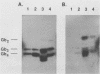
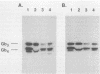
Abstract
Edema disease (ED) of weanling pigs is caused by an infection with Escherichia coli that produces Shiga-like toxin II variant (SLT-IIv). Pathology identical to that caused by ED can be duplicated in pigs that are injected with less than 10 ng of purified SLT-IIv per kg of body weight. Therefore, SLT-IIv was mutated to create an immunoreactive form of the toxin that was significantly reduced in enzymatic activity. Initially, purified SLT-IIv was treated with formaldehyde which abrogated cytotoxic activity. Pigs were vaccinated with the toxoid (100 micrograms) to determine whether a toxoid was a viable vaccine candidate and whether young pigs were capable of mounting an immune response. Although the pigs developed a neutralizing antibody titer (1:128 to 1:512) 28 days postinjection, they also lost weight and developed ED lesions. The deleterious effect of the toxoid appeared to result from residual enzymatic activity or a reversion to a toxic form. An alternative method, site-directed mutagenesis, was employed to consistently reduce the enzymatic activity of SLT-IIv. Glutamate at position 167 of the mature A subunit was replaced by aspartate (E167D), and arginine at position 170 was replaced by lysine (R170K). These mutations reduced cytotoxic activity 10(4)-fold and 10-fold, respectively, while the enzymatic activities were decreased 400-fold and 5-fold, respectively. The activity of a toxin that contained both mutations (SLT-IIvE167D/R170K) closely resembled that of SLT-IIvE167D. When position 167 was replaced by glutamine (E167Q), the cytotoxic activity decreased 10(6)-fold and the enzymatic activity decreased approximately 1,500-fold. Pigs that were vaccinated with purified, mutant toxin designated SLT-IIvE167Q developed a neutralizing antibody titer of 1:512 21 days postinjection, and their tissues were free of ED lesions. These data suggest that SLT-IIvE167Q may represent an effective vaccine against ED.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyd B., Richardson S., Gariepy J. Serological responses to the B subunit of Shiga-like toxin 1 and its peptide fragments indicate that the B subunit is a vaccine candidate to counter action of the toxin. Infect Immun. 1991 Mar;59(3):750–757. doi: 10.1128/iai.59.3.750-757.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clugston R. E., Nielsen N. O., Smith D. L. Experimental edema disease of swine (E. coli enterotoxemia). 3. Pathology and pathogenesis. Can J Comp Med. 1974 Jan;38(1):34–43. [PMC free article] [PubMed] [Google Scholar]
- Endo Y., Tsurugi K., Yutsudo T., Takeda Y., Ogasawara T., Igarashi K. Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur J Biochem. 1988 Jan 15;171(1-2):45–50. doi: 10.1111/j.1432-1033.1988.tb13756.x. [DOI] [PubMed] [Google Scholar]
- Frankel A., Schlossman D., Welsh P., Hertler A., Withers D., Johnston S. Selection and characterization of ricin toxin A-chain mutations in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Feb;9(2):415–420. doi: 10.1128/mcb.9.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A., Welsh P., Richardson J., Robertus J. D. Role of arginine 180 and glutamic acid 177 of ricin toxin A chain in enzymatic inactivation of ribosomes. Mol Cell Biol. 1990 Dec;10(12):6257–6263. doi: 10.1128/mcb.10.12.6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry M. K., Dalrymple J. M. Quantitative microtiter cytotoxicity assay for Shigella toxin. J Clin Microbiol. 1980 Sep;12(3):361–366. doi: 10.1128/jcm.12.3.361-366.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovde C. J., Calderwood S. B., Mekalanos J. J., Collier R. J. Evidence that glutamic acid 167 is an active-site residue of Shiga-like toxin I. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2568–2572. doi: 10.1073/pnas.85.8.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. P., Deresiewicz R. L., Calderwood S. B. Mutational analysis of the Shiga toxin and Shiga-like toxin II enzymatic subunits. J Bacteriol. 1990 Jun;172(6):3346–3350. doi: 10.1128/jb.172.6.3346-3350.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmali M. A. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989 Jan;2(1):15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmali M. A., Petric M., Lim C., Fleming P. C., Arbus G. S., Lior H. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J Infect Dis. 1985 May;151(5):775–782. doi: 10.1093/infdis/151.5.775. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacLeod D. L., Gyles C. L., Wilcock B. P. Reproduction of edema disease of swine with purified Shiga-like toxin-II variant. Vet Pathol. 1991 Jan;28(1):66–73. doi: 10.1177/030098589102800109. [DOI] [PubMed] [Google Scholar]
- Marques L. R., Moore M. A., Wells J. G., Wachsmuth I. K., O'Brien A. D. Production of Shiga-like toxin by Escherichia coli. J Infect Dis. 1986 Aug;154(2):338–341. doi: 10.1093/infdis/154.2.338. [DOI] [PubMed] [Google Scholar]
- Mata L. J., Gangarosa E. J., Cáceres A., Perera D. R., Mejicanos M. L. Epidemic Shiga bacillus dysentery in Central America. I. Etiologic investigations in Guatemala, 1969. J Infect Dis. 1970 Sep;122(3):170–180. doi: 10.1093/infdis/122.3.170. [DOI] [PubMed] [Google Scholar]
- Morrison D. M., Tyrrell D. L., Jewell L. D. Colonic biopsy in verotoxin-induced hemorrhagic colitis and thrombotic thrombocytopenic purpura (TTP). Am J Clin Pathol. 1986 Jul;86(1):108–112. doi: 10.1093/ajcp/86.1.108. [DOI] [PubMed] [Google Scholar]
- O'Brien A. D., Holmes R. K. Shiga and Shiga-like toxins. Microbiol Rev. 1987 Jun;51(2):206–220. doi: 10.1128/mr.51.2.206-220.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., LaVeck G. D. Purification and characterization of a Shigella dysenteriae 1-like toxin produced by Escherichia coli. Infect Immun. 1983 May;40(2):675–683. doi: 10.1128/iai.40.2.675-683.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel J. E., Perera L. P., Ward S., O'Brien A. D., Ginsburg V., Krivan H. C. Comparison of the glycolipid receptor specificities of Shiga-like toxin type II and Shiga-like toxin type II variants. Infect Immun. 1990 Mar;58(3):611–618. doi: 10.1128/iai.58.3.611-618.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlossman D., Withers D., Welsh P., Alexander A., Robertus J., Frankel A. Role of glutamic acid 177 of the ricin toxin A chain in enzymatic inactivation of ribosomes. Mol Cell Biol. 1989 Nov;9(11):5012–5021. doi: 10.1128/mcb.9.11.5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storsaeter J., Olin P., Renemar B., Lagergård T., Norberg R., Romanus V., Tiru M. Mortality and morbidity from invasive bacterial infections during a clinical trial of acellular pertussis vaccines in Sweden. Pediatr Infect Dis J. 1988 Sep;7(9):637–645. doi: 10.1097/00006454-198809000-00008. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]