Abstract
Purpose
Senile cataracts are associated with oxidation, fragmentation, cross-linking, insolubilization, and yellow pigmentation of lens crystallins. This process is partially explained by advanced glycation endproducts (AGEs) from ascorbic acid (ASA), as unequivocally demonstrated in our hSVCT2 transgenic mouse(PNAS 103:16912, 2006). We now present the first pharmacological intervention study against ascorbylation in these mice.
Methods
Five groups of mice (10 mice/group) were fed from two to nine months a diet containing 0.1% (wt/wt) aminoguanidine (AG), pyridoxamine (PM), penicillamine (PA), and nucleophilic compounds NC-I and NC-II. AGEs were determined in crystallin digests using HPLC, LC-MS or GC-MS. In vitro incubations of lens protein extract with ASA or dehydroascorbic aicd (DHA) were also performed.
Results
ASA level increased ~10 fold in all groups and was unaffected by treatment. AGEs were several fold increased in transgenic compared to control lenses. Body weight, food intake, lenticular glutathione and glycated lysine level were unaltered. In vitro, all compounds inhibited AGE formation. In vivo, NC-I and NC-II significantly decreased protein fluorescence at λex335/em385 (p=0.045, 0.017, respectively) and λex370/em440 (p=0.029, 0.007, respectively). Other inhibitors had no effect. After 7 months, only NC-1 and NC-2 induced a 50 % reduction in pentosidine (n.s, p=0.035 respectively). NC-1 also decreased carboxymethyllysine (CML) (p=0.032) and carboxyethyllysine (CEL) (p= n.s). Fluorescent crosslink K2P was decreased by NC-1, NC-2, AG and PM (p= n.s).
Conclusions
Pharmacologically blocking protein ascorbylation with absorbable guanidino compounds is feasible and may represent a new strategy for the delay of age-related nuclear sclerosis of the lens.
Introduction
Advanced glycation end products (AGEs) are formed non-enzymatically by the reaction of reducing sugars and oxoaldehydes, with amino groups in proteins, lipids and nucleic acids through a series of reactions (Maillard Reaction) and their levels have been documented to increase with age in many tissues1–3. Reducing sugars are degraded prior to or during their interaction with proteins in order to form AGEs4–6, and in fact many AGEs are formed from reactive degradation intermediates such as methylglyoxal and glyoxal. From this point of view, advanced glycation can be considered as a set of posttranslational, nonenzymatic modifications of proteins by reactive carbonyl compounds.
With age, various postsynthetic modifications occur in human lens crystallins. These are particularly associated with the water-insoluble (WI) proteins and are characterized by the presence high levels of yellow chromophores and non-tryptophan fluorescence7,8, pigmentation9, aggregation10, insolubilization11, fragmentation, and crosslinking12. By and large these modifications are greatly enhanced in senile and brunescent cataractous lenses13. Various theories and mechanisms have been hypothesized to explain these changes, including oxygen free radicals and oxidation14,15, glycation (Maillard reaction)16 and tryptophan oxidation products17,18. In recent years, because of the presence of high concentrations of ascorbate in the human lens, considerable interest has focused on the potential role of ascorbate as a mediator of postsynthetic modifications of crystallins in the aging lens. It was found that many phenomena observed in aging and cataractous lenses could be duplicated by the reaction of lens crystallins with ascorbate19,20. This hypothesis has been unequivocally confirmed with our recently established mouse model of lens crystallin aging in which the human Sodium Dependent Vitamin C transporter 2 (hSVCT2) was overexpressed under the control of the mouse αA-crystallin promoter fused to a chick lens δ-crystallin enhancer 21. With age, there was an increase in ascorbic acid oxidation products and crystallin-bound AGEs pentosidine, carboxymethyllysine (CML), vesperlysine A and K2P. We also noticed that at 12 months mouse lenses had acquired a yellow color similar to that observed in old human lenses. This animal model compressed lens crystallin chemical aging from 70 years in the human to 12 months in the mouse, providing us the opportunity to test potential pharmacological inhibitors of lens crystallin aging by the Maillard reaction in vivo.
In the present study, we treated 2 months old transgenic mice with five different candidate inhibitors and analyzed lens crystallin-bound fluorescence and AGEs markers after seven months of treatment. Similar experiments were carried out in vitro.
Methods
Animals
All animal experiments were conducted in accordance with procedures approved by the Case Western Reserve University Animal Care Committee and conformed to the ARVO Statement for use of Animals in Ophthalmic and Vision Research. Animals were housed under diurnal lighting condition and allowed free access to food and water. hSVCT2 transgenic mice were generated as described previously21. Genomic DNA from mouse tails was isolated and subjected to PCR screening using specifically designed primers. The genetic background of these transgenic mice is C57BL/6 after at least 8 generations of cross-breeding with wild type C57BL/6 mice.
Treatment of hSVCT2 mice with candidate ascorbylation inhibitors
Pyridoxamine dihydrochloride (P9380), DL-penicillamine (P5125), aminoguanidine (A7259), and the guanidino compounds NC-I and NC-II were all purchased from Sigma Company (St. Louis, MI). The structure is displayed in Figure. 1. The mouse diet with 0.1% w/w inhibitors listed above was produced by Bio-Serv company using standard diet Isopro 3000 (Prolab 5P75; LabDiet, Richmond, IN). Doses of the inhibitors were chosen based on similar studies with nucleophilic AGE inhibitors. Transgenic and age-matched control mice (50/50 male/female), 10 mice per group, were maintained on a standard mouse diet or special medical diet started at 2 months and continued till 9 months of age. The body weight and food intake were monitored monthly. Nine months old mice were sacrificed, eyes were removed and decapsulated to release the lenses. The lenses were processed for AGE determination as described below.
Figure 1.

The structure of tested inhibitors (A) and the potential inhibitory mechanism of action of guanidino compounds against ascorbylation products (B).
In vitro incubation of lens crystallins with ascorbylation inhibitors
In order to best mimick the in vivo formation of ascorbylation products, two models of incubation conditions were tested. Model A consisted of 5mg of calf lens protein homogenate (CLP) incubated with 1mM of DHA, with or without 1.5mM inhibitors, under anaerobic condition in Chelex treated 5mM sodium phosphate buffer (pH 7.0) at 37°C for 7 days. DHA and inhibitors were refreshed daily to take into account the short half-life of DHA. Model B consisted of one time addition of 25mM ASA with or without 15mM inhibitors in aerobic conditions, but with Chelex treated buffer for 7 days in order to slow down but not eliminate the oxidation of ASA. At the end of the incubations the protein was dialyzed against phosphate buffer saline (PBS) for 24 hours at 4°C, then twice against water for 48 hours at 4°C and lyophilized. Half of incubated protein (~2.5mg) was used for enzymatic proteolysis as previously described 21. The other half of protein was hydrolysis with 6N HCl. Both enzymatic digest and hydrolysates were used for AGEs determination.
Measurement of Ascorbic acid and Glutathione
Lenses were homogenized in 200μl of 10% trichloroacetic acid (TCA). The supernatant was used for derivatization with dimethyl-o-phenylene-diamine to determine ascorbic acid level and for derivatization with 1-fluoro-2,4-dinitrobenzene to determine glutathione (GSH) level as previously reported21.
Enzymatic Digestion of Lens Proteins
The TCA precipitate of mouse lens protein was washed twice with 500μl of ethyl ether and allowed to dry at room temperature for 10 min. The pellet was reconstituted in an Eppendorf tube with 500μl of 5.0 mM argon-exchanged, Chelex-treated phosphate buffer (pH 7.0). The protein pellet was enzymatic digested with series of protease as previously described21. Digestion efficiency varied from 64% to 75%. Corresponding enzyme blanks incubated without added protein were used as background control. After digestion, the samples were reconstituted with water for fluorescence measurement, dried, reconstituted with 0.01 M heptafluorobutyric acid (HFBA) in water, and subjected to HPLC assay for determination of K2P as described 21. The protein concentrations of the samples were analyzed by means of a ninhydrin assay expressed as leucine equivalent after enzymatic digestion and also after 6N HCl hydrolysis in order to evaluate the digestion efficiency. The reported values may underestimate the true AGE content since at best 80% digestion efficieny was obtained.
Fluorescence Spectroscopy
The fluorescence at λex/em 370/440nm and 335/385nm of the enzymatic lens protein digest was measured with a spectrofluorometer (821-F; Jasco, Easton, MD). The data were expressed as fluorescence units per unit protein measured as leucine equivalent.
Determination of advanced glycation endproducts
K2P, pentosidine, CML, CEL and furosine were analyzed in this study. The acid labile K2P was determined in lens protein digest as reported in our previous study21. Pentosidine, CML, CEL and furosine were determined in lens protein acid hydrolysate as previously reported21.
Statistical Analysis
All values were expressed as means ±SD. Statistical significance of differences in mean values was assessed by repeated-measures ANOVA or Student’s t test. P values of <0.05 were considered statistically significant.
Results
Parameters of Homeostasis
In order to better interpret our data we first determined a number of parameters of homeostasis in the five treatment groups and compared the data with untreated wild type and control mice. As shown in Fig. 2A, ascorbic acid was elevated in all hSVCT2 mice to levels similar to those of the human lens (2mM), and were unaffected by treatment. Similarly, lenticular glucose concentrations as reflected by furosine, i.e. fructose-lysine, were not significantly different in any of the groups (Fig. 2B), suggesting that the tested drugs did not impair lenticular glucose levels. Finally, and most importantly, there was no change in lenticular glutathione concentration (Fig. 2C), which could be a confounding factor for the interpretation of the inhibition data.
Figure 2.
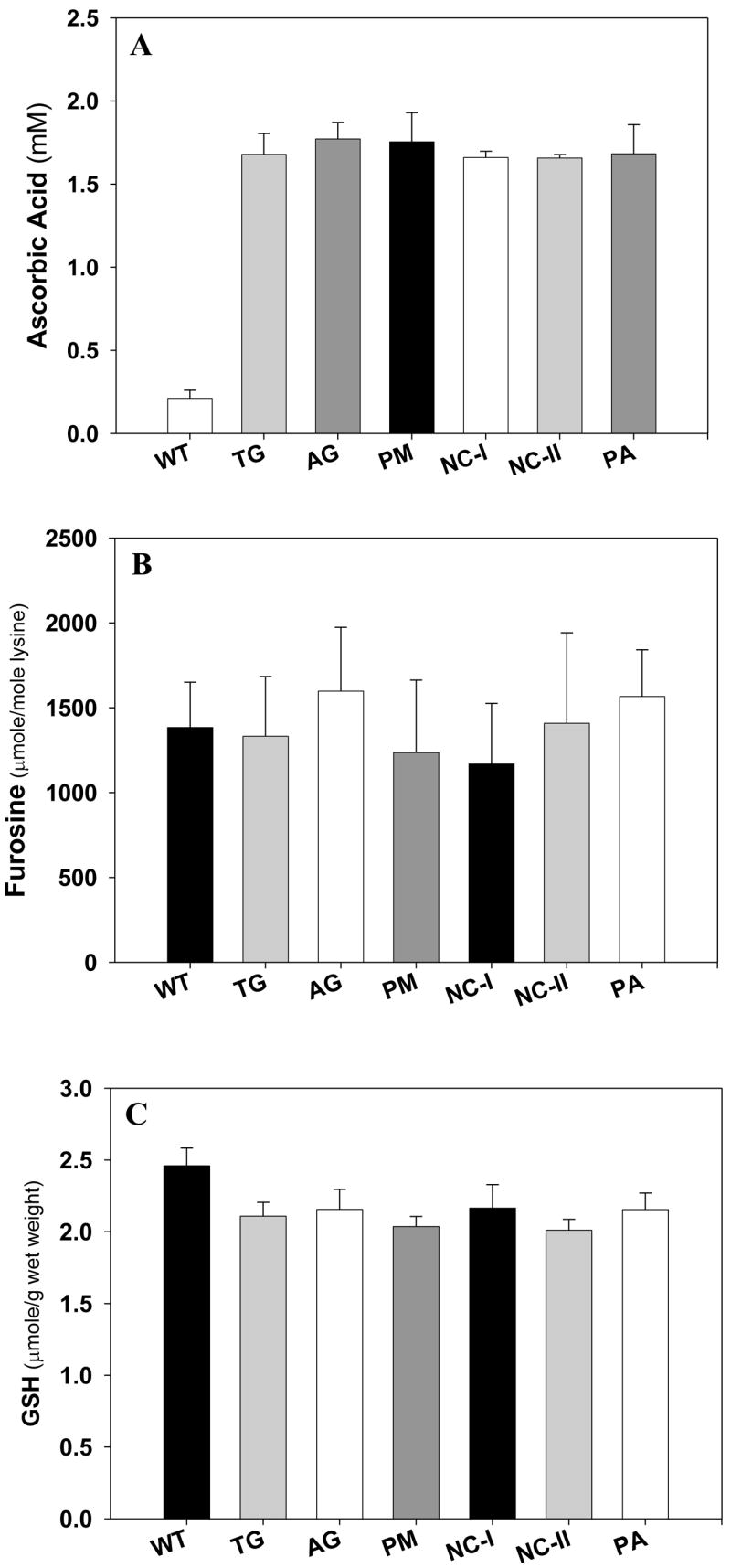
Lens ascorbic acid, glutathione (GSH) and protein bond furosine level in vivo. Mouse lens was homogenized and extracted by 10% TCA. The supernatant was used for GSH and ASA assay and the protein pellet was hydrolyzed for fructosamine (furosine assay), a marker mean glucose levels. (a). Lens ascorbic acid level. (b) lens GSH level. (c) furosine level. Student’s t test was used for all comparisons. (n=10 mice per group).
Testing for Ascorbylation Inhibition In vivo and In vitro
The five candidate inhibitors aminoguanidine, pyridoxamine, penicillamine, and the guanidino compounds NC-I and NC-II were given to the mice at a concentration of 0.1% (wt/wt/) in powdered lab chow. In addition to testing the effects of the inhibitors in vivo, these were also tested in vitro with ascorbic acid (Model B) and dehydroascorbic acid (Model A). The latter was applied with repetitive supplementation to account for its short half-life. Below we present the data in Fig. 3 and 4 whereby the in vitro data obtained with ASA are displayed in the main figure, and those obtained with DHA are shown in the inset.
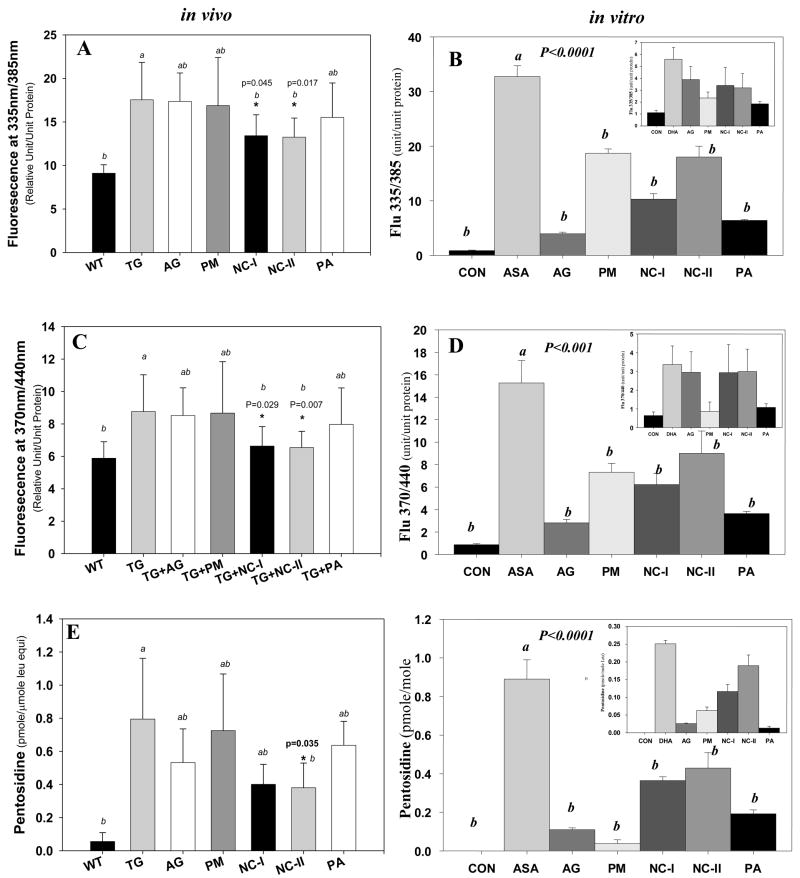
Figure 3.
Levels of protein-bound fluorescence and pentosidine in transgenic mouse lens protein(in vivo, panels A,C,E) and calf lens protein extract (CLP) (in vitro, panels B,D,F) incubated with ASA or DHA (inset) with and without inhibitor treatment. (A) and (B) fluorescence at λex/em 335/385 and at λex/em 370/440. (E) and (F) pentosidine. One-Way ANOVA was used followed by Post-Hoc analysis for all comparisons (n=10 per group).
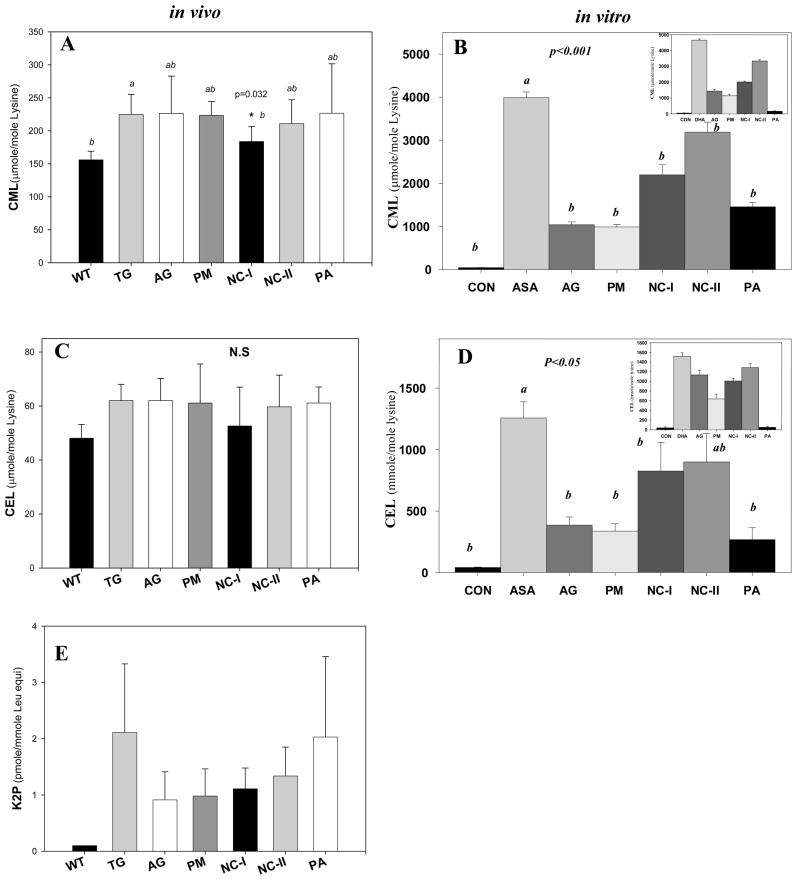
Figure 4.
Additional AGE levels in both in vitro and in vivo intervention studies. (A) Mouse lens protein CML levels were significantly reduced by NC-I (p=0.032). (B) CLP bound CML level was inhibited by all five inhibitors during in vitro incubation with ASA or DHA(inset). (C) Mouse lens protein CEL was mildly suppressed by NC-I (p = N.S.) vs. regular diet alone. (D) CLP bound CEL level was inhibited by all five inhibitors during in vitro incubation with ASA. (E) Cross-link K2P content in treated mice was lowered by all inhibitors (p=N.S) except PA. One-Way ANOVA was used followed by Post-Hoc analysis for all comparisons (n=10 per group).
Effect of inhibitors on Protein-bound fluorescence at λex/em335/385 nm and 370/440 nm
Although fluorescence is not specific, the similarity of the fluorescence spectra of various synthetic AGEs (including pentosidine and vesperlysine A) with those of human lens proteins supports the assumption that AGEs are the main fluorescent species which increase with age in the human lens 22. Usually, fluorescence is monitored for AGEs at λex/em 335/385nm and λex/em 370/440nm. Both types of protein-bound fluorescence were highly elevated in the lens protein digest from transgenic vs. wild type mice (Fig. 3A and C), as well as in the ASA incubated calf lens crystallins (Fig. 3B and D). After 7 months of intervention, NC-I was able to significantly reduce the fluorescence at both λex/em 335/385 (p=0.045) (Fig. 3A) and λex/em 370/440 (p=0.029) (Fig. 3C). NC-II had similar effect at both wavelengths (λex/em 335/385, p=0.017; λex/em 370/440, p=0.007) (Fig. 3A and C). Surprisingly, aminoguanidine (AG), penicillamine (PA) and pyridoxamine (PM) showed no fluorescence reduction at neither wavelength (Fig. 3A and C). In contrast to the in vivo data, in Model B (ASA) all inhibitors suppressed both types of fluorescence in vitro, though to various degrees (Fig 3B and 3D). Moreover, aminoguanidine and penicillamine, which had no effect in vivo, suppressed it efficiently in vitro. Interestingly NC-I, NC-II and pyridoxamine (PM) all inhibited fluorescence at both wavelengths to the about same extent, but only NC-1 and NC-2 had a significant in vivo effect. Model A (DHA) behaved similarly to ASA for fluorescence at 385 nm (Fig. 3B, inset), but the latter was curiously not suppressed by the guanidino compounds including aminoguanidine.
The inhibition pattern of pentosidine formation in vivo (Fig. 3E) is similar to the data in Fig. 3C. This is not entirely surprising since the excitation/emission wavelengths are similar for both parameters. Again the most potent inhibitors were NC-I and NC-II, with a positive trend for aminoguanidine. Paradoxically, NC-I and NC-II were relatively weak inhibitors in both in vitro models compared to the other inhibitors (Fig 3F and inset). These discrepancies raise issues that will be addressed in the Discussion.
As we move to the glycoxidation/lipoxidation ascorbylation products CML and CEL we note that they are vigorously formed from both ASA and DHA (Fig. 4B, 4D and insets) as previously reported23, but that only NC-I had significant in vivo suppressive activity toward carboxymethyllysine (CML) (Fig. 4A)._A trend toward suppression of carboxyethyllysine (CEL) was observed (Fig. 4C). In vitro, there was an overall similar pattern of CML and CEL inhibition in both models (insets 4B and 4D), whereby NC-I was the weakest inhibitor. Quite remarkably, PA was the most potent in vitro inhibitor with no activity whatsoever in vivo.
Finally, all inhibitors had a tendency to suppress the fluorophore K2P in vivo (Fig 4E). Statistical significance was not reached due to the high standard errors in the control. No in vitro data could not be obtained due to interference with other UV peaks in the chromatogram.
We found no significance differences in animal body weight, food and water intake upon treatment with any of the inhibitors (data no shown).
Discussion
The availability of an animal model that can rapidly accumulate age-related chemical modifications identical with those occurring in the human lens is crucial to the development of pharmacological agents that can delay the aging of lens crystallins, and perhaps cataractogenesis as well. In that regard the hSCVT2 mouse unequivocally confirmed that part of the yellowing, fluorescence and AGEs found in old and cataractous human lens crystallins is related to ascorbic acid and its oxidation products 21. This was already postulated many years ago by Bensch, Ortwerth and us7,12,24. The chemical pathways by which ASA is thought to generate glycating agents likely initiate from dehydroascorbic acid (DHA), which is highly elevated in the hSVCT2 mouse 21. DHA can either directly react with nucleophiles25, or it can delactonize into 2,3-diketogulonate which spontaneously degrades into xylosone and erythrulose26,27 (Fig. 1B). Our in vitro modeling reactions now unequivocally confirmed that most compounds that are elevated in mouse lens can be duplicated by in vitro incubation of crystallins with DHA.
To approach the question of pharmacological inhibition, we selected pyridoxamine (PM) and aminoguanidine (AG), ie. two AGE inhibitors that have been extensively studied both in vitro and in vivo28–30. Studies revealed that PM and AG can dramatically inhibit AGE formation in diabetic animals. We also chose penicillamine (PA), a thiol compound that has been shown to inhibit AGE formation in diabetic rat tendons 31 and in cultured rabbit proximal tubular epithelial cells32, as well as AGE formation in bovine eyes incubated with glucose or glucose-6-phosphate33. Finally, we chose two promising nucleophiles with a guanidino group in the structure that can trap dicarbonyl compounds and block AGE formation, provisorily named NC-I and NC-II. Guanidino compounds can also serve as free radical scavengers34 and increased levels were found in brain from hyperargininemia patients35,36. However, their role in this condition as well as in normal humans is still unclear36.
At the outset a major finding in this study is that all five inhibitors showed inhibition of ascorbylation in vitro in both models, though to various degrees. By and large, aminoguanidine, pyridoxamine and penicillamine were the most potent inhibitors for specific AGEs, i.e. pentosidine, CML and CEL. The most surprising finding, however, is that NC-I and NC-II, which had the weakest in vitro activity, had the best in vivo activity, notably NC-I.
Of particular interest are the data with K2P. This molecule is a fluorescent and UV active lysine-lysine crosslink and one of the major modifications in old and cataractous human lenses37. In our previous study, there was a dramatic increase in K2P between 9 and 12 mos of age, suggesting the data might have reached significance if we had extended the study by another three months. The uniform trend toward its suppression by all nucleophiles except for penicillamine (Fig. 3C) suggests similar effects would have been observed in vitro. As previously mentioned, however, overlapping UV peaks precluded us from interpreting the in vitro data. Nevertheless, the in vivo suppressive effect of the drugs appears to be strong.
A more difficult issue to address is the discrepancy between the in vivo and in vitro effects of NC-I and NC-II versus PM, AG and PA. The latter three compounds’ metal chelation ability may have contributed toward their strong in vitro anti-AGE property, as previously reported by Price et al. (New Reference: PRICE BAYNES JBC 2001). Another possibility is that NC-I and NC-II have been shown elsewhere to penetrate the blood barrier, likely via an active transport. It may thus be possible that these agents achieved millimolar concentrations providing thus strong dicarbonyl compound trapping activity. Nevertheless, the fact that AG and PM lowered K2P suggests that these nucleophiles are also able to reach the lens. It is clear that while we postulate that NC-I and NC-II act as trapping agents (Fig. 2B), other biological effects such as decrease in reactive oxygen species formation cannot be excluded at this time.
The overall conclusion from the above data is that the existing lenticular concentrations of natural nucleophiles or GSH itself, here determined at about 2.5 mM, does not suffice to scavenge the ascorbylation precursors, and that the tested experimental drugs most likely act by trapping the reactive carbonyls originating from ascorbic acid. While the exact nature of the trapped molecules remains to be identified, this conclusion is supported by the fact that the drugs impaired neither lenticular glucose, nor glutathione or ascorbic acid levels themselves. If that had been the case, very different interpretation would have been needed.
The above data may have important translational significance for the potential prevention of senile cataracts. The nuclear fraction of the human lens shows a steady increase in blue and green fluorophores with age that are attributable to UV-light induced tryptophan photodegradation reaction38, kynurenine oxidation products that bind lens crystallins 39 and non-enzymatic glycation/ascorbylation products 40. Lerman et al. proposed that the decreased transmission of visible light in the aging lens and in nuclear cataract is mainly due to the accumulation of lens fluorogens rather than configurational changes of lens protein38. More recently, several glycation experiments have demonstrated that lens crystallin chaperone activity is impaired by certain glycation products41,42. It is thus possible that the fluorophores act as surrogate markers for the total damage to lens crystallins and thus participate in destabilizing lens crystallins and eventually cataractogenesis. Indeed, Lens Opacities Classification System (LOCS) studies indicate a significant correlation between lens opacity, discoloration and autofluorescence43–45. Therefore, prevention of lens discoloration and fluorogen formation may have the potential to delay lens opacification and cataractogenesis. Intringuingly, however, the trapping of methylgyoxal by guanidino compounds could have deleterious effects, if indeed the reported modification of arginine residues by methylglyoxal improves the chaperone activity of the crystallins46,47. Further experiments with our animal model are in progress in that regard.
In summary, five potential ascorbylation inhibitors were tested in hSVCT2 transgenic mouse model of lens chemical aging. Two inhibitors NC-I and NC-II were found to significantly block advanced glycation end products (AGEs) formation. NC-I, a guanidino- compound capable of being delivered into the lens may surprisingly reveal itself as potent anticataract agent in clinical studies.
Acknowledgments
This work was supported by National Eye Institute Grant EY07099 and NEI Center grant P30 VSRC EY11373 to Case Western Reserve University. We thank Dr. David Sell for assistance with statistical analysis.
References
- 1.Sell DR, Lane MA, Johnson WA, Masoro EJ, Mock OB, Reiser KM, Fogarty JF, Cutler RG, Ingram DK, Roth GS, Monnier VM. Longevity and the genetic determination of collagen glycoxidation kinetics in mammalian senescence. Proc Natl Acad Sci U S A. 1996;93:485–90. doi: 10.1073/pnas.93.1.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horie K, Miyata T, Yasuda T, Takeda A, Yasuda Y, Maeda K, Sobue G, Kurokawa K. Immunohistochemical localization of advanced glycation end products, pentosidine, and carboxymethyllysine in lipofuscin pigments of Alzheimer’s disease and aged neurons. Biochem Biophys Res Commun. 1997;236:327–32. doi: 10.1006/bbrc.1997.6944. [DOI] [PubMed] [Google Scholar]
- 3.Oudes AJ, Herr CM, Olsen Y, Fleming JE. Age-dependent accumulation of advanced glycation end-products in adult Drosophila melanogaster. Mech Ageing Dev. 1998;100:221–9. doi: 10.1016/s0047-6374(97)00146-2. [DOI] [PubMed] [Google Scholar]
- 4.Puttaiah S, Zhang Y, Pilch HA, Pfahler C, Oya-Ito T, Sayre LM, Nagaraj RH. Detection of dideoxyosone intermediates of glycation using a monoclonal antibody: characterization of major epitope structures. Arch Biochem Biophys. 2006;446:186–96. doi: 10.1016/j.abb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Tauer A, Bender TO, Fleischmann EH, Niwa T, Jorres A, Pischetsrieder M. Fate of the glucose degradation products 3-deoxyglucosone and glyoxal during peritoneal dialysis. Mol Nutr Food Res. 2005;49:710–5. doi: 10.1002/mnfr.200400111. [DOI] [PubMed] [Google Scholar]
- 6.Cai W, Gao QD, Zhu L, Peppa M, He C, Vlassara H. Oxidative stress-inducing carbonyl compounds from common foods: novel mediators of cellular dysfunction. Mol Med. 2002;8:337–46. [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng R, Lin B, Lee KW, Ortwerth BJ. Similarity of the yellow chromophores isolated from human cataracts with those from ascorbic acid-modified calf lens proteins: evidence for ascorbic acid glycation during cataract formation. Biochim Biophys Acta. 2001;1537:14–26. doi: 10.1016/s0925-4439(01)00051-5. [DOI] [PubMed] [Google Scholar]
- 8.Mota MC, Carvalho P, Ramalho JS, Cardoso E, Gaspar AM, Abreu G. Protein glycation and in vivo distribution of human lens fluorescence. Int Ophthalmol. 1994;18:187–93. doi: 10.1007/BF00951795. [DOI] [PubMed] [Google Scholar]
- 9.Roberts DK, Winters JE, Castells DD, Clark CA, Teitelbaum BA. Pigmented striae of the anterior lens capsule and age-associated pigment dispersion of variable degree in a group of older African-Americans: an age, race, and gender matched study. Int Ophthalmol. 2001;24:313–22. doi: 10.1023/b:inte.0000006762.32723.17. [DOI] [PubMed] [Google Scholar]
- 10.Srivastava OP. Age-related increase in concentration and aggregation of degraded polypeptides in human lenses. Exp Eye Res. 1988;47:525–43. doi: 10.1016/0014-4835(88)90092-9. [DOI] [PubMed] [Google Scholar]
- 11.Kamei A. Characterization of water-insoluble proteins in normal and cataractous human lens. Jpn J Ophthalmol. 1990;34:216–24. [PubMed] [Google Scholar]
- 12.Nagaraj RH, Sell DR, Prabhakaram M, Ortwerth BJ, Monnier VM. High correlation between pentosidine protein crosslinks and pigmentation implicates ascorbate oxidation in human lens senescence and cataractogenesis. Proc Natl Acad Sci U S A. 1991;88:10257–61. doi: 10.1073/pnas.88.22.10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng R, Lin B, Ortwerth BJ. Separation of the yellow chromophores in individual brunescent cataracts. Exp Eye Res. 2003;77:313–25. doi: 10.1016/s0014-4835(03)00131-3. [DOI] [PubMed] [Google Scholar]
- 14.Linetsky M, James HL, Ortwerth BJ. The generation of superoxide anion by the UVA irradiation of human lens proteins. Exp Eye Res. 1996;63:67–74. doi: 10.1006/exer.1996.0092. [DOI] [PubMed] [Google Scholar]
- 15.Huang L, Estrada R, Yappert MC, Borchman D. Oxidation-induced changes in human lens epithelial cells. 1 Phospholipids. Free Radic Biol Med. 2006;41:1425–32. doi: 10.1016/j.freeradbiomed.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 16.Biemel KM, Friedl DA, Lederer MO. Identification and quantification of major maillard cross-links in human serum albumin and lens protein. Evidence for glucosepane as the dominant compound. J Biol Chem. 2002;277:24907–15. doi: 10.1074/jbc.M202681200. [DOI] [PubMed] [Google Scholar]
- 17.Korlimbinis A, Truscott RJ. Identification of 3-hydroxykynurenine bound to proteins in the human lens. A possible role in age-related nuclear cataract. Biochemistry. 2006;45:1950–60. doi: 10.1021/bi051744y. [DOI] [PubMed] [Google Scholar]
- 18.Truscott RJ, Wood AM. Tryptophan metabolism and the reactive metabolite hypothesis for human cataract. Dev Ophthalmol. 1994;26:83–6. doi: 10.1159/000423767. [DOI] [PubMed] [Google Scholar]
- 19.Cheng R, Lin B, Ortwerth BJ. Rate of formation of AGEs during ascorbate glycation and during aging in human lens tissue. Biochim Biophys Acta. 2002;1587:65–74. doi: 10.1016/s0925-4439(02)00069-8. [DOI] [PubMed] [Google Scholar]
- 20.Atalay A, Ogus A, Bateman O, Slingsby C. Vitamin C induced oxidation of eye lens gamma crystallins. Biochimie. 1998;80:283–8. doi: 10.1016/s0300-9084(98)80068-0. [DOI] [PubMed] [Google Scholar]
- 21.Fan X, Reneker LW, Obrenovich ME, Strauch C, Cheng R, Jarvis SM, Ortwerth BJ, Monnier VM. Vitamin C mediates chemical aging of lens crystallins by the Maillard reaction in a humanized mouse model. Proc Natl Acad Sci U S A. 2006;103:16912–7. doi: 10.1073/pnas.0605101103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das BK, Sun TX, Akhtar NJ, Chylack LT, Jr, Liang JJ. Fluorescence and immunochemical studies of advanced glycation-related lens pigments. Invest Ophthalmol Vis Sci. 1998;39:2058–66. [PubMed] [Google Scholar]
- 23.Slight SH, Prabhakaram M, Shin DB, Feather MS, Ortwerth BJ. The extent of N epsilon-(carboxymethyl)lysine formation in lens proteins and polylysine by the autoxidation products of ascorbic acid. Biochim Biophys Acta. 1992;1117:199–206. doi: 10.1016/0304-4165(92)90080-e. [DOI] [PubMed] [Google Scholar]
- 24.Bensch KG, Fleming JE, Lohmann W. The role of ascorbic acid in senile cataract. Proc Natl Acad Sci U S A. 1985;82:7193–6. doi: 10.1073/pnas.82.21.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seidel W, Pischetsrieder M. Immunochemical detection of N2-[1-(1-carboxy)ethyl]guanosine, an advanced glycation end product formed by the reaction of DNA and reducing sugars or L-ascorbic acid in vitro. Biochim Biophys Acta. 1998;1425:478–84. doi: 10.1016/s0304-4165(98)00101-9. [DOI] [PubMed] [Google Scholar]
- 26.Simpson GL, Ortwerth BJ. The non-oxidative degradation of ascorbic acid at physiological conditions. Biochim Biophys Acta. 2000;1501:12–24. doi: 10.1016/s0925-4439(00)00009-0. [DOI] [PubMed] [Google Scholar]
- 27.Reihl O, Lederer MO, Schwack W. Characterization and detection of lysine-arginine cross-links derived from dehydroascorbic acid. Carbohydr Res. 2004;339:483–91. doi: 10.1016/j.carres.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Stitt A, Gardiner TA, Alderson NL, Canning P, Frizzell N, Duffy N, Boyle C, Januszewski AS, Chachich M, Baynes JW, Thorpe SR. The AGE inhibitor pyridoxamine inhibits development of retinopathy in experimental diabetes. Diabetes. 2002;51:2826–32. doi: 10.2337/diabetes.51.9.2826. [DOI] [PubMed] [Google Scholar]
- 29.Tanimoto M, Gohda T, Kaneko S, Hagiwara S, Murakoshi M, Aoki T, Yamada K, Ito T, Matsumoto M, Horikoshi S, Tomino Y. Effect of pyridoxamine (K-163), an inhibitor of advanced glycation end products, on type 2 diabetic nephropathy in KK-A(y)/Ta mice. Metabolism. 2007;56:160–7. doi: 10.1016/j.metabol.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 30.Lin YT, Tseng YZ, Chang KC. Aminoguanidine prevents fructose-induced arterial stiffening in Wistar rats: aortic impedance analysis. Exp Biol Med (Maywood) 2004;229:1038–45. doi: 10.1177/153537020422901008. [DOI] [PubMed] [Google Scholar]
- 31.Elgawish A, Glomb M, Friedlander M, Monnier VM. Involvement of hydrogen peroxide in collagen cross-linking by high glucose in vitro and in vivo. J Biol Chem. 1996;271:12964–71. doi: 10.1074/jbc.271.22.12964. [DOI] [PubMed] [Google Scholar]
- 32.Verbeke P, Perichon M, Friguet B, Bakala H. Inhibition of nitric oxide synthase activity by early and advanced glycation end products in cultured rabbit proximal tubular epithelial cells. Biochim Biophys Acta. 2000;1502:481–94. doi: 10.1016/s0925-4439(00)00071-5. [DOI] [PubMed] [Google Scholar]
- 33.Stevens A. The effectiveness of putative anti-cataract agents in the prevention of protein glycation. J Am Optom Assoc. 1995;66:744–9. [PubMed] [Google Scholar]
- 34.Nakai K, Kadiiska MB, Jiang JJ, Stadler K, Mason RP. Free radical production requires both inducible nitric oxide synthase and xanthine oxidase in LPS-treated skin. Proc Natl Acad Sci U S A. 2006;103:4616–21. doi: 10.1073/pnas.0510352103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Deyn PP, Marescau B, D’Hooge R, Possemiers I, Nagler J, Mahler C. Guanidino compound levels in brain regions of non-dialyzed uremic patients. Neurochem Int. 1995;27:227–37. doi: 10.1016/0197-0186(95)00041-6. [DOI] [PubMed] [Google Scholar]
- 36.Mizutani N, Hayakawa C, Ohya Y, Watanabe K, Watanabe Y, Mori A. Guanidino compounds in hyperargininemia. Tohoku J Exp Med. 1987;153:197–205. doi: 10.1620/tjem.153.197. [DOI] [PubMed] [Google Scholar]
- 37.Cheng R, Feng Q, Argirov OK, Ortwerth BJ. K2P--a novel cross-link from human lens protein. Ann N Y Acad Sci. 2005;1043:184–94. doi: 10.1196/annals.1333.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lerman S, Kuck JF, Jr, Borkman R, Saker E. Accleration of an aging parameter (fluorogen) in the ocular lens. Ann Ophthalmol. 1976;8:558–61. [PubMed] [Google Scholar]
- 39.Aquilina JA, Carver JA, Truscott RJ. Oxidation products of 3-hydroxykynurenine bind to lens proteins: relevance for nuclear cataract. Exp Eye Res. 1997;64:727–35. doi: 10.1006/exer.1996.0258. [DOI] [PubMed] [Google Scholar]
- 40.Monnier VM, Cerami A. Nonenzymatic browning in vivo: possible process for aging of long-lived proteins. Science. 1981;211:491–3. doi: 10.1126/science.6779377. [DOI] [PubMed] [Google Scholar]
- 41.Blakytny R, Harding JJ. Prevention of the fructation-induced inactivation of glutathione reductase by bovine alpha-crystallin acting as a molecular chaperone. Ophthalmic Res. 1996;28(Suppl 1):19–22. doi: 10.1159/000267938. [DOI] [PubMed] [Google Scholar]
- 42.Kumar MS, Reddy PY, Kumar PA, Surolia I, Reddy GB. Effect of dicarbonyl-induced browning on alpha-crystallin chaperone-like activity: physiological significance and caveats of in vitro aggregation assays. Biochem J. 2004;379:273–82. doi: 10.1042/BJ20031633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chylack LT, Jr, Leske MC, McCarthy D, Khu P, Kashiwagi T, Sperduto R. Lens opacities classification system II (LOCS II) Arch Ophthalmol. 1989;107:991–7. doi: 10.1001/archopht.1989.01070020053028. [DOI] [PubMed] [Google Scholar]
- 44.Chylack LT, Jr, Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL, Friend J, McCarthy D, Wu SY. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol. 1993;111:831–6. doi: 10.1001/archopht.1993.01090060119035. [DOI] [PubMed] [Google Scholar]
- 45.Siik S, Chylack LT, Jr, Friend J, Wolfe J, Teikari J, Nieminen H, Airaksinen PJ. Lens autofluorescence and light scatter in relation to the lens opacities classification system, LOCS III. Acta Ophthalmol Scand. 1999;77:509–14. doi: 10.1034/j.1600-0420.1999.770504.x. [DOI] [PubMed] [Google Scholar]
- 46.Biswas A, Miller A, Oya-Ito T, Santhoshkumar P, Bhat M, Nagaraj RH. Effect of site-directed mutagenesis of methylglyoxal-modifiable arginine residues on the structure and chaperone function of human alphaA-crystallin. Biochemistry. 2006;45:4569–77. doi: 10.1021/bi052574s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Puttaiah S, Biswas A, Staniszewska M, Nagaraj RH. Methylglyoxal inhibits glycation-mediated loss in chaperone function and synthesis of pentosidine in alpha-crystallin. Exp Eye Res. 2007;84:914–21. doi: 10.1016/j.exer.2007.01.013. [DOI] [PubMed] [Google Scholar]