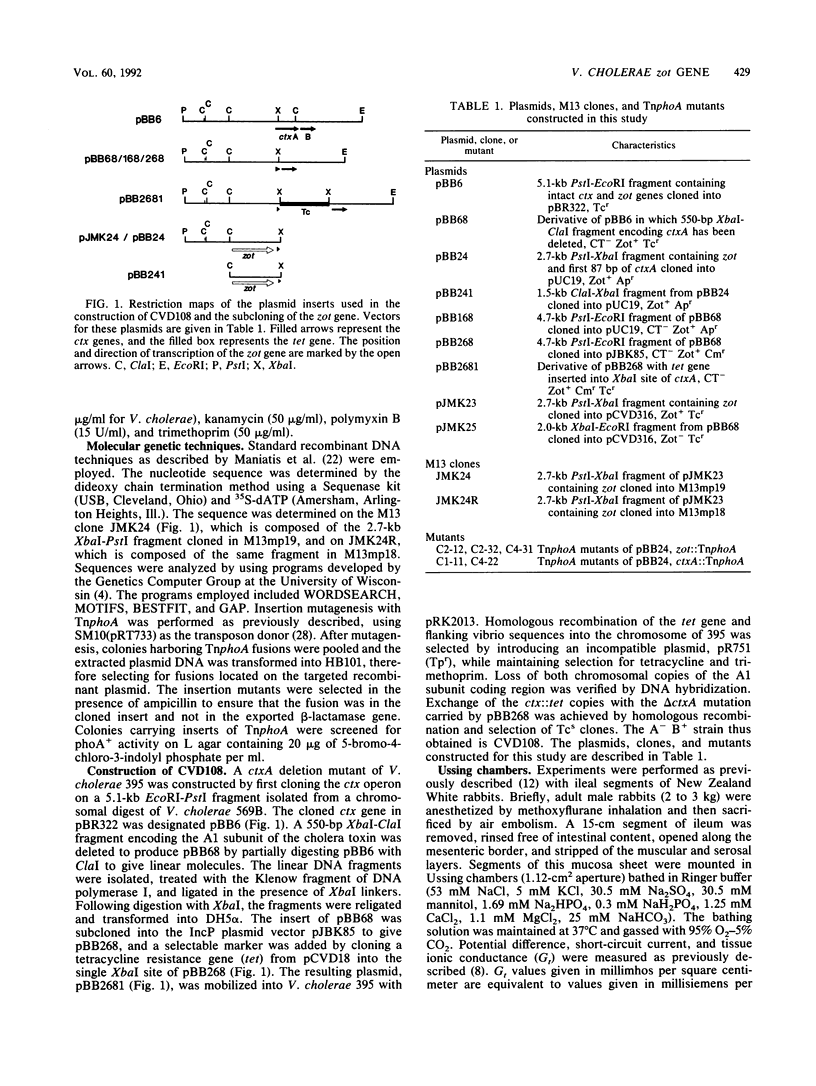
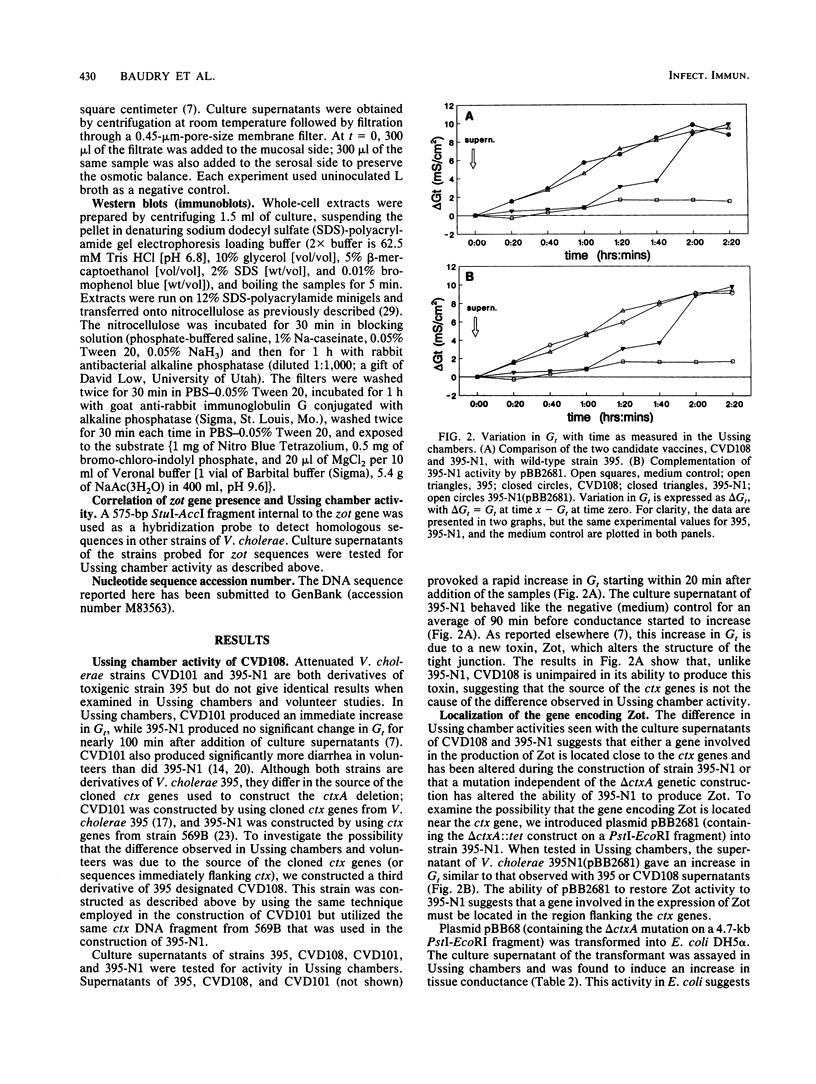
Abstract
Live oral candidate cholera vaccines have previously been constructed by deletion of Vibrio cholerae sequences encoding the enzymatically active A subunit of the cholera toxin. However, volunteer studies have shown that these non-cholera toxin-producing strains still provoke mild to moderate diarrhea in some individuals. We recently reported the identification of a second toxin produced by V. cholerae which may be responsible for this residual diarrhea (A. Fasano, B. Baudry, D. W. Pumplin, S. S. Wasserman, B. D. Tall, J. M. Ketley, and J. B. Kaper, Proc. Natl. Acad. Sci. USA 88:5242-5246, 1991). This new toxigenic factor increases the permeability of rabbit ileal mucosa by affecting the structure of the intercellular tight junctions (zonula occludens). We now report the identification and cloning of the gene encoding this new toxin. This gene, named zot (for zonula occludens toxin), consists of a 1.3-kb open reading frame which could potentially encode a 44.8-kDa polypeptide. The location of the zot gene encoding the new toxin is immediately upstream of the ctx operon encoding cholera toxin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove C. H., Wang S. Z., Price S. B., Phelps C. J., Lyerly D. M., Wilkins T. D., Johnson J. L. Molecular characterization of the Clostridium difficile toxin A gene. Infect Immun. 1990 Feb;58(2):480–488. doi: 10.1128/iai.58.2.480-488.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A., Baudry B., Pumplin D. W., Wasserman S. S., Tall B. D., Ketley J. M., Kaper J. B. Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5242–5246. doi: 10.1073/pnas.88.12.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., Fromm D., McColl I. Ion transport in rabbit ileal mucosa. I. Na and Cl fluxes and short-circuit current. Am J Physiol. 1971 May;220(5):1388–1396. doi: 10.1152/ajplegacy.1971.220.5.1388. [DOI] [PubMed] [Google Scholar]
- Gill D. M., Meren R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3050–3054. doi: 10.1073/pnas.75.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M. The arrangement of subunits in cholera toxin. Biochemistry. 1976 Mar 23;15(6):1242–1248. doi: 10.1021/bi00651a011. [DOI] [PubMed] [Google Scholar]
- Guandalini S., Fasano A., Migliavacca M., Verga M. C., Mastrantonio Gianfrilli P., Ferrara A., Alessio M., Malamisura B., Galati P., Pantosti A. Pathogenesis of postantibiotic diarrhoea caused by Clostridium difficile: an in vitro study in the rabbit intestine. Gut. 1988 May;29(5):598–602. doi: 10.1136/gut.29.5.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht G., Pothoulakis C., LaMont J. T., Madara J. L. Clostridium difficile toxin A perturbs cytoskeletal structure and tight junction permeability of cultured human intestinal epithelial monolayers. J Clin Invest. 1988 Nov;82(5):1516–1524. doi: 10.1172/JCI113760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington D. A., Hall R. H., Losonsky G., Mekalanos J. J., Taylor R. K., Levine M. M. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J Exp Med. 1988 Oct 1;168(4):1487–1492. doi: 10.1084/jem.168.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Finkelstein R. A. Selection and characteristics of a Vibrio cholerae mutant lacking the A (ADP-ribosylating) portion of the cholera enterotoxin. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2052–2056. doi: 10.1073/pnas.76.4.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. B., Lockman H., Baldini M. M., Levine M. M. Recombinant nontoxinogenic Vibrio cholerae strains as attenuated cholera vaccine candidates. Nature. 1984 Apr 12;308(5960):655–658. doi: 10.1038/308655a0. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Black R. E., Clements M. L., Cisneros L., Saah A., Nalin D. R., Gill D. M., Craig J. P., Young C. R., Ristaino P. The pathogenicity of nonenterotoxigenic Vibrio cholerae serogroup O1 biotype El Tor isolated from sewage water in Brazil. J Infect Dis. 1982 Mar;145(3):296–299. doi: 10.1093/infdis/145.3.296. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Herrington D., Ketley J., Losonsky G., Tacket C. O., Tall B., Cryz S. Safety, immunogenicity, and efficacy of recombinant live oral cholera vaccines, CVD 103 and CVD 103-HgR. Lancet. 1988 Aug 27;2(8609):467–470. doi: 10.1016/s0140-6736(88)90120-1. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Herrington D., Losonsky G., Morris J. G., Clements M. L., Black R. E., Tall B., Hall R. Volunteer studies of deletion mutants of Vibrio cholerae O1 prepared by recombinant techniques. Infect Immun. 1988 Jan;56(1):161–167. doi: 10.1128/iai.56.1.161-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Vancell R., Beaty G., Stefani E., Rodríguez-Boulan E. E., Cereijido M. Changes in paracellular and cellular ionic permeabilities of monolayers of MDCK cells infected with influenza or vesicular stomatitis viruses. J Membr Biol. 1984;81(3):171–180. doi: 10.1007/BF01868711. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J., Swartz D. J., Pearson G. D., Harford N., Groyne F., de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983 Dec 8;306(5943):551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miller V. L., Taylor R. K., Mekalanos J. J. Cholera toxin transcriptional activator toxR is a transmembrane DNA binding protein. Cell. 1987 Jan 30;48(2):271–279. doi: 10.1016/0092-8674(87)90430-2. [DOI] [PubMed] [Google Scholar]
- Morris J. G., Jr, Takeda T., Tall B. D., Losonsky G. A., Bhattacharya S. K., Forrest B. D., Kay B. A., Nishibuchi M. Experimental non-O group 1 Vibrio cholerae gastroenteritis in humans. J Clin Invest. 1990 Mar;85(3):697–705. doi: 10.1172/JCI114494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]



