5 cells retained most of the PDE activity (80 ± 5%; n = 3). The cytosolic cAMP-PDE activity was mainly due to type 4 PDEs, because rolipram inhibited it by 82 ± 3% (n = 3).
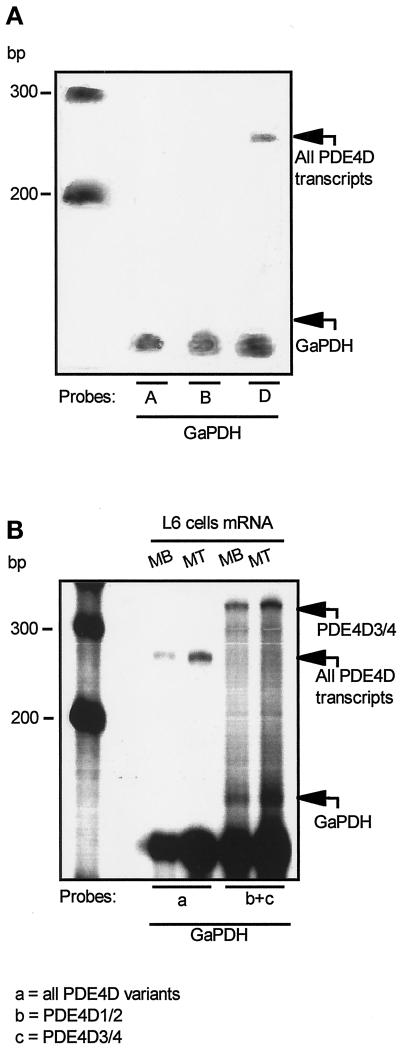
To determine which genes and isoforms are expressed in L6-C5 cells, a panel of probes corresponding to three of the four different PDE4 genes (PDE4A, PDE4B, and PDE4D) was used in RPA experiments. A first set of riboprobes was complementary to the sequence encoding the catalytic region of each isoform, which is conserved in all variants deriving from a given gene. As shown in Figure 4A, only the PDE4D gene was expressed in L6-C5 cells, whereas PDE4A and PDE4B transcripts were not present. Because it has been previously shown that at least five transcripts are encoded by the PDE4D gene in different rat cell types (Sette et al., 1994b,c; Vicini and Conti 1997; Jin et al., 1998), we investigated which PDE4D isoforms are expressed in L6-C5 myoblasts. For this purpose, two probes that are complementary to the unique 5′ ends of the PDE4D1/2 and PDE4D3/4 isoforms, respectively, were used. Figure 4B shows that only PDE4D3 and/or PDE4D4 mRNA are expressed. An identical pattern of PDE4D expression was observed in both myoblasts (cultured in serum-free medium) and myotubes (cultured in serum-free medium and 0.1 μM AVP) (Figure 4B), suggesting that no other PDE4D isoform is expressed during cell differentiation. These data were confirmed by RT-PCR experiments using specific primer pairs allowing the selective amplification of sequences of the transcripts originating from the expression of each of the four PDE4 genes. Only the presence of PDE4D gene transcripts could be detected in L6-C5 myoblasts as well as in AVP-differentiated myotubes (our unpublished results).
Figure 4.
RNase protection analysis of the PDE4D genes expressed in L6-C5 myoblasts and myotubes. L6-C5 cells were cultured for 24 h in complete medium and then shifted to serum-free medium in the absence (myoblasts, MB) or presence of 0.1 μM AVP (myotubes, MT). After 5 d, Poly(A)+ RNA was extracted, and RPA was performed as detailed in MATERIALS AND METHODS. Autoradiographies of the PAGE gels of protected fragments are shown. (A) The experiment was performed on myoblast mRNA with one of three probes recognizing all the products of PDE4A (expected size of protected fragment, 215 bp), PDE4B (expected size of protected fragment, 153 bp), and PDE4D genes (size of the protected fragment, 270 bp). mRNA integrity was assessed by glyceraldehyde-3-phosphate dehydrogenase–protected fragments (size, 130 bp). (B) The experiment was performed with either a probe recognizing a region common to all PDE4D transcripts (a) or a mixture of probes recognizing, respectively, the 5′ end of PDE4D1 and PDE4D2 transcripts (expected sizes of protected fragments, respectively, 293 and 170 bp) (b) and the 5′ end of PDE4D3 and PDE4D4 transcripts (size of protected fragment, 350 bp) (c). The size of the major band indicates that a fragment corresponding to PDE4D3/4 was protected, whereas no band corresponding to the size of the PDE4D1/2-specific probe could be detected.
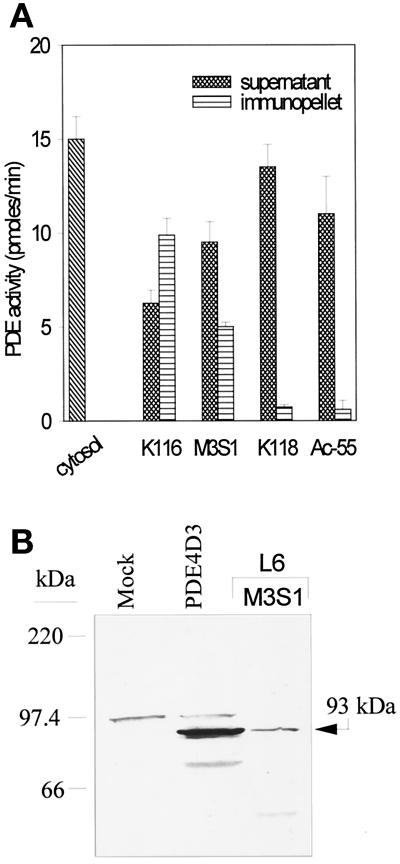
To confirm at the level of protein expression the data obtained with RPA analysis of PDE4 mRNAs and RT-PCR, we used different anti-PDE4 antibodies in immunoprecipitation and immunoblotting experiments. The non–isoform-selective antiserum K116, which recognizes all PDE4 isoforms (Sette et al., 1994a), immunoprecipitated 66% of total cytosolic cAMP-PDE activity (corresponding to ∼80% of rolipram-sensitive PDE activity; Figure 5A). However, among the isoform-selective antibodies used (Sette et al., 1994a), only the anti-PDE4D M3S1 antibody (which recognizes all the PDE variants originating from the expression of gene PDE4D) was able to immunoprecipitate a significant amount of cAMP-PDE activity (∼30% of total and 40% of rolipram-sensitive cAMP-PDE activity), whereas neither the anti-PDE4A Ac-55 antiserum nor the anti-PDE4B K118 antibody immunoprecipitated significant amounts of PDE activity (Figure 5A). Western blot analysis of the immunopellets with the K116 antibody showed the presence of a single protein, with an apparent molecular mass of 93 kDa, immunoprecipitated with M3S1 (Figure 5B). The 93-kDa protein migrated at the same level as a recombinant PDE4D3 isoform expressed in 293 cells (Figure 5B). Neither a 67- nor a 74-kDa band was observed, confirming the lack of expression of PDE4D2 and PDE4D1 at the protein level. The 93-kDa band was not detected when the K118 or Ac-55 immunopellets were analyzed (our unpublished results). These data strongly support the conclusion that the major PDE4 protein present in L6-C5 myoblasts is the PDE4D3 isoform.
Figure 5.
Characterization of PDE4 proteins expressed in L6-C5 cells. (A) Selective immunoprecipitation of PDE4D. L6-C5 cells were homogenized in hypotonic homogenization buffer as detailed in MATERIALS AND METHODS. Aliquots of the soluble fraction obtained after centrifugation for 15 min at 15,000 × g were immunoprecipitated with the different antibodies. PDE activity was assayed in the starting cytosolic fraction, in the supernatants, and in the immunopellets. The experiment was performed three times with similar results. (B) Western blot analysis of PDE4 proteins expressed in L6-C5 cells. Immunopellet of the L6-C5 cell soluble fraction precipitated with M3S1 antibody was subjected to Western blot analysis, using K116 antiserum for the immunodetection of blots. For comparison, recombinant PDE4D3 protein obtained in 293 cells as previously described (Sette et al., 1994c) was analyzed, in parallel with an extract of mock-transfected 293 cells.
PDE4 Activity Is Stimulated after AVP Treatment
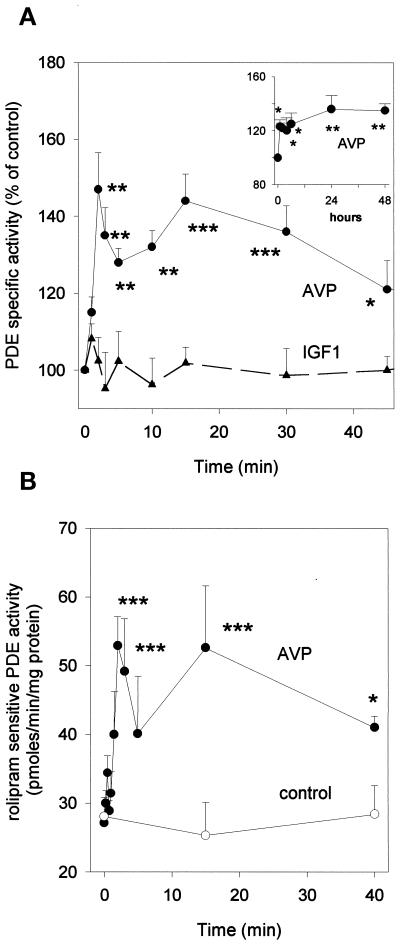
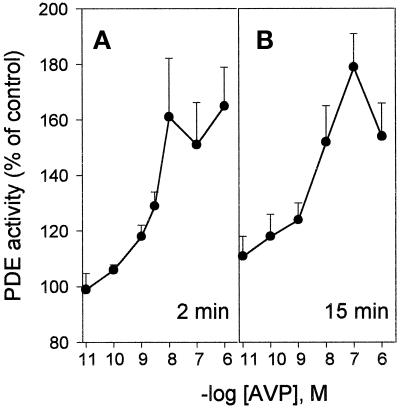
The strong inhibition of differentiation elicited by PDE4 inhibitors suggests that the PDE4D3 isoform plays a key role in the control of cAMP levels in L6-C5 cells. We therefore evaluated the hypothesis that AVP-induced differentiation is accompanied by changes in PDE4 activity. AVP treatment of L6-C5 cells induced a biphasic increase of total cAMP-PDE activity: a first transient peak occurred after 2 min of stimulation, followed by a slower increase reaching a maximum after 15 min. Activity remained significantly elevated even after 48 h from AVP addition (Figure 6, A and inset). The AVP-induced PDE activation was restricted to the type 4 component of activity, because no significant change in activity was observed after AVP treatment when PDE was assayed in the presence of rolipram (our unpublished results). Conversely, when the rolipram-sensitive fraction of PDE activity was considered, AVP-induced stimulation was more pronounced and clearly showed a biphasic pattern, with 90% increase at 2 min and 110% increase at 15 min of treatment (Figure 6B). Both phases of PDE activation were dependent on AVP concentration: the early phase (2 min of stimulation) showed a maximal response at 10 nM AVP and an EC50 of 2.5 nM; the later phase (15 min of stimulation) showed a maximal response at 100 nM AVP and an EC50 of 3.6 nM (Figure 7, A and B).
Figure 6.
PDE4 activity is stimulated by AVP-treatment of L6-C5 cells. (A) Effects of AVP and IGF1 on total cAMP-PDE activity of L6-C5 cells. AVP (1 μM), IGF1 (1 nM), or saline (control) was added at time 0 to L6-C5 myoblasts, which had been cultured for 2 d in serum-free medium. Cells were harvested at the indicated times (an extended time course up to 48 h is shown in the inset), and immediately homogenized and assayed for total cAMP-PDE activity. Data are means ± SEM for nine or more samples in at least five different experiments for AVP (●) and five or more samples in three different experiments for IGF1 treatment (▴). (B) Effect of AVP treatment on PDE4 activity of L6-C5 cells. Cells were treated with 1 μM AVP ([●) or left untreated (○) for the indicated times. They were then rapidly homogenized and assayed for cAMP-PDE activity, in the presence and absence of 10 μM rolipram. PDE4 activity was evaluated as the rolipram-inhibited fraction of PDE activity. Data are means ± SEM for n = 5–7 samples from four different experiments. *, Significance at p < 0.05; **, p < 0.02; ***, p < 0.01.
Figure 7.
Dose-dependent effects of AVP on the rapid and slow components of PDE activation. L6-C5 cells were treated with increasing concentrations of AVP. Total cAMP-PDE activity was measured after 2 min (A) and 15 min (B) of AVP stimulation. Data represent the means of four to nine measurements performed in three independent experiments.
Interestingly IGF1, which is unable by itself to induce differentiation in L6-C5 cells in serum-free medium (Minotti et al., 1998), did not induce modifications of PDE activity for up to 60 min of stimulation (Figure 6A).
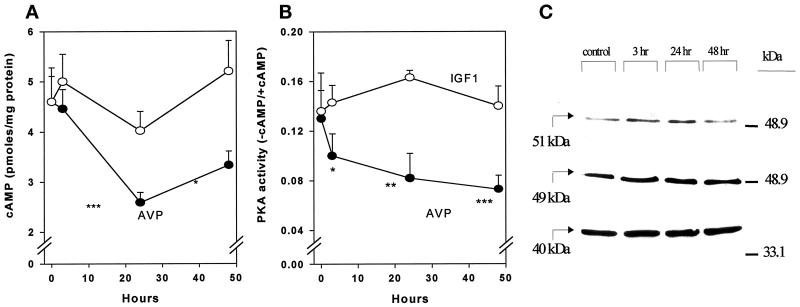
cAMP Level and PKA Activity Decrease under AVP Treatment
To evaluate the physiological consequences of PDE activation induced by AVP treatment of L6-C5 cells, we measured cAMP level and PKA activity at different times after hormone addition. AVP treatment induced a reduction in cAMP concentration, which was maximal at 24 h (Figure 8A). AVP also induced a significant decrease in PKA activity ratio, which was evident after 3 h of hormonal stimulation and continued up to 48 h after the onset of treatment (Figure 8B). Addition of 10 μM rolipram to the cell culture medium totally prevented the AVP-dependent decrease of PKA activity (our unpublished results). The PKA activity ratio decrease was not related to modifications in PKA protein levels, as assessed by Western blot analysis of the expression of the catalytic and regulatory subunits RIα and RIIα (Figure 8C), and can thus be attributed to decreased cAMP availability. Such decreases in cAMP concentration and PKA activity are consistent with the observation of an elevated capacity of myoblasts to hydrolyze cAMP after AVP stimulation and could be part of the mechanism that leads to myoblast differentiation.
Figure 8.
AVP treatment of L6-C5 cells induces a decrease of cAMP and PKA activity without affecting PKA expression. (A) cAMP concentration was measured after incubation of cells with 1 μM AVP or 1 nM IGF1 for the indicated times. The cells were treated at different times, and all the samples (control and AVP treated) were harvested and assayed at the same time, i.e., 2 d after shifting to serum-free medium. cAMP was measured as described in MATERIALS AND METHODS. Results are the means ± SEM of at least three independent measurements performed in triplicate. *, Significance at p < 0.05; ***, p < 0.01. (B) PKA activity was measured after incubation of the cells as described above. PKA assays were conducted on cell soluble extracts prepared as detailed in MATERIALS AND METHODS. Results are the means ± SEM of six measurements performed in three different experiments for AVP and four measurements in two different experiments for IGF1. *, Significance at p < 0.05; **, p < 0.02; ***, p < 0.01. (C) PKA catalytic subunit (40 kDa) and regulatory subunit RIα and RIIα (49 and 51 kDa, respectively) expression was evaluated on cells treated as described above. Cell samples were prepared by adding directly concentrated sample buffer to 10-cm dishes. Western blot analysis was carried out using specific mAbs for the immunodetection of blots. Total protein concentrations were determined by Bradford (1976) protein assay, and well loading was normalized.
By contrast, treatment of L6-C5 cells with IGF1, which does not increase PDE4 activity, induced no significant change in either cAMP levels or PKA activity (Figure 8, A and B).
Effect of PDE4D3 Overexpression on L6-C5 Cell Differentiation
To confirm whether the increase in PDE4D3 activity is involved in the differentiation process, this enzyme was overexpressed by transient transfection in L6-C5 myoblasts, and the expression of both myogenin (a muscle-specific transcription factor whose nuclear accumulation represents an early marker of myogenic differentiation) and sarcomeric myosin (a marker of terminal myogenic differentiation) was evaluated. After 48 h of culture in serum-free medium, with or without AVP addition, transfected cells were immunostained with anti-myogenin antibody. As shown in Figure 9A, cultures of cells transfected with PDE4D3 showed a marked increase of myogenin-positive nuclei (b and d) compared with mock-transfected cell cultures (a and c). This increase was evident in untreated cells (a vs. b) as well as in AVP-treated cells (c vs. d). When transfected L6-C5 cells were further cultured for 6 d, a significant increase in myotube formation and in the expression of myosin was observed in cells overexpressing PDE4D3 compared with mock-transfected cells, by immunochemical staining of the cells (Figure 9B) as well as by ELISA quantification of myosin in cell extracts (Table 1). In this case, too, overexpression of PDE4D3 induced a higher expression of the differentiation marker in both AVP-treated and untreated cells (Figure 9B and Table 1).
Figure 9.
Effect of PDE4D3 overexpression on L6-C5 cell differentiation. L6-C5 myoblasts were transiently transfected in suspension by using Fugene 6 with 1 μg of pCMV5 expression vector containing rat PDE4D3 cDNA (Sette et al., 1994c) or the empty vector and plated in 10% FCS-containing medium. After 24 h, the medium was replaced by serum-free medium with or without addition of 0.1 μM AVP. (A) Immunofluorescence analysis of the expression of myogenin in transfected L6-C5 cells. Mock-transfected cells (a and c) and pCMV5-PDE4D3-transfected cells (b and d) were left untreated (a and b) or were treated with 0.1 μM AVP for 48 h (c and d). Myogenin was detected by using the anti-myogenin antibody F5D. (B) Immunochemical detection of myosin expression in transfected L6-C5 cells. Mock-transfected cells (a and c) and pCMV5-PDE4D3-transfected cells (b and d) were left untreated (a and b) or were treated with 0.1 μM AVP for 6 d (c and d). Myosin was detected by using the anti-myosin antibody MF20.
Table 1.
Effect of PDE4D3 overexpression on L6 cell differentiation (myosin accumulation)
| Culture medium | Mock transfected (ng/μg DNA) | PDE4D3 transfected (ng/μg DNA) |
|---|---|---|
| 1% BSA | 45 ± 2.25 | 100 ± 1.1a |
| 1% BSA + 0.1 μM AVP | 320 ± 25.3 | 530 ± 9.1a |
L6 myoblasts were transiently transfected in suspension by using Fugene 6 with 1 μg of the pCMV5-PDE4D3 plasmid or the empty vector and plated in 10% FCS medium. After 24 h, the medium was replaced as indicated, with or without addition of 0.1 μM AVP. After 6 d of culture, the cells were solubilized, and their myosin content was measured by ELISA. Results are the means ± SD of six measurements performed in two independent experiments.
Significantly different from mock-transfected cells, p < 0.01.
DISCUSSION
This work demonstrates that type 4 PDEs play a role in the control of myogenic differentiation of L6-C5 myoblasts. We show that selective inhibition of PDE4 enzymes by two specific, structurally unrelated inhibitors, namely rolipram (Schwabe et al., 1976) and RS 23544 (Alvarez et al., 1995), suppresses differentiation induced by AVP in these cells. Indeed, it prevents translocation of the muscle-specific transcription factor myogenin to the nucleus, the formation of myotubes, and ultimately the expression of the terminal differentiation marker CK. We also demonstrate that AVP stimulation of L6-C5 cells leads to activation of PDE4 and a decrease of cAMP level and PKA activity. Because PKA activation is considered a negative signal for myoblast differentiation, our data suggest that AVP-dependent down-regulation of PKA activity allows the full expression of the myogenic program in L6-C5 cells. The importance of the role played by PDE4 in the control of myogenic differentiation is further supported by experiments showing that overexpression of the PDE4D3 isoform in L6-C5 cells increased their differentiation (both spontaneous and AVP induced), as evaluated by means of markers of both early phases of differentiation (nuclear accumulation of myogenin), and terminal differentiation (myotube formation and myosin expression).
The ability of PDE4 inhibitors to completely prevent differentiation can be ascribed to increased cAMP intracellular levels and PKA activation, because the PDE4-specific inhibitor rolipram induced a large increase of endogenous cAMP levels together with PKA activation in the absence of stimulation of adenylyl cyclase. Furthermore, addition of a cAMP cell-permeable analogue, 8-Br-cAMP, which is slowly hydrolyzed by PDEs, partially reproduced the inhibition of differentiation. The negative regulation of myogenic differentiation by cAMP and PKA has been previously established in several cellular models, including L6 cells, induced to differentiate by reducing the serum concentration of the culture medium, or by adding insulin (Wahrman et al., 1973; Hu and Olson 1988; Salminen et al., 1991; Winter et al., 1993). The present results allow extension of this concept to the model of AVP-induced myogenic differentiation. We have previously shown that AVP induction of myogenesis in L6-C5 cells is related to an accumulation of the muscle transcription factor myogenin in the cell nucleus (Minotti et al., 1998). We now observe that, in the presence of rolipram, myogenin is not detectable in myoblast nuclei, although AVP treatment increases the amount of myogenin. This result suggests that the cAMP signal transduction pathway controls the subcellular localization of myogenin. No information is available yet about the mechanism by which cAMP regulates myogenin import, although phosphorylation steps may conceivably be involved. Nuclear myogenin import could be an important control point in regulating myogenic differentiation.
Our results highlight the importance of the function of type 4 PDEs in the regulation of cAMP levels during L6-C5 myoblast differentiation. Indeed, inhibitors specific for other classes of PDEs, milrinone and zaprinast (which respectively inhibit type 3 and type 5 PDEs), were devoid of inhibitory effects. It is thus conceivable that in L6-C5 cells a functional pool of cAMP under the sole control of PDE4 is able to modulate the differentiative response. Both rolipram and RS 23544 appear as very potent inhibitors of AVP-induced myogenic differentiation. Half-maximal inhibition of differentiation, as evaluated by CK activity, was achieved at a rolipram concentration of 10 nM. This can be ascribed to an effect of the drug directed to PDE4 species present under a “high affinity rolipram state” (Huston et al., 1996; Sette and Conti 1996) sensitive to nanomolar concentrations of rolipram (reviewed by Souness and Rao 1997; Houslay et al., 1998).
Identification of type 4 PDE expressed in L6-C5 cells by RPA, RT-PCR, immunoprecipitation, and Western blot analysis with isoform-selective anti-PDE4 antibodies led to the conclusion that only the PDE4D gene is expressed in these cells, confirming previous observations based only on RT-PCR data (Kovala et al., 1994). Furthermore, we found that the PDE4D3 isoform, one of the five variants encoded by the PDE4D gene (Sette et al., 1994c; Bolger et al., 1997), is the major isoform present in these cells. This result is only apparently in contrast with the observation made by others that PDE4D1 mRNA is the major species expressed in L6 myoblasts. The latter data were obtained after a prolonged pharmacological treatment of the cells by cAMP-elevating agents (Kovala et al., 1994). Because it is well established that the PDE4D1 and PDE4D2 isoforms, but not the PDE4D3 isoform, are up-regulated by cAMP at the transcriptional level in different cell types (Sette et al., 1994b,c; Vicini and Conti 1997), we can infer that the major type 4 PDE in the absence of a cAMP-elevating pharmacological treatment is the PDE4D3 isoform in undifferentiated myoblasts as well as in differentiated myotubes (Figure 4B).
L6-C5 myoblasts only express the V1 receptors for AVP, which have no direct relationships with adenylyl cyclase regulation (Wakelam et al., 1987) but trigger the activation of phospholipases C and D (Naro et al., 1997). AVP seems to modulate cAMP levels and PKA activity in these cells by modifying cAMP hydrolysis rather than by acting on cAMP synthesis. Indeed two phases of PDE activation were apparent in response to AVP addition: a fast activation, which peaked at 2 min, and a slower activation, which peaked at 15 min and was significantly maintained even after 48 h of hormonal treatment. That the AVP-induced PKA activity decrease is mainly dependent on PDE4 activation and unrelated to changes in adenylyl cyclase activity is confirmed by the observation that rolipram, when added to the culture medium, totally suppressed the kinase activity reduction (our unpublished results).
In addition to PKA-dependent phosphorylation (Sette et al., 1994b; Sette and Conti 1996), PDE4D3 is specifically activated by PA, the product of phospholipase D action, in a cell-free system (Némoz et al., 1997; Grange et al., 1998). Both phospholipid binding and PKA phosphorylation sites reside in the same amino-terminal region, because isoforms lacking this region, such as PDE4D1 and PDE4D2, are not activated by PKA phosphorylation or by phospholipid interaction (Sette et al., 1994b; Némoz et al., 1997). Because phospholipase D activation is a primary signaling event triggered by AVP in L6-C5 cells (Naro et al., 1997), the possibility exists that PDE4D3 is stimulated by PA, which rapidly accumulates in the cells under AVP stimulation. Thus both PA-induced stimulation and phosphorylation processes might be responsible for the increase in PDE4 activity observed in L6-C5 cells. The EC50 value observed for the first phase of AVP-induced PDE stimulation (2.5 nM) is compatible with the 0.4 nM EC50 previously reported for AVP activation of phospholipase D (Naro et al., 1997), suggesting that the rapid PDE stimulation could be linked to phospholipase D activation and production of PA. Furthermore, the kinetics of PA production by AVP-stimulated L6-C5 cells are in agreement with this hypothesis, because the PA level reaches its maximum by 1 min (Naro et al., 1997). Experiments are in progress to more clearly define the mechanism(s) involved.
In view of the profound effect of PDE4 inhibition on the differentiation process, it can be postulated that an increase in PDE4 activity plays a physiological role in lowering the intracellular cAMP concentration, thus preventing inhibition of the myogenic program by cAMP. The sustained decrease in both cAMP level and PKA activity that we observed in AVP-treated myoblasts supports this hypothesis. Furthermore, the observation that overexpression of the type 4 PDE isoform PDE4D3 could positively influence myogenic differentiation provides confirmation of this model. Thus, PDE4 activation appears an essential step in myogenesis, and the lack of effect of IGF1 in inducing terminal myogenic differentiation in the absence of serum or AVP (Minotti et al., 1998) may be attributed to its inability to induce stimulation of PDE4 activity. This supports the conclusion that cAMP levels must be tightly controlled during myogenesis and that PDE4 plays a key role in such control.
ACKNOWLEDGMENTS
We thank Dr. Bianca Maria Scicchitano for the construction of the myogenin promoter-luciferase construct, Dr. Raffaella Curci for expert assistance, and Prof. Giulio Cossu for helpful discussion and suggestions. This work was supported in part by grants from the Italian Ministry of University and Scientific and Technological Research (to S.A.), from the Associazione Italiana per la Ricerca sul Cancro AIRC (to M.M.) and from Consiglio Nazionale delle Ricerche grants 96.0600.PF39 (to M.M.) and 96.00651.39 (to S.A.). The exchanges between the collaborating institutions were supported by a Consiglio Nazionale delle Ricerche–Institut National de la Santé et de la Recherche Médicale joint program grant (to G.N. and S.A.).
Abbreviations:
- AVP
Arg8-vasopressin
- β-gal
β-galactosidase
- 8-Br-cAMP
8-bromo-cAMP
- CK
creatine kinase
- CMV
cytomegalovirus
- DME
Dulbecco’s modified Eagle’s
- IGF1
insulin-like growth factor 1
- PA
phosphatidic acid
- PDE
phosphodiesterase
- PKA
protein kinase A
- PKI
protein kinase inhibitor
- RPA
RNase protection assay
- RT
reverse transcriptase
REFERENCES
- Alvarez R, Sette C, Yang D, Eglen RM, Wilhelm R, Shelton ER, Conti M. Activation and selective inhibition of a cyclic AMP-specific phosphodiesterase, PDE4D-3. Mol Pharmacol. 1995;48:616–622. [PubMed] [Google Scholar]
- Bader D, Masaki T, Fischman DA. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol. 1982;95:763–770. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- Bolger GB, Erdogan S, Jones RE, Loughney K, Scotland G, Hoffmann R, Wilkinson I, Farrell C, Houslay MD. Characterization of five different proteins produced by alternatively spliced mRNAs from the human cAMP-specific phosphodiesterase PDE4D gene. Biochem J. 1997;328:539–548. doi: 10.1042/bj3280539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger GB, Michaeli T, Martins T, St. John T, Steiner B, Rodgers L, Riggs M, Wigler M, Ferguson K. A family of human phosphodiesterases homologous to the dunce learning and memory gene product of Drosophila melagonaster are potential targets for antidepressant drugs. Mol Cell Biol. 1993;13:6558–6571. doi: 10.1128/mcb.13.10.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brasier AR, Tate JE, Habener JF. Optimized use of the firefly luciferase assay as a reporter gene in mammalian cell lines. Biotechniques. 1989;7:1116–1122. [PubMed] [Google Scholar]
- Braun T, Bober E, Buschhausen-Denker G, Kothz S, Grzeschik KH, Arnold HH. Differential expression of myogenic determination genes in muscle cells: possible autoactivation by the Myf gene products. EMBO J. 1989;8:3617–3625. doi: 10.1002/j.1460-2075.1989.tb08535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg CH, Linkhart TA, Olwin BB, Hauschka SD. Growth factor control of skeletal muscle differentiation: committment to terminal differentiation occurs in G1 phase and is repressed by fibroblast growth factor. J Cell Biol. 1987;105:949–956. doi: 10.1083/jcb.105.2.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colicelli J, Birchmeier C, Michaeli T, O’Neill K, Riggs M, Wigler M. Isolation and characterization of a mammalian gene encoding a high-affinity cAMP phosphodiesterase. Proc Natl Acad Sci USA. 1989;86:3599–3603. doi: 10.1073/pnas.86.10.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti M, Némoz G, Sette C, Vicini E. Recent progress in understanding the hormonal regulation of phosphodiesterases. Endocr Rev. 1995;16:370–389. doi: 10.1210/edrv-16-3-370. [DOI] [PubMed] [Google Scholar]
- Cossu G, Tajbakhsh S, Buckingham M. How is myogenesis initiated in the embryo? Trends Genet. 1996;12:218–223. doi: 10.1016/0168-9525(96)10025-1. [DOI] [PubMed] [Google Scholar]
- Cusella De Angelis MG, et al. MyoD, myogenin independent differentiation of primordial myoblasts in mouse somites. J Cell Biol. 1992;116:1243–1255. doi: 10.1083/jcb.116.5.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL, Takayasu H, Eberwine M, Myres J. Cloning and characterization of mammalian homologs of the Drosophila dunce+ gene. Proc Natl Acad Sci USA. 1989;86:3604–3608. doi: 10.1073/pnas.86.10.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson DG, Cheng TC, Cserjesi P, Chakraborty T, Olson EN. Analysis of the myogenin promoter reveals an indirect pathway for positive autoregulation mediated by the muscle-specific enhancer factor MEF-2. Mol Cell Biol. 1992;12:3665–3677. doi: 10.1128/mcb.12.9.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engert JC, Berglund EB, Rosenthal N. Proliferation precedes differentiation in IGF-I stimulated myogenesis. J Cell Biol. 1996;135:431–440. doi: 10.1083/jcb.135.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florini JR. Hormonal control of muscle growth. Muscle Nerve. 1987;10:577–598. doi: 10.1002/mus.880100702. [DOI] [PubMed] [Google Scholar]
- Florini JR, Ewton DZ, Magri KA. Hormones, growth factors, and myogenic differentiation. Annu Rev Physiol. 1991;53:201–216. doi: 10.1146/annurev.ph.53.030191.001221. [DOI] [PubMed] [Google Scholar]
- Grange M, Picq M, Prigent AF, Lagarde M, Némoz G. Regulation of PDE-4 cAMP phosphodiesterases by phosphatidic acid. Cell Biochem Biophys. 1998;29:1–17. doi: 10.1007/BF02737825. [DOI] [PubMed] [Google Scholar]
- Houslay MD, Sullivan M, Bolger GB. The multienzyme PDE4 cyclic adenosine monophosphate-specific phosphodiesterase family: intracellular targeting, regulation, and selective inhibition by compounds exerting ant-inflammatory and antidepressant actions. Adv Pharmacol. 1998;44:225–342. doi: 10.1016/s1054-3589(08)60128-3. [DOI] [PubMed] [Google Scholar]
- Hu JS, Olson EN. Regulation of differentiation of the BC3H1 muscle cell line through cAMP-dependent and -independent pathways. J Biol Chem. 1988;263:19670–19677. [PubMed] [Google Scholar]
- Huston E, Pooley L, Julien P, Scotland G, McPhee I, Sullivan M, Bolger GB, Houslay MD. The human cyclic AMP-specific phosphodiesterase PDE-46 (HSPDE4A4B) expressed in transfected COS7 cells occurs as both particulate and cytosolic species that exhibit distinct kinetics of inhibition by the antidepressant rolipram. J Biol Chem. 1996;271:31334–31344. doi: 10.1074/jbc.271.49.31334. [DOI] [PubMed] [Google Scholar]
- Iona S, Cuomo M, Bushnik T, Naro F, Sette C, Hess M, Shelton ER, Conti M. Characterization of the rolipram-sensitive, cAMP specific phosphodiesterases: identification and differential expression of immunologically distinct forms in the rat brain. Mol Pharmacol. 1998;53:23–32. doi: 10.1124/mol.53.1.23. [DOI] [PubMed] [Google Scholar]
- Jin SLC, Bushnik T, Lan L, Conti M. Subcellular localization of rolipram-sensitive, cAMP-specific phosphodiesterases. J Biol Chem. 1998;273:19672–19678. doi: 10.1074/jbc.273.31.19672. [DOI] [PubMed] [Google Scholar]
- Kovala T, Lorimer IAJ, Brickenden AM, Ball EH, Sanwal BD. Protein kinase A regulation of cAMP phosphodiesterase expression in rat skeletal myoblasts. J Biol Chem. 1994;269:8680–8685. [PubMed] [Google Scholar]
- Li L, Heller-Harrison R, Czech M, Olson EN. Cyclic AMP-dependent protein kinase activity inhibits the activity of myogenic helix-loop-helix proteins. Mol Cell Biol. 1992;12:4478–4485. doi: 10.1128/mcb.12.10.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludolph DC, Konieczny SF. Transcription factor families: muscling in on the myogenic program. FASEB J. 1995;9:1595–1604. doi: 10.1096/fasebj.9.15.8529839. [DOI] [PubMed] [Google Scholar]
- Minotti S, Scicchitano BM, Nervi C, Scarpa S, Lucarelli M, Molinaro M, Adamo S. Vasopressin and insulin-like growth factors synergistically induce myogenesis in serum-free medium. Cell Growth & Differ. 1998;9:155–163. [PubMed] [Google Scholar]
- Nadal-Ginard B. Commitment, fusion and biochemical differentiation of a myogenic cell line in the absence of DNA synthesis. Cell. 1978;15:855–864. doi: 10.1016/0092-8674(78)90270-2. [DOI] [PubMed] [Google Scholar]
- Naro F, Donchenko V, Minotti S, Zolla L, Molinaro M, Adamo S. Role of phospholipase C and D signaling pathways in vasopressin-dependent myogenic differentiation. J Cell Physiol. 1997;171:34–42. doi: 10.1002/(SICI)1097-4652(199704)171:1<34::AID-JCP5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Naro F, Fazzini A, Citro G, Malatesta F, Antonini G, Sarti P, Brunori M, Franconi F, Giotti A. Immunoquantitation of cytochrome c in cardiac perfusate. J Immunoassay. 1991;12:251–262. doi: 10.1080/01971529108055070. [DOI] [PubMed] [Google Scholar]
- Naro F, Zhang R, Conti M. Developmental regulation of unique cyclic adenosine 3′-5′-monophosphate-specific phosphodiesterase variants during rat spermatogenesis. Endocrinology. 1996;137:2464–2472. doi: 10.1210/endo.137.6.8641200. [DOI] [PubMed] [Google Scholar]
- Némoz G, Sette C, Conti M. Selective activation of rolipram-sensitive, cAMP-specific phosphodiesterase isoforms by phosphatidic acid. Mol Pharmacol. 1997;51:242–249. doi: 10.1124/mol.51.2.242. [DOI] [PubMed] [Google Scholar]
- Nervi C, Benedetti L, Minasi A, Molinaro M, Adamo S. Arginine-vasopressin induces differentiation of skeletal myogenic cells and up-regulation of myogenin and myf-5. Cell Growth & Differ. 1995;6:81–89. [PubMed] [Google Scholar]
- Olson EN, Sternberg E, Hu JS, Spizz G, Wilcox C. Regulation of myogenic differentiation by type β transforming growth factor. J Cell Biol. 1986;103:1799–1805. doi: 10.1083/jcb.103.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill MC, Stockdale FE. A kinetic analysis of myogenesis in vitro. J Cell Biol. 1972;52:52–65. doi: 10.1083/jcb.52.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Braun T, Buchberger A, Jurs S, Winter B, Arnold HH. Transcription of the muscle regulatory gene MYF4 is regulated by serum components, peptide growth factors and signaling pathways involving G proteins. J Cell Biol. 1991;115:905–917. doi: 10.1083/jcb.115.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe U, Miyake M, Ohga Y, Daly JW. 4-(-3-Cyclopentyloxy-4-methoxyphenyl)-2-pyrrolidone (ZK 62711): a potent inhibitor of adenosine cyclic 3′ 5′-mono-phosphate-phosphodiesterases in homogenates and tissue slices from rat brain. Mol Pharmacol. 1976;12:900–910. [PubMed] [Google Scholar]
- Sette C, Conti M. Phosphorylation and activation of a cAMP-specific phosphodiesterase by the cAMP-dependent protein kinase. Involvement of serine 54 in the enzyme activation. J Biol Chem. 1996;271:16526–16534. doi: 10.1074/jbc.271.28.16526. [DOI] [PubMed] [Google Scholar]
- Sette C, Iona S, Conti M. The short-term activation of a rolipram-sensitive, cAMP-specific phosphodiesterase by thyroid-stimulating hormone in thyroid FRTL-5 cells is mediated by a cAMP dependent phosphorylation. J Biol Chem. 1994a;269:9245–9252. [PubMed] [Google Scholar]
- Sette C, Vicini E, Conti M. Modulation of cellular responses by hormones: role of cAMP specific, rolipram sensitive phosphodieserases. Mol Cell Endocrinol. 1994b;100:75–79. doi: 10.1016/0303-7207(94)90282-8. [DOI] [PubMed] [Google Scholar]
- Sette C, Vicini E, Conti M. The ratPDE3/IVd phosphodiesterase gene codes for multiple proteins differentially activated by cAMP-protein kinase. J Biol Chem. 1994c;269:18271–18274. [PubMed] [Google Scholar]
- Soderling SH, Bayuga SJ, Beavo JA. Cloning and characterization of a cAMP-specific cyclic nucleotide phosphodiesterase. Proc Natl Acad Sci USA. 1998;95:8991–8996. doi: 10.1073/pnas.95.15.8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souness JE, Rao S. Proposal for pharmacologically distinct conformers of PDE4 cyclic AMP phosphodiesterases. Cell Signalling. 1997;9:227–236. doi: 10.1016/s0898-6568(96)00173-8. [DOI] [PubMed] [Google Scholar]
- Swinnen JV, Joseph DR, Conti M. Molecular cloning of rat homologues of the Drosophila melanogaster dunce cAMP phosphodiesterase: evidence for a family of genes. Proc Natl Acad Sci USA. 1989;86:5325–5329. doi: 10.1073/pnas.86.14.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teti A, Naro F, Molinaro M, Adamo S. Transduction of the arginine-vasopressin signal in skeletal myogenic cells. Am J Physiol. 1993;265:C113–C121. doi: 10.1152/ajpcell.1993.265.1.C113. [DOI] [PubMed] [Google Scholar]
- Thompson WJ, Brooker G, Appleman MM. Assay of cyclic nucleotide phosphodiesterase with radioactive substrates. Methods Enzymol. 1974;38:205–212. doi: 10.1016/0076-6879(74)38033-0. [DOI] [PubMed] [Google Scholar]
- Vicini E, Conti M. Characterization of an intronic promoter of a cyclic adenosine 3′,5′-monophosphate (cAMP)-specific phosphodiesterase gene that confers hormone and cAMP inducibility. Mol Endocrinol. 1997;11:839–850. doi: 10.1210/mend.11.7.9941. [DOI] [PubMed] [Google Scholar]
- Wahrman JP, Winand R, Luzzatti D. Effect of cyclic AMP on growth and morphological differentiation of an established myogenic cell line. Nature. 1973;245:112–113. doi: 10.1038/newbio245112a0. [DOI] [PubMed] [Google Scholar]
- Wakelam MJO, Patterson S, Hanley MR. L6 skeletal muscle cells have functional V1 vasopressin receptors coupled to stimulated inositol phospholipid metabolism. FEBS Lett. 1987;210:181–184. doi: 10.1016/0014-5793(87)81333-9. [DOI] [PubMed] [Google Scholar]
- Winter B, Braun T, Arnold HH. cAMP-dependent protein kinase represses myogenic differentiation and the activity of the muscle-specific helix-loop-helix transcription factors Myf-5 and MyoD. J Biol Chem. 1993;268:9869–9878. [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci USA. 1968;61:477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]