Abstract
We investigate the voltage-driven translocation dynamics of individual DNA molecules through solid-state nanopores in the diameter range 2.7–5 nm. Our studies reveal an order of magnitude increase in the translocation times when the pore diameter is decreased from 5 to 2.7 nm, and steep temperature dependence, nearly threefold larger than would be expected if the dynamics were governed by viscous drag. As previously predicted for an interaction-dominated translocation process, we observe exponential voltage dependence on translocation times. Mean translocation times scale with DNA length by two power laws: for short DNA molecules, in the range 150–3500 bp, we find an exponent of 1.40, whereas for longer molecules, an exponent of 2.28 dominates. Surprisingly, we find a transition in the fraction of ion current blocked by DNA, from a length-independent regime for short DNA molecules to a regime where the longer the DNA, the more current is blocked. Temperature dependence studies reveal that for increasing DNA lengths, additional interactions are responsible for the slower DNA dynamics. Our results can be rationalized by considering DNA/pore interactions as the predominant factor determining DNA translocation dynamics in small pores. These interactions markedly slow down the translocation rate, enabling higher temporal resolution than observed with larger pores. These findings shed light on the transport properties of DNA in small pores, relevant for future nanopore applications, such as DNA sequencing and genotyping.
INTRODUCTION
Nanopores are an emerging class of single-molecule sensors capable of probing the properties of nucleic acids and proteins with high-throughput and resolution (1–3). In a nanopore experiment, voltage is applied across a thin insulating membrane containing a nanoscale pore, and the ion current of an electrolyte flowing through the pore is measured. Upon introduction of charged biopolymers to the solution, the local electrical field drives individual molecules through the nanopore. Passage of biopolymers through the pore causes distinct ion current signals, with amplitudes that directly correspond to their properties. Among single-molecule sensors, nanopores are unique because molecules can be probed without chemical modification and/or surface immobilization, thus preserving structure/function and allowing very high throughput. These attractive features have set the stage for the development of novel nanopore-based applications, such as detection of genetic variability, probing DNA-protein interactions, and low-cost, high-throughput DNA sequencing (4–6).
Central to all nanopore methods is the need for control over the translocation process at a level that allows spatial information to be resolved at the nanometer scale, within the finite time resolution imposed by instrumental bandwidth. Ultimately, fundamental understanding of the factors governing the DNA translocation dynamics, and its relationship with the magnitude and fluctuations of the blocked current signal, is necessary to achieve this goal. To date, most DNA translocation studies have been performed using the toxin α-hemolysin (α-HL), which can only admit single-stranded (ss) nucleic acids (7–9). The linear dependence of the most probable translocation time (tP) on ssDNA length (l), and the lack of strong sticking interactions between the nucleic acids and α-HL, have supported the idea that the translocation process can be approximated by a mean sliding velocity 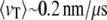 (measured for ssDNA at 120 mV and room temperature), or an average translocation rate
(measured for ssDNA at 120 mV and room temperature), or an average translocation rate  (where N is the number of nucleotides). This rate provides sufficient temporal resolution for detecting a few bases within instrumental bandwidth limits (9,10). However, prospective biotechnological nanopore applications require size tunability and membrane robustness, not available with phospholipid-embedded protein channels.
(where N is the number of nucleotides). This rate provides sufficient temporal resolution for detecting a few bases within instrumental bandwidth limits (9,10). However, prospective biotechnological nanopore applications require size tunability and membrane robustness, not available with phospholipid-embedded protein channels.
Recent progress in the fabrication of nanoscale materials has enabled the reproducible formation of artificial, well-defined nanopores in thin, solid-state membranes (11–13). Most DNA translocation studies have focused on relatively large pores (8–20 nm), for which average translocation dynamics were markedly faster than those reported for α-HL (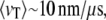 or 30 ns/bp) (14–18). Broad dwell-time distributions for DNA translocation have been previously reported with smaller solid-state nanopores (2–3 nm) (14,19), although the source of broadening and the nature of the dwell-time events were not investigated experimentally. To slow the translocation dynamics, several experimental parameters have been modified, including viscosity, temperature, and voltage. However, these parameters also reduce the open-pore current, thereby degrading the blocked current signal (20). Moreover, an increase in the bulk viscosity or a reduction of the driving voltage reduces DNA diffusion to the pore and the capture probability, respectively, therefore decreasing the overall throughput (21).
or 30 ns/bp) (14–18). Broad dwell-time distributions for DNA translocation have been previously reported with smaller solid-state nanopores (2–3 nm) (14,19), although the source of broadening and the nature of the dwell-time events were not investigated experimentally. To slow the translocation dynamics, several experimental parameters have been modified, including viscosity, temperature, and voltage. However, these parameters also reduce the open-pore current, thereby degrading the blocked current signal (20). Moreover, an increase in the bulk viscosity or a reduction of the driving voltage reduces DNA diffusion to the pore and the capture probability, respectively, therefore decreasing the overall throughput (21).
In this article, we focus on the use of nanopore/DNA interactions as an alternative means to slow down DNA translocation through nanopores, by using nanopores only slightly larger than a double-stranded DNA (dsDNA) cross section. Theoretically, interactions have been proposed to dominate the dynamics for both α-HL (22) and synthetic (19,23,24) nanopores, in particular for nanopore dimensions slightly larger than the molecular cross section (2.2 nm for dsDNA).
DNA analysis using nanopores via single-file threading (i.e., by unfolded entry) is highly attractive, potentially allowing detection of subtle variations in local DNA structure as it transverses the pore, for example, single- and double-stranded regions on a DNA template. To promote unfolded DNA entry while simultaneously maximizing DNA/surface interactions, we have focused in this study on solid-state nanopores in the range 2.7–5 nm. Our results show that small variations in the nanopore diameter strongly affect average translocation times, the threading probability, and the event current amplitude. Also, translocation times exhibit steep temperature dependence, nearly three times larger than expected from viscosity changes. Our results clearly show that DNA/pore interactions are the dominant contributing factor governing DNA translocation through small pores, revealing a more complex DNA translocation dynamics than previously observed.
MATERIALS AND METHODS
Nanopores were fabricated in 25- to 30-nm-thick, low-stress SiN windows (25 μm × 25 μm) supported by a Si chip (Protochips, Raleigh, NC), using a focused electron beam (13). Extensive transmission electron microscopic tomography studies revealed an hourglass nanopore profile with an effective thickness of ∼⅓ the membrane thickness (∼10 nm for the 30-nm membrane in this study). Nanopore chips were cleaned and assembled on a custom-designed cell under controlled atmosphere (see Wanunu and Meller (3) for details). After the addition of degassed and filtered 1 M KCl electrolyte (buffered with 10 mM Tris-HCl to pH 8.5), the nanopore cell was placed in a custom-designed chamber featuring thermoelectric regulation within ±0.1°C, rapid thermal equilibration (<5 min), and an effective electromagnetic shield. Ag/AgCl electrodes were immersed into each chamber of the cell and connected to an Axon 200B headstage. All measurements were taken inside a dark Faraday cage. DNA was introduced to the cis chamber, and a positive voltage of 300 mV was applied to the trans chamber in all experiments.
For the DNA length dependence studies we used a series of pure, linear DNA fragments with lengths in the range 150–20,000 bp (NoLimits, Fermentas, Burlington, Ontario, Canada). Agarose gel electrophoresis confirmed the purity of each DNA sample (see the Supplementary Material, Fig. S1 in Data S1). All DNA samples were heated to 70°C for 10 min before use. Our solid-state nanopore setup is displayed schematically in Fig. 1 a. Upon addition of dsDNA into the cis chamber (Fig. 1 b, green arrow), we observe distinct, stochastic current blockade events, the rates of which scale with DNA concentration. A trace of current blockade events with an expanded time axis is shown in the inset to Fig. 1 b. Several parameters are defined here: the event duration (or dwell-time), 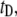 the mean blocked-pore current,
the mean blocked-pore current, 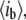 and the dimensionless fractional current,
and the dimensionless fractional current, 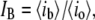 where
where  is the open-pore current.
is the open-pore current.  is the event amplitude (e.g.,
is the event amplitude (e.g., 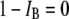 when the pore is fully open, and thus,
when the pore is fully open, and thus, 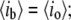 in a similar way, a fully blocked pore corresponds to
in a similar way, a fully blocked pore corresponds to  or
or 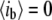 ). The use of normalized units facilitates the comparison of event amplitudes between measurements using different pore sizes or other conditions that alter the open-pore current (e.g., temperature). All measurements reported in this article were performed using a 75 kHz low-pass filter, and sampled using a 16-bit/250 KHz DAQ card. Under these conditions, the maximum error in
). The use of normalized units facilitates the comparison of event amplitudes between measurements using different pore sizes or other conditions that alter the open-pore current (e.g., temperature). All measurements reported in this article were performed using a 75 kHz low-pass filter, and sampled using a 16-bit/250 KHz DAQ card. Under these conditions, the maximum error in 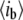 determination for the shortest dwell-times we can measure (12 μs) is <3%, as determined experimentally (Fig. S2, Data S1).
determination for the shortest dwell-times we can measure (12 μs) is <3%, as determined experimentally (Fig. S2, Data S1).
FIGURE 1.
(a) Schematic illustration of a solid-state nanopore device for probing DNA translocation dynamics (not to scale). DNA molecules are driven through the nanopore by an applied voltage while the ion current of an electrolyte is measured. Dynamic voltage control is used to automatically unclog the pore when a molecule remains in the pore for >5 s. Hourglass-shaped nanopores with diameters in the range 3 < d < 8 nm and effective thickness of ∼10 nm were used (see text). (b) A typical ion-current trace for a 4-nm pore, before and after the introduction of 5 nM 400-bp DNA to the cis chamber (green arrow). The transient current-blockade events correspond to single-molecule translocation of DNA. The inset displays a magnified translocation event, in which the relevant parameters used in this article are defined.
Continuous-time recordings of a 4-nm pore at 300 mV (1 M KCl, 21°C, pH 8.5) for different concentrations of 400-bp DNA in the cis chamber are shown in Fig. 2 a. As expected from this stochastic process, we find that delay times between successive events (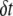 ) follow monoexponential distributions (25), with timescales corresponding to average event rates. For the same DNA fragment, the event rate grows linearly with DNA concentration, as shown in Fig. 2 b. PCR experiments were performed to verify that DNA molecules cross the membrane (from cis to trans) only upon application of positive voltage to the trans chamber (Fig. S3, Data S1).
) follow monoexponential distributions (25), with timescales corresponding to average event rates. For the same DNA fragment, the event rate grows linearly with DNA concentration, as shown in Fig. 2 b. PCR experiments were performed to verify that DNA molecules cross the membrane (from cis to trans) only upon application of positive voltage to the trans chamber (Fig. S3, Data S1).
FIGURE 2.
Translocation recordings for a 400-bp DNA fragment using a 4-nm pore at 1 M KCl, pH 8.5, 300 mV. (a) Continuous current recordings showing blockade events at the indicated DNA concentrations. (b) Normalized distributions of time delay between successive events for different concentrations, using the same pore and DNA as in a, with monoexponential fits to the distributions. (c) A plot of the average event rate as a function of DNA concentration, showing a linear dependence.
RESULTS
We first describe our data and the methods used to analyze blocked-current and dwell-time distributions, and then discuss the effect of pore size on the DNA capture probability, the translocation dynamics, and the blocked current. The last Results section is focused on dependence of the translocation dynamics on DNA length, temperature, and voltage. See Table 1 for a glossary of symbols.
General properties of the dwell-time and blocked-ion-current distributions
In Fig. 3, we display semilog scatter plots of 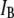 versus
versus 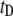 for 8000-bp DNA using 8-nm and 4-nm pores (blue and red markers,
for 8000-bp DNA using 8-nm and 4-nm pores (blue and red markers, 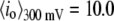 and 2.5 nA, respectively). Three main features are apparent. 1), In the 8-nm pore, one-tenth of the open-pore current is blocked by the DNA (
and 2.5 nA, respectively). Three main features are apparent. 1), In the 8-nm pore, one-tenth of the open-pore current is blocked by the DNA (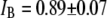 ), whereas in 4-nm pores, more than half of the open-pore current is blocked, i.e.,
), whereas in 4-nm pores, more than half of the open-pore current is blocked, i.e.,  ), Similar to results from previous studies using large pores, events in the 8-nm pore exhibit a substantial fraction of bilevel events, attributed to partially folded DNA entering the pore (14,16). In contrast, we find that events with 4-nm pores are exclusively on a single level, residing in one of two
), Similar to results from previous studies using large pores, events in the 8-nm pore exhibit a substantial fraction of bilevel events, attributed to partially folded DNA entering the pore (14,16). In contrast, we find that events with 4-nm pores are exclusively on a single level, residing in one of two 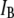 populations, as discussed later. 3), We observe a shift in
populations, as discussed later. 3), We observe a shift in 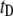 of nearly two orders of magnitude when the nanopore size is decreased from 8 nm to 4 nm. Although a quantitative analysis of the dwell-time dynamics is provided later, we note that if the translocation time were to simply scale with frictional drag in the pore (
of nearly two orders of magnitude when the nanopore size is decreased from 8 nm to 4 nm. Although a quantitative analysis of the dwell-time dynamics is provided later, we note that if the translocation time were to simply scale with frictional drag in the pore ( where d is the pore diameter and
where d is the pore diameter and 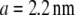 is the hydrodynamic diameter of dsDNA) (17), one would expect a mere threefold increase in translocation times. Thus, the striking difference in
is the hydrodynamic diameter of dsDNA) (17), one would expect a mere threefold increase in translocation times. Thus, the striking difference in 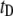 qualitatively suggests a nontrivial, powerful dependence of pore size on the translocation dynamics, which we investigate in this article.
qualitatively suggests a nontrivial, powerful dependence of pore size on the translocation dynamics, which we investigate in this article.
FIGURE 3.
Semilog 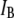 versus
versus  scatter plots measured for 8000-bp DNA at the indicated nanopore diameters (V = 300 mV, T = 21.0 ± 0.1°C). Two salient features emerge upon decreasing the pore size: 1), a decrease in
scatter plots measured for 8000-bp DNA at the indicated nanopore diameters (V = 300 mV, T = 21.0 ± 0.1°C). Two salient features emerge upon decreasing the pore size: 1), a decrease in  (from 0.9 to 0.5); and 2), a drastic increase in
(from 0.9 to 0.5); and 2), a drastic increase in 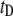 of nearly two orders of magnitude.
of nearly two orders of magnitude.
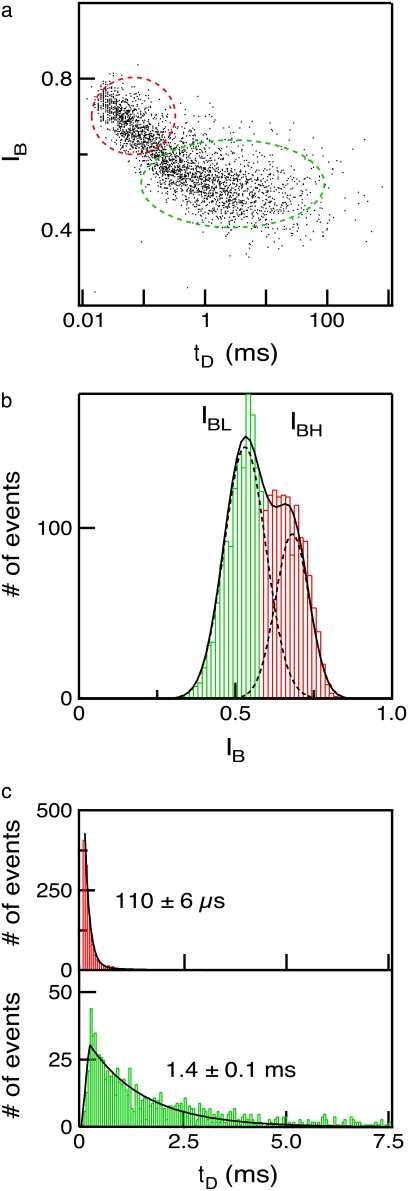
In Fig. 4, we present a summary of 2744 events collected for 6000-bp DNA using a 4-nm pore. A 2D scatter plot of 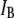 versus
versus 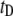 (Fig. 4 a) shows a broad distribution of
(Fig. 4 a) shows a broad distribution of  values (0.4–0.8) and dwell times (20 μs to 100 ms). Moreover, we note that
values (0.4–0.8) and dwell times (20 μs to 100 ms). Moreover, we note that 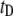 values are not randomly distributed, but rather correlate with the
values are not randomly distributed, but rather correlate with the 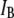 level: on average, shorter events block the pore less than long events. This trend, clearly observed over a time range of 50–500 μs, occurs well within the temporal resolution of our system (∼12 μs, see Fig. S2). To quantitatively correlate the event duration with the current blockage level, we present in Fig. 4 b an
level: on average, shorter events block the pore less than long events. This trend, clearly observed over a time range of 50–500 μs, occurs well within the temporal resolution of our system (∼12 μs, see Fig. S2). To quantitatively correlate the event duration with the current blockage level, we present in Fig. 4 b an 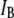 distribution for all events in the scatter plot. This distribution unambiguously shows two peaks and is well approximated using a double-Gaussian function. The appearance of two
distribution for all events in the scatter plot. This distribution unambiguously shows two peaks and is well approximated using a double-Gaussian function. The appearance of two  peaks is a typical feature of our nanopore experiments, for all examined DNA lengths and temperatures. From the double-Gaussian fit parameters, we split the event populations into low-level (peak at
peaks is a typical feature of our nanopore experiments, for all examined DNA lengths and temperatures. From the double-Gaussian fit parameters, we split the event populations into low-level (peak at  green) and high-level (peak at
green) and high-level (peak at 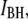 red) blockades, where the low-level blockades correspond to greater current blockage by the DNA and vice versa. To probe the dwell-time characteristics of a population, we chose a cutoff that excludes >99% of events in the other population: e.g., a pure
red) blockades, where the low-level blockades correspond to greater current blockage by the DNA and vice versa. To probe the dwell-time characteristics of a population, we chose a cutoff that excludes >99% of events in the other population: e.g., a pure  population is obtained with a cutoff at
population is obtained with a cutoff at 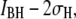 where
where 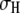 is the std of the
is the std of the  Gaussian.
Gaussian.
FIGURE 4.
Translocation of a 6000-bp DNA fragment using a 4-nm pore (300 mV, 21°C, n = 2744 events). (a) A 2D scatter plot of 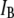 versus
versus 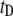 for all the events, highlighting two distinct populations (red and green ovals). (b)
for all the events, highlighting two distinct populations (red and green ovals). (b) 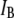 histogram of all the events, revealing two normal distributions,
histogram of all the events, revealing two normal distributions, 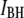 (red) and
(red) and 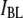 (green). (c) Histograms of the segregated events based on their respective
(green). (c) Histograms of the segregated events based on their respective 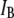 population, yielding distinct dwell-time distributions for events in the
population, yielding distinct dwell-time distributions for events in the  (upper) and
(upper) and  (lower) populations (solid lines are fits to the distributions; see text).
(lower) populations (solid lines are fits to the distributions; see text).
Upon segregation of the events by their respective 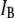 populations, we find that the corresponding dwell-time distributions for the two populations are markedly different: The
populations, we find that the corresponding dwell-time distributions for the two populations are markedly different: The  population, which consists of nearly half the events, exclusively contains short
population, which consists of nearly half the events, exclusively contains short  values, and the distribution can be well approximated by an exponential function with decay constant
values, and the distribution can be well approximated by an exponential function with decay constant  (Fig. 4 c, upper). In contrast, dwell times for the
(Fig. 4 c, upper). In contrast, dwell times for the 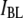 population are much longer. We find that the
population are much longer. We find that the 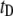 distribution can be approximated by a sharply increasing function for
distribution can be approximated by a sharply increasing function for 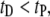 and a broad biexponential tail for
and a broad biexponential tail for 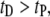 with time constants
with time constants 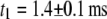 and
and 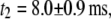 where
where 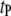 ∼ 200 ± 12 μs denotes the peak of the distribution (Fig. 4 c, lower). Since the vast majority of
∼ 200 ± 12 μs denotes the peak of the distribution (Fig. 4 c, lower). Since the vast majority of  events are spread over the broad tail of the distribution (i.e.,
events are spread over the broad tail of the distribution (i.e., 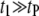 ), it follows that the average dwell time is primarily determined by a weighted sum of
), it follows that the average dwell time is primarily determined by a weighted sum of 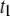 and
and 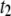 (i.e., not by events with
(i.e., not by events with 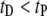 ). As discussed in detail below, the relative frequency of the long
). As discussed in detail below, the relative frequency of the long 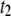 events gradually increases with DNA length, becoming the dominant population for DNA longer than several thousand basepairs (in Fig. 3, for example, the broad dwell-time distribution for 8000-bp DNA using the 4-nm pore is comprised of >90%
events gradually increases with DNA length, becoming the dominant population for DNA longer than several thousand basepairs (in Fig. 3, for example, the broad dwell-time distribution for 8000-bp DNA using the 4-nm pore is comprised of >90% 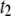 events). Small changes in the cutoff values for
events). Small changes in the cutoff values for 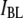 and
and  had negligible impact on determination of the timescales. As demonstrated in the next section, the two
had negligible impact on determination of the timescales. As demonstrated in the next section, the two 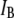 levels correspond to either collisions or full translocations.
levels correspond to either collisions or full translocations.
Effect of pore size on DNA capture probability, blocked current values, and translocation times
Fig. 5 shows characteristic 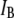 histograms for three pores with
histograms for three pores with 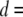 3.1 nm, 4.0 nm, and 4.6 nm (Fig. 5, upper, middle, and lower, respectively), measured using a 400-bp DNA fragment (300 mV, 21.0°C). As explained above, double-Gaussian fits are used to determine
3.1 nm, 4.0 nm, and 4.6 nm (Fig. 5, upper, middle, and lower, respectively), measured using a 400-bp DNA fragment (300 mV, 21.0°C). As explained above, double-Gaussian fits are used to determine 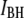 and
and 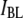 values, as well as the relative fraction of events in each population, using:
values, as well as the relative fraction of events in each population, using: 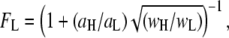 where
where 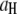 and
and 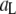 are the high and low amplitudes, respectively, and
are the high and low amplitudes, respectively, and 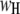 and
and 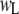 are the high and low widths respectively. The dashed lines display the individual normal distributions for
are the high and low widths respectively. The dashed lines display the individual normal distributions for 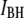 and
and 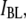 as determined from the fits. We find that
as determined from the fits. We find that 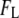 increases from 0.36 ± 0.03 to 0.83 ± 0.01 as the pore diameter increases from 3.1 to 4.6 nm. This trend is schematically illustrated in green for bins predominantly belonging to the
increases from 0.36 ± 0.03 to 0.83 ± 0.01 as the pore diameter increases from 3.1 to 4.6 nm. This trend is schematically illustrated in green for bins predominantly belonging to the 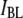 population and in red for those belonging predominantly to the
population and in red for those belonging predominantly to the 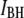 population. We also note that both
population. We also note that both 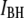 and
and  gradually increase with the nanopore size.
gradually increase with the nanopore size.
FIGURE 5.
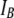 histograms for 400-bp DNA at three different nanopore diameters (d). (Insets) Transmission electron microscope images of the nanopores (scale bars, 2 nm). The current histograms clearly show two normal populations, described by a sum of two Gaussian functions (solid black curves). Each Gaussian function (dashed lines) is used to estimate mean
histograms for 400-bp DNA at three different nanopore diameters (d). (Insets) Transmission electron microscope images of the nanopores (scale bars, 2 nm). The current histograms clearly show two normal populations, described by a sum of two Gaussian functions (solid black curves). Each Gaussian function (dashed lines) is used to estimate mean 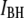 and
and  values, as well as the low current fraction,
values, as well as the low current fraction, 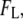 as defined in the text. Red and green colors are used to highlight the shift in relative populations as a function of d (n denotes the number of events).
as defined in the text. Red and green colors are used to highlight the shift in relative populations as a function of d (n denotes the number of events).
Additional experiments, using 25 different nanopores (2.7–5 nm) and performed under the same conditions, are shown in Fig. 6. The values of 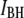 and
and 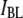 follow a clearly increasing trend with
follow a clearly increasing trend with 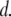 A purely geometrical estimation of the blocked ion current is given by the ratio of the hydrodynamic cross section of B-form dsDNA (
A purely geometrical estimation of the blocked ion current is given by the ratio of the hydrodynamic cross section of B-form dsDNA (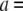 2.2 nm) to the pore diameter:
2.2 nm) to the pore diameter:
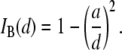 |
(1) |
It is remarkable that Eq. 1 (Fig. 6, dashed line), which does not involve any scaling factors or fitting parameters, coincides extremely well with measured 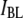 values, at the same time clearly deviating from the trend of
values, at the same time clearly deviating from the trend of 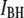 values. Referring back to Fig. 4, we recall that events associated with the
values. Referring back to Fig. 4, we recall that events associated with the 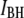 population have an extremely short tD distribution, in contrast to the much broader distribution observed in events of the
population have an extremely short tD distribution, in contrast to the much broader distribution observed in events of the 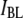 population. In the last Results section, we show that characteristic timescales associated with
population. In the last Results section, we show that characteristic timescales associated with  strongly depend on DNA length, whereas timescales associated with
strongly depend on DNA length, whereas timescales associated with 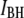 exhibit weak length dependence.
exhibit weak length dependence.
FIGURE 6.
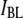 (green) and
(green) and  (red) values for a series of 25 nanopores with different diameters in the range 2.7–4.6 nm, measured using a 400-bp fragment (each
(red) values for a series of 25 nanopores with different diameters in the range 2.7–4.6 nm, measured using a 400-bp fragment (each 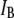 pair is based on a histogram of >1500 events, as in Fig. 4). The dashed line is the theoretical
pair is based on a histogram of >1500 events, as in Fig. 4). The dashed line is the theoretical 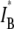 curve based on Eq. 1, with
curve based on Eq. 1, with 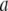 = 2.2 nm, showing excellent agreement with
= 2.2 nm, showing excellent agreement with 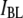 and clearly deviating from
and clearly deviating from 
The above findings lead us to postulate that events in population 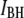 correspond to unsuccessful threading attempts (collisions), whereas events in population
correspond to unsuccessful threading attempts (collisions), whereas events in population 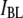 represent DNA translocations, as supported by 1), the excellent agreement of
represent DNA translocations, as supported by 1), the excellent agreement of 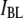 with Eq. 1, and clear deviation of
with Eq. 1, and clear deviation of  values from it, 2), the shift in
values from it, 2), the shift in 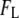 as a function of nanopore size (i.e., more collisions for decreasing nanopore size); 3), the superlinear dependence of DNA length on
as a function of nanopore size (i.e., more collisions for decreasing nanopore size); 3), the superlinear dependence of DNA length on 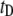 values for events in the
values for events in the 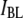 population, and the weak dependence of length on
population, and the weak dependence of length on  values in the
values in the 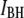 population (see last Results section). Our hypothesis is in accordance with previous investigations of ssDNA translocation through α-HL, which concluded that short (∼10-μs) and shallow events (
population (see last Results section). Our hypothesis is in accordance with previous investigations of ssDNA translocation through α-HL, which concluded that short (∼10-μs) and shallow events ( ) are random collisions with the pore entrance, whereas longer events (
) are random collisions with the pore entrance, whereas longer events (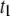 and
and 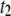 ) are translocations (7,9,10,26).
) are translocations (7,9,10,26).
Recalling Fig. 3, the vast difference in dwell times between 8-nm pores and 4-nm pores implies that nanopore size plays a crucial role on the dynamics. We expect that as 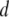 approaches the diameter of the DNA cross section, small variations in size would strongly affect the extent of DNA/nanopore interactions, but would have negligible effects on the biopolymer configurational energy outside the pore, or on the collision timescale,
approaches the diameter of the DNA cross section, small variations in size would strongly affect the extent of DNA/nanopore interactions, but would have negligible effects on the biopolymer configurational energy outside the pore, or on the collision timescale, 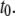 Finer insight into the size dependence is given in Fig. 7 a, which shows
Finer insight into the size dependence is given in Fig. 7 a, which shows 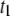 and
and 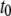 as a function of
as a function of 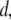 measured using a 400-bp fragment. We observe a striking increase in
measured using a 400-bp fragment. We observe a striking increase in 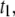 by a factor of ∼13, when
by a factor of ∼13, when 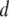 is reduced from 5.0 to 2.7 nm, well above that expected due to drag inside the pore (a factor of 5.3). Meanwhile,
is reduced from 5.0 to 2.7 nm, well above that expected due to drag inside the pore (a factor of 5.3). Meanwhile,  has marginal influence on
has marginal influence on 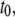 supporting our assignment of
supporting our assignment of 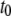 to the collision timescale, and
to the collision timescale, and 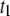 to the timescale of full DNA translocations. We note that
to the timescale of full DNA translocations. We note that 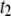 shows pore-size dependence similar to that of
shows pore-size dependence similar to that of 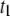 (not shown); however, the
(not shown); however, the  population is a minority for 400-bp DNA, and extracted
population is a minority for 400-bp DNA, and extracted 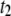 values are associated with large uncertainty.
values are associated with large uncertainty.
FIGURE 7.
Plots of the collision timescale (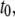 open circles) and the translocation timescale (
open circles) and the translocation timescale (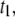 solid circles) for 400-bp DNA as a function of nanopore diameters (d) in the range 2.7–5 nm. The lines are guides to the eye.
solid circles) for 400-bp DNA as a function of nanopore diameters (d) in the range 2.7–5 nm. The lines are guides to the eye.
Dependence of the translocation dynamics on DNA length, blocked current level, voltage, and temperature
We now shift our attention to the dwell-time distributions of events in population  as a function of DNA length, ranging from 150 to 20,000 bp (300 mV, 21.0°C). We chose to concentrate on 4-nm pores for this study, because the majority of events for these pores are in the
as a function of DNA length, ranging from 150 to 20,000 bp (300 mV, 21.0°C). We chose to concentrate on 4-nm pores for this study, because the majority of events for these pores are in the 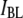 population, and folding is not expected to occur. We extracted characteristic timescales from the dwell-time distributions for a representative set of DNA lengths. A typical distribution for N = 2000 bp is shown in Fig. 8 (see Fig. S4 for distributions of other DNA lengths). For comparison, monoexponential (dashed line) and double-exponential (solid line) fits are overlaid on the distribution. It is evident that the monoexponential functions poorly fit our data, reflected in poor reduced
population, and folding is not expected to occur. We extracted characteristic timescales from the dwell-time distributions for a representative set of DNA lengths. A typical distribution for N = 2000 bp is shown in Fig. 8 (see Fig. S4 for distributions of other DNA lengths). For comparison, monoexponential (dashed line) and double-exponential (solid line) fits are overlaid on the distribution. It is evident that the monoexponential functions poorly fit our data, reflected in poor reduced 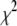 values (
values (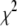 > 3 for all DNA lengths above 400 bp), whereas double-exponential fits yield reduced
> 3 for all DNA lengths above 400 bp), whereas double-exponential fits yield reduced 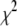 values in the range ∼1.0 ± 0.2 for all datasets (for each distribution, optimum bin size was chosen to determine both timescales simultaneously). Using models involving three or more exponentials did not improve the goodness of the fits. Although our approach to fit the data is partly empirical, we point out that the tails of translocation distributions are well approximated by exponentially decaying functions. The overlap of two broad populations and a collision timescale with small solid-state pores complicates the determination of exact
values in the range ∼1.0 ± 0.2 for all datasets (for each distribution, optimum bin size was chosen to determine both timescales simultaneously). Using models involving three or more exponentials did not improve the goodness of the fits. Although our approach to fit the data is partly empirical, we point out that the tails of translocation distributions are well approximated by exponentially decaying functions. The overlap of two broad populations and a collision timescale with small solid-state pores complicates the determination of exact 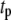 values, whereas extracted decay timescales are highly robust. We note that with the exception of the shortest DNA (150 bp), translocation timescales
values, whereas extracted decay timescales are highly robust. We note that with the exception of the shortest DNA (150 bp), translocation timescales  and
and 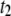 were well-resolved from corresponding collision timescales (
were well-resolved from corresponding collision timescales ( ).
).
FIGURE 8.
Representative dwell-time histogram (1755 events taken from  population) for a 2000-bp DNA fragment (4-nm pore, 300 mV, 21°C). A monoexponential fit to the tail of the first-passage time distribution (dashed line) yielded poor fits (
population) for a 2000-bp DNA fragment (4-nm pore, 300 mV, 21°C). A monoexponential fit to the tail of the first-passage time distribution (dashed line) yielded poor fits ( ), whereas a double-exponential tail fit (solid line) yields excellent agreement with the data, as indicated by a reduced
), whereas a double-exponential tail fit (solid line) yields excellent agreement with the data, as indicated by a reduced 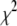 value of 1.05. Similar distributions of other representative DNA lengths are shown in Fig. S4 (Data S1).
value of 1.05. Similar distributions of other representative DNA lengths are shown in Fig. S4 (Data S1).
Fig. 9 a shows a log-log plot of the three timescales as a function of DNA length. Error bars were determined from the reduced 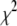 analysis in each fit, considering the statistical error of each bin in the dwell-time histogram. As previously noted,
analysis in each fit, considering the statistical error of each bin in the dwell-time histogram. As previously noted, 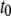 exhibits extremely weak length dependence (dashed line). Since the timescales for molecular collisions are governed by DNA diffusion, we can expect a weak length scaling of
exhibits extremely weak length dependence (dashed line). Since the timescales for molecular collisions are governed by DNA diffusion, we can expect a weak length scaling of 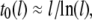 as our data indicates. In contrast, the translocation timescales
as our data indicates. In contrast, the translocation timescales 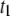 and
and  exhibit a strong dependence on
exhibit a strong dependence on 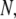 displaying a soft transition between two power laws:
displaying a soft transition between two power laws: 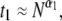 where
where  = 1.40 ± 0.05, and
= 1.40 ± 0.05, and  where
where 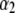 = 2.28 ± 0.05 (Fig. 9 a, solid lines). By defining the relative fraction of long to short events as
= 2.28 ± 0.05 (Fig. 9 a, solid lines). By defining the relative fraction of long to short events as 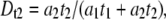 where
where 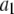 and
and 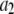 are the amplitudes of the double-exponential fits, we find that a transition from a
are the amplitudes of the double-exponential fits, we find that a transition from a 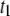 -dominated regime to a
-dominated regime to a 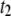 -dominated regime occurs near 3500 bp (see Fig. 9 b). For each DNA length, the dominant timescale (representing >50% of events) is displayed with a solid marker. Apart from the gradual shift to
-dominated regime occurs near 3500 bp (see Fig. 9 b). For each DNA length, the dominant timescale (representing >50% of events) is displayed with a solid marker. Apart from the gradual shift to 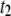 timescales, a clear deviation in our extracted
timescales, a clear deviation in our extracted 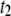 timescales for 400-bp and 1200-bp DNA molecules is observed, which may be a result of error stemming from the low fractions of
timescales for 400-bp and 1200-bp DNA molecules is observed, which may be a result of error stemming from the low fractions of 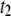 events for these DNA lengths.
events for these DNA lengths.
FIGURE 9.
(a) Log-log plot of DNA translocation timescales as a function of DNA length (N) measured using a 4-nm pore: 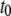 (open diamonds) attributed to collisions with the pore,
(open diamonds) attributed to collisions with the pore, 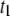 (circles) attributed to translocations, which follows a power law with
(circles) attributed to translocations, which follows a power law with  = 1.40, and
= 1.40, and 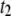 (squares) attributed to long translocation events, which exhibit a power law with
(squares) attributed to long translocation events, which exhibit a power law with 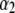 = 2.28. Open markers designate the minor timescale in the population (<50% of events), whereas solid markers represent the dominant timescale. (b) Semilog plot of
= 2.28. Open markers designate the minor timescale in the population (<50% of events), whereas solid markers represent the dominant timescale. (b) Semilog plot of 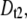 the fraction of events in the
the fraction of events in the 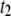 population (see text), as a function of N. The plot shows a gradual transition from
population (see text), as a function of N. The plot shows a gradual transition from 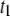 -dominated distributions to
-dominated distributions to 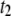 -dominated distributions occurring at N ∼ 3500 bp.
-dominated distributions occurring at N ∼ 3500 bp.
Fig. 10 displays the dependence of 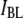 on the DNA length, using 4-nm pores. If one relates
on the DNA length, using 4-nm pores. If one relates 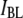 solely to the geometric blockage imposed by the DNA (a good approximation under high salt conditions, see Fig. 6),
solely to the geometric blockage imposed by the DNA (a good approximation under high salt conditions, see Fig. 6),  is expected to be independent of N. This is supported by our data: for 150
is expected to be independent of N. This is supported by our data: for 150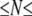 2000 bp, we find that
2000 bp, we find that  = 0.65 ± 0.05, close to the expected value of
= 0.65 ± 0.05, close to the expected value of 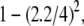 or 0.70. However, for molecules >1200 bp, we observe a regular decrease in
or 0.70. However, for molecules >1200 bp, we observe a regular decrease in 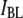 with increasing
with increasing 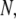 which has not been previously observed (similar behavior is observed for the dependence of
which has not been previously observed (similar behavior is observed for the dependence of  on N, not shown for clarity). This surprising decrease in
on N, not shown for clarity). This surprising decrease in  for long DNA molecules suggests that a greater fraction of ions is displaced from the pore and its vicinity during translocation.
for long DNA molecules suggests that a greater fraction of ions is displaced from the pore and its vicinity during translocation.
FIGURE 10.
Semilog plot of the dependence of the blocked current,  on N, displaying a transition from N-independent to N-dependent regimes at N > 1200 bp. The line for N > 1200 bp is a power law fit with an exponent of 0.49 ± 0.10.
on N, displaying a transition from N-independent to N-dependent regimes at N > 1200 bp. The line for N > 1200 bp is a power law fit with an exponent of 0.49 ± 0.10.
The observed dependence of the translocation times on DNA length suggests that DNA/pore interactions govern the translocation process. As predicted by recent studies, in the limit of strong interactions we expect nonlinear dependence of translocation times on the applied voltage (22). In Fig. 11, we display a set of measurements of  values for a 400-bp fragment versus applied voltage. As seen in the figure, translocation times strongly decrease with increasing voltage and can be well approximated by an exponential function (dashed line). This behavior is expected if DNA/pore interactions are biased by the applied field.
values for a 400-bp fragment versus applied voltage. As seen in the figure, translocation times strongly decrease with increasing voltage and can be well approximated by an exponential function (dashed line). This behavior is expected if DNA/pore interactions are biased by the applied field.
FIGURE 11.
Voltage dependence on the translocation dynamics measured for a 400-bp DNA fragment (experiments carried out with a 3.5-nm pore at 21°C). The dashed line is an exponential fit to the data.
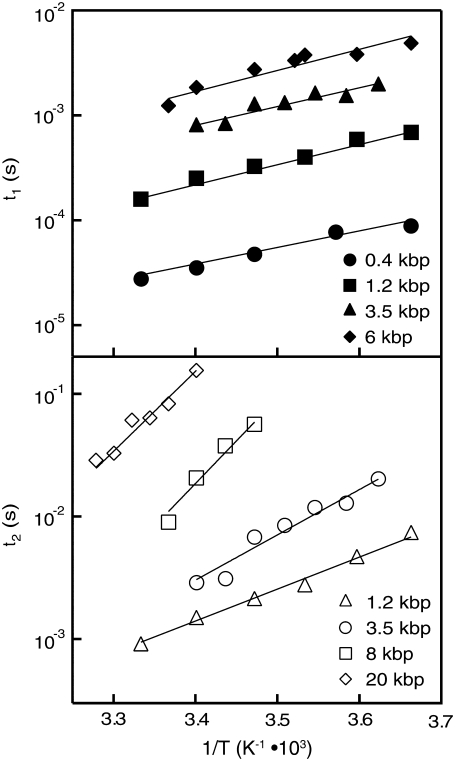
Finally, we investigated the role of temperature on the translocation dynamics. Fig. 12 displays a semilog plot of 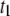 (a) and
(a) and 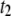 (b) for selected DNA lengths as a function of
(b) for selected DNA lengths as a function of 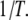 A simplified Arrhenius model for the temperature dependence (
A simplified Arrhenius model for the temperature dependence ( ) yields similar effective energy barriers for all DNA lengths,
) yields similar effective energy barriers for all DNA lengths, 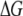 ∼ 12.0 ± 0.5 kBT (or 7.1 ± 0.3 kcal/mol) for
∼ 12.0 ± 0.5 kBT (or 7.1 ± 0.3 kcal/mol) for 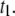 The invariance of
The invariance of 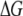 with N affirms our hypothesis that interactions within the pore dominate the dynamics, since such interactions should not be length-dependent. In contrast,
with N affirms our hypothesis that interactions within the pore dominate the dynamics, since such interactions should not be length-dependent. In contrast, 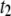 displays increasing
displays increasing 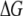 values for increasing
values for increasing  with
with 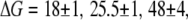 and 45 ± 2 kBT for 1200, 3500, 8000, and 20,000 bp, respectively. This can be rationalized by considering the extent of interactions of the translocating DNA coil with the membrane, which is expected to show length dependence. It should be noted that the slowing down observed with reduced temperature in both
and 45 ± 2 kBT for 1200, 3500, 8000, and 20,000 bp, respectively. This can be rationalized by considering the extent of interactions of the translocating DNA coil with the membrane, which is expected to show length dependence. It should be noted that the slowing down observed with reduced temperature in both 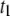 and
and 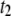 cannot be attributed to increased fluid viscosity: cooling the electrolyte from 30°C to 0°C results in slowing down by a factor of ∼7, whereas in this range of temperatures, viscosity merely increases by ∼2.7.
cannot be attributed to increased fluid viscosity: cooling the electrolyte from 30°C to 0°C results in slowing down by a factor of ∼7, whereas in this range of temperatures, viscosity merely increases by ∼2.7.
FIGURE 12.
Temperature dependence on the translocation dynamics: semilog plot of 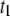 (upper) and
(upper) and 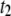 (lower) values for 4-nm pores at the indicated DNA lengths as a function of 1/T. The lines are Arrhenius fits to the data, with slopes corresponding to energy barriers of ∼12.0 ± 0.5 kBT for
(lower) values for 4-nm pores at the indicated DNA lengths as a function of 1/T. The lines are Arrhenius fits to the data, with slopes corresponding to energy barriers of ∼12.0 ± 0.5 kBT for 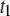 and 18–48 kBT for
and 18–48 kBT for 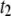 (see text).
(see text).
DISCUSSION
Our understanding of the factors governing voltage-driven DNA translocation through solid-state nanopores is to date still lagging. In this article, we systematically analyzed the translocation dynamics as a function of nanopore size, DNA length, voltage, and temperature, in a range where DNA can only enter the nanopore in an unfolded (single-file) configuration. Our main findings can be summarized as follows: first, subtle decreases of the nanopore size result in decreased threading probabilities, markedly larger  values, and marginal impact on the collision timescale
values, and marginal impact on the collision timescale 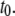 These results, as well as the striking correlation between timescales and current blockage (Fig. 4) and the agreement between the expected
These results, as well as the striking correlation between timescales and current blockage (Fig. 4) and the agreement between the expected 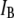 based on nanopore size and the measured
based on nanopore size and the measured  confirm that low-level, deep blocking events (i.e., events in
confirm that low-level, deep blocking events (i.e., events in 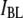 ) correspond to translocations, whereas shallow events are due to fast collisions. We show here that the translocation time histograms bear resemblance to translocation distributions obtained for ssDNA through α-HL, with two major distinctions: 1), distributions for solid-state nanopores exhibit much broader decays; and 2), monoexponential functions fail to fit the distribution tails, whereas double-exponential functions (with timescales
) correspond to translocations, whereas shallow events are due to fast collisions. We show here that the translocation time histograms bear resemblance to translocation distributions obtained for ssDNA through α-HL, with two major distinctions: 1), distributions for solid-state nanopores exhibit much broader decays; and 2), monoexponential functions fail to fit the distribution tails, whereas double-exponential functions (with timescales 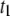 and
and 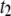 ) yield excellent fits.
) yield excellent fits.
For short dsDNA molecules, where the 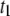 timescale is dominant, we note first that the appearance of broad
timescale is dominant, we note first that the appearance of broad 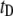 distributions (where
distributions (where 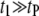 ) distinguishes the solid-state nanopore system from the ssDNA/α-HL case, where
) distinguishes the solid-state nanopore system from the ssDNA/α-HL case, where 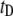 distributions are relatively narrow. In addition, in contrast to the linear dependence of translocation times on DNA length (l) reported for α-HL (27), we find a power-law dependence of
distributions are relatively narrow. In addition, in contrast to the linear dependence of translocation times on DNA length (l) reported for α-HL (27), we find a power-law dependence of 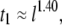 similar to findings of recent experimental (17,18) and theoretical (28) studies, which reveal/predict a power law of 1.27–1.34 for dsDNA translocation through 8- to 20-nm pores. In particular, our data correspond well to recent Monte-Carlo simulations by Vocks and co-workers, which have predicted for a polymer performing Rouse dynamics a power law scaling of
similar to findings of recent experimental (17,18) and theoretical (28) studies, which reveal/predict a power law of 1.27–1.34 for dsDNA translocation through 8- to 20-nm pores. In particular, our data correspond well to recent Monte-Carlo simulations by Vocks and co-workers, which have predicted for a polymer performing Rouse dynamics a power law scaling of 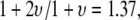 where
where 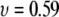 is the Flory exponent of the polymer (29).
is the Flory exponent of the polymer (29).
The order of magnitude increase in measured translocation times with decreasing pore diameter implies that interactions (or drag) inside the pore are the predominant factors governing translocation dynamics. Our voltage and temperature studies suggest that DNA/pore interactions are the main factor governing translocation: for the same pore size, we find an exponential dependence of voltage on mean translocation times, as well as steep Arrhenius temperature dependence on both 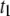 and
and  timescales, much larger than the expected slowing down due to viscosity. These findings isolate DNA/pore interactions as the prevailing mechanism controlling translocation dynamics in small pores.
timescales, much larger than the expected slowing down due to viscosity. These findings isolate DNA/pore interactions as the prevailing mechanism controlling translocation dynamics in small pores.
What, then, is the nature of the DNA/nanopore interactions? A process involving a single, strong binding event (per translocation) may look plausible at first, suggesting Arrhenius-like kinetics. However, a single binding-unbinding mechanism is highly unlikely, as it would be incompatible with the regularity in translocation time dependence on l. On the other hand, the observed dynamics is compatible with a process involving a series of many thermally activated jumps over small energy barriers (possibly each ∼12 kBT). In this case, translocation times are expected to scale linearly with the number of energy jumps, thus growing linearly with l. A detailed model for this process is beyond the scope of the current manuscript. We note, however, that in developing a model, one has to take into account the fact that any energy barrier is highly biased by the strong electrical force, which under the conditions employed in our experiment amounts to at least 70 pN, or ∼18  assuming a Manning screening factor of ∼50% inside solid-state nanopores (30).
assuming a Manning screening factor of ∼50% inside solid-state nanopores (30).
For long DNA biopolymers, we observe the appearance of a much longer timescale 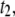 which has a steeper power law,
which has a steeper power law,  Although the source of this timescale is a subject for further study, we propose that it is related to additional interactions between external parts of the DNA (i.e., not the DNA region in the nanopore) and the SiN membrane. This is supported by the increasing fraction of
Although the source of this timescale is a subject for further study, we propose that it is related to additional interactions between external parts of the DNA (i.e., not the DNA region in the nanopore) and the SiN membrane. This is supported by the increasing fraction of  -timescale events with increasing DNA length. Although short polymers (several Kuhn lengths) are less likely to interact with the membrane, our observation of a minor
-timescale events with increasing DNA length. Although short polymers (several Kuhn lengths) are less likely to interact with the membrane, our observation of a minor 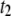 population for short DNA molecules suggests a more complex translocation mode with small pores (e.g., DNA loop interacting with the pore mouth), with the probability increasing with DNA length. Indeed, longer DNA molecules can form more and more interaction sites with the membrane, leading to a prominent
population for short DNA molecules suggests a more complex translocation mode with small pores (e.g., DNA loop interacting with the pore mouth), with the probability increasing with DNA length. Indeed, longer DNA molecules can form more and more interaction sites with the membrane, leading to a prominent 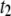 timescale. We note that average translocation times obtained using 4-nm pores are still shorter than the self-relaxation time for dsDNA, as approximated by Rouse or Zimm dynamics (31), implying that the “frozen” polymer configuration at the initial moment of threading will determine the translocation dynamics. This may explain the mixture of
timescale. We note that average translocation times obtained using 4-nm pores are still shorter than the self-relaxation time for dsDNA, as approximated by Rouse or Zimm dynamics (31), implying that the “frozen” polymer configuration at the initial moment of threading will determine the translocation dynamics. This may explain the mixture of  and
and 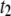 events with our pores, corresponding to biopolymers that interact only inside the pore and those which also interact with the membrane, respectively.
events with our pores, corresponding to biopolymers that interact only inside the pore and those which also interact with the membrane, respectively.
A striking observation is that  values are constant for DNA molecules up to 2000 bp, whereas they decrease for longer molecules. Although there are several possible interpretations for this observation, the external DNA coil above the pore mouth may provide additional resistance to ion flow, further decreasing
values are constant for DNA molecules up to 2000 bp, whereas they decrease for longer molecules. Although there are several possible interpretations for this observation, the external DNA coil above the pore mouth may provide additional resistance to ion flow, further decreasing 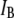 from its expected geometrical contribution inside the pore. To check our hypothesis, we crudely estimate the increase in the access resistance to the pore by assuming that the long DNA coil forms a sphere of radius
from its expected geometrical contribution inside the pore. To check our hypothesis, we crudely estimate the increase in the access resistance to the pore by assuming that the long DNA coil forms a sphere of radius 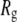 and resistivity
and resistivity 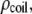 slightly higher than the bulk resistivity. Since the change in current due to the presence of this sphere near the pore opening is proportional to
slightly higher than the bulk resistivity. Since the change in current due to the presence of this sphere near the pore opening is proportional to 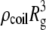 and inversely proportional to its area (access resistance),
and inversely proportional to its area (access resistance), 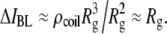 Ignoring all prefactors and using
Ignoring all prefactors and using 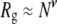 to estimate the polymer's radius of gyration, it follows that the decrease in
to estimate the polymer's radius of gyration, it follows that the decrease in 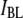 for long DNA will scale as
for long DNA will scale as 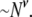 Although this estimation is clearly a crude one, fitting our data in Fig. 10 to a power law yields an exponent of 0.49 ± 0.10 for N > 2000 bp (dashed line), in qualitative agreement with our rudimentary prediction.
Although this estimation is clearly a crude one, fitting our data in Fig. 10 to a power law yields an exponent of 0.49 ± 0.10 for N > 2000 bp (dashed line), in qualitative agreement with our rudimentary prediction.
In summary, the focus of our study is the dynamics of dsDNA translocation through solid-state nanopores as a function of their size, temperature, voltage, and DNA length. High-bandwidth measurements have allowed us to resolve short collisions from full translocations, which clearly differ by their blocked-current levels. By decreasing the nanopore size or temperature, we observe an increase in the translocation time of more than an order of magnitude (e.g., ∼0.5 ms for 1200 bp), as compared with larger pores, attributable to increased DNA/nanopore interactions. However, smaller pore sizes yield broader, more complex DNA translocation distributions, and a reduced fraction of full translocations to collision. A finer control on the interaction between biological molecules and inorganic pores may be needed to achieve the spatial/temporal resolution required for DNA sequencing and genotyping applications. Manipulating the surface properties of nanopores by coating with inorganic or organic materials (21,32) may achieve this goal. Most important, this study highlights some of the advantages and complexities associated with strong DNA/nanopore interactions in small pores. Although theory and computer simulations have predicted that interaction of polynucleic acids with nanopores can markedly affect the dynamics, we hope our results can stimulate further theoretical and experimental studies, required for a full understanding of the dynamics of DNA translocation through small nanopores.
TABLE 1.
Glossary of symbols
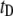 |
Event dwell-time |
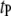 |
Most probable translocation time |
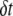 |
Time delay between two successive events |
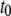 |
Collision timescale |
 |
Short translocation timescale |
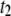 |
Long translocation timescale |
| l | DNA length |
| N | Number of DNA nucleotides or basepairs |
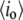 |
Open-pore current level |
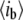 |
Blocked-pore current level |
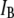 |
Fractional blocked current |
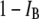 |
Normalized event amplitude |
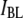 |
Low-level current blockade |
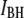 |
High-level current blockade |
SUPPLEMENTARY MATERIAL
To view all of the supplemental files associated with this article, visit www.biophysj.org.
Supplementary Material
Acknowledgments
We acknowledge valuable input from D. Branton, D. R. Nelson, Y. Rabin, A. Parsegian, and S. Bezrukov. We thank G. V. Soni and A. Squires for reading the manuscript.
We acknowledge support from the Center for Nanoscale Systems at Harvard University, as well as awards from the National Institutes of Health (HG-004128) and the National Science Foundation (PHY-0646637 and NIRT-0403891).
Editor: Taekjip Ha.
References
- 1.Dekker, C. 2007. Solid-state nanopores. Nat. Nanotechnol. 2:209–215. [DOI] [PubMed] [Google Scholar]
- 2.Healy, K. 2007. Nanopore-based single-molecule DNA analysis. Nanomedicine. 2:459–481. [DOI] [PubMed] [Google Scholar]
- 3.Wanunu, M., and A. Meller. 2008. Single-molecule analysis of nucleic acids and DNA-protein interactions using nanopores. In Single-Molecule Techniques: A Laboratory Manual. P. Selvin, and T. J. Ha, editors. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 395–420.
- 4.Hornblower, B., A. Coombs, R. D. Whitaker, A. Kolomeisky, S. J. Picone, A. Meller, and M. Akeson. 2007. Single-molecule analysis of DNA-protein complexes using nanopores. Nat. Methods. 4:315–317. [DOI] [PubMed] [Google Scholar]
- 5.Zwolak, M., and M. Di Ventra. 2007. Physical approaches to DNA sequencing and detection. Rev. Mod. Phys. 80:141–165. [Google Scholar]
- 6.Soni, G. V., and A. Meller. 2007. Progress toward ultrafast DNA sequencing using solid-state nanopores. Clin. Chem. 53:1996–2001. [DOI] [PubMed] [Google Scholar]
- 7.Kasianowicz, J. J., E. Brandin, D. Branton, and D. W. Deamer. 1996. Characterization of individual polynucleotide molecules using a membrane channel. Proc. Natl. Acad. Sci. USA. 93:13770–13773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akeson, M., D. Branton, J. J. Kasianowicz, E. Brandin, and D. W. Deamer. 1999. Microsecond time-scale discrimination among polycytidylic acid, polyadenylic acid, and polyuridylic acid as homopolymers or as segments within single RNA molecules. Biophys. J. 77:3227–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meller, A., L. Nivon, E. Brandin, J. Golovchenko, and D. Branton. 2000. Rapid nanopore discrimination between single polynucleotide molecules. Proc. Natl. Acad. Sci. USA. 97:1079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meller, A., L. Nivon, and D. Branton. 2001. Voltage-driven DNA translocations through a nanopore. Phys. Rev. Lett. 86:3435–3438. [DOI] [PubMed] [Google Scholar]
- 11.Li, J., D. Stein, C. McMullan, D. Branton, M. J. Aziz, and J. A. Golovchenko. 2001. Ion-beam sculpting at nanometre length scales. Nature. 412:166–169. [DOI] [PubMed] [Google Scholar]
- 12.Storm, A. J., J. H. Chen, X. S. Ling, H. W. Zandbergen, and C. Dekker. 2003. Fabrication of solid-state nanopores with single-nanometre precision. Nat. Mater. 2:537–540. [DOI] [PubMed] [Google Scholar]
- 13.Kim, M. J., M. Wanunu, D. C. Bell, and A. Meller. 2006. Rapid fabrication of uniformly sized nanopores and nanopore arrays for parallel DNA analysis. Adv. Mater. 18:3149–3153. [Google Scholar]
- 14.Li, J. L., M. Gershow, D. Stein, E. Brandin, and J. A. Golovchenko. 2003. DNA molecules and configurations in a solid-state nanopore microscope. Nat. Mater. 2:611–615. [DOI] [PubMed] [Google Scholar]
- 15.Chen, P., J. J. Gu, E. Brandin, Y. R. Kim, Q. Wang, and D. Branton. 2004. Probing single DNA molecule transport using fabricated nanopores. Nano Lett. 4:2293–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Storm, A. J., J. H. Chen, H. W. Zandbergen, and C. Dekker. 2005. Translocation of double-strand DNA through a silicon oxide nanopore. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 71:051903. [DOI] [PubMed] [Google Scholar]
- 17.Storm, A. J., C. Storm, J. H. Chen, H. Zandbergen, J. F. Joanny, and C. Dekker. 2005. Fast DNA translocation through a solid-state nanopore. Nano Lett. 5:1193–1197. [DOI] [PubMed] [Google Scholar]
- 18.Fologea, D., E. Brandin, J. Uplinger, D. Branton, and J. Li. 2007. DNA conformation and base number simultaneously determined in a nanopore. Electrophoresis. 28:3186–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heng, Z. B., C. Ho, T. Kim, R. Timp, A. Aksimentiev, Y. V. Grinkova, S. Sligar, K. Schulten, and G. Timp. 2004. Sizing DNA using a nanometer-diameter pore. Biophys. J. 87:2905–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fologea, D., J. Uplinger, B. Thomas, D. S. McNabb, and J. Li. 2005. Slowing DNA translocation in solid-state nanopore. Nano Lett. 5:1734–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen, P., T. Mitsui, D. B. Farmer, J. Golovchenko, R. G. Gordon, and D. Branton. 2004. Atomic layer deposition to fine-tune the surface properties and diameters of fabricated nanopores. Nano Lett. 4:1333–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lubensky, D. K., and D. R. Nelson. 1999. Driven polymer translocation through a narrow pore. Biophys. J. 77:1824–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aksimentiev, A., Z. B. Heng, G. Timp, and K. Schulten. 2004. Microscopic kinetics of DNA translocation through synthetic nanopores. Biophys. J. 87:2086–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeh, I. C., and G. Hummer. 2004. Nucleic acid transport through carbon nanotube membranes. Proc. Natl. Acad. Sci. USA. 101:12177–12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meller, A., and D. Branton. 2002. Single molecule measurements of DNA transport through a nanopore. Electrophoresis. 23:2583–2591. [DOI] [PubMed] [Google Scholar]
- 26.Bates, M., M. Burns, and A. Meller. 2003. Dynamics of DNA molecules in a membrane channel probed by active control techniques. Biophys. J. 84:2366–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meller, A. 2003. Dynamics of polynucleotide transport through nanometre-scale pores. J. Phys.: Condens. Matter. 15:R581–R607. [Google Scholar]
- 28.Luo, K., T. Ala-Nissila, and S.-C. Ying. 2006. Polymer translocation through a nanopore under an applied extrnal filed. J. Chem. Phys. 124:114704. [DOI] [PubMed] [Google Scholar]
- 29.Vocks, H., D. Panja, G. T. Barkema, and R. C. Ball. 2008. Pore-blockade times for field-driven polymer translocation. J. Phys.: Condens. Matter. 20:095224. [Google Scholar]
- 30.Keyser, U. F., B. N. Koeleman, S. Van Dorp, D. Krapf, R. M. M. Smeets, S. G. Lemay, N. H. Dekker, and C. Dekker. 2006. Direct force measurements on DNA in a solid-state nanopore. Nat. Phys. 2:473–477. [Google Scholar]
- 31.Shusterman, R., S. Alon, T. Gavrinyov, and O. Krichevsky. 2004. Monomer dynamics in double- and single-stranded DNA polymers. Phys. Rev. Lett. 92:048303. [DOI] [PubMed] [Google Scholar]
- 32.Wanunu, M., and A. Meller. 2007. Chemically modified solid-state nanopores. Nano Lett. 7:1580–1585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.