Abstract
Arabidopsis flowers in long day (LD) in response to signals transported from the photoinduced leaf to the shoot apex. These LD signals may include protein of the gene FLOWERING LOCUS T (FT) while in short day (SD) with its slower flowering, signalling may involve sucrose and gibberellin. Here, it is shown that after 5 weeks growth in SD, a single LD up-regulated leaf blade expression of FT and CONSTANS (CO) within 4–8 h, and flowers were visible within 2–3 weeks. Plants kept in SDs were still vegetative 7 weeks later. This LD response was blocked in ft-1 and a co mutant. Exposure to different LD light intensities and spectral qualities showed that two LD photoresponses are important for up-regulation of FT and for flowering. Phytochrome is effective at a low intensity from far-red (FR)-rich incandescent lamps. Independently, photosynthesis is active in an LD at a high intensity from red (R)-rich fluorescent lamps. The photosynthetic role of a single high light LD is demonstrated here by the blocking of the flowering and FT increase on removal of atmospheric CO2 or by decreasing the LD light intensity by 10-fold. These conditions also reduced leaf blade sucrose content and photosynthetic gene expression. An SD light integral matching that in a single LD was not effective for flowering, although there was reasonable FT-independent flowering after 12 SD at high light. While a single photosynthetic LD strongly amplified FT expression, the ability to respond to the LD required an additional but unidentified photoresponse. The implications of these findings for studies with mutants and for flowering in natural conditions are discussed.
Keywords: Arabidopsis, CONSTANS, far-red light, flowering, FT, long day, photosynthesis, sucrose
Introduction
Flowering of Arabidopsis thaliana (L.) Heynh. is regulated environmentally by daylength and cold (Boss et al., 2004; Searle and Coupland, 2004; Imaizumi and Kay, 2006: Turck et al., 2008). Its light response in long days (LD) involves phytochrome (PHY) and the blue photoreceptors (Goto et al., 1991; Reed et al., 1994; Bagnall and King, 2001; Endo et al., 2007, and references therein). These photoreceptors interact with endogenous oscillators to activate expression in leaf blade vascular tissue of two ‘floral’ genes, CONSTANS (CO) and FLOWERING LOCUS T (FT) (Halliday et al., 2003; Imaizumi et al., 2003; Takada and Goto, 2003; An et al., 2004; Valverde et al., 2004; Endo et al., 2007).
Genetically, the link between FT and flowering is shown by the delayed flowering in ft mutants and early flowering in overexpression lines (Koorneef et al., 1991; Kardailsky et al., 1999; Kobayashi et al., 1999; Yoo et al., 2005). Furthermore, recent evidence implies LD floral signalling by FT protein which may act as a signal transmitted from the leaf to the shoot apex in Arabidopsis, Cucurbita spp, and rice (Corbesier et al., 2007; Jaeger and Wigge, 2007; Lin et al., 2007; Mathieu et al., 2007; Tamaki et al., 2007). At the shoot apex, FT then interacts in a putative transcriptional complex with protein of the FD gene (Abe et al., 2005; Wigge et al., 2005).
Far-red (FR) light acting via phytochrome up-regulates FT expression in Arabidopsis (Cerdän and Chory, 2003; Halliday et al., 2003; Valverde et al., 2004) and, in parallel, FR promotes flowering (Reed et al., 1994; Bagnall and King, 2001, and references therein). Red (R) light acting via phytochrome blocks the FT increase and flowering is delayed. Paradoxically, however, high light intensities from R-rich fluorescent lamps are traditionally used to show LD up-regulation of FT (Suarez-Lopez et al., 2001; Imaizumi et al., 2003; Takada and Goto, 2003; Valverde et al., 2004; Abe et al., 2005; Wigge et al., 2005; Yoo et al., 2005).
Here, for Arabidopsis this paradox is explained by showing independent LD photoregulation of both FT and flowering by photosynthesis in high intensity R-rich light and by phytochrome in low intensity FR-rich light. Importantly, to assess cause and effect in the link between photosynthesis, FT, and flowering, conditions were used which give rapid flowering after exposure to a single LD. In a limited way, the question of sucrose regulation of the extremely late short day (SD) flowering of Arabidopsis (Ericksson et al., 2006) is also addressed.
Materials and methods
Plant material, growing conditions, and light treatments
Plants of A. thaliana (L.) Heynh. ecotype Columbia and various mutant lines in Columbia were grown in 8 h SDs at 22 °C under an irradiance of 100 μmol m−2 s−1 from fluorescent lamps. There was a very limited flowering by 3 weeks (up to 3% of 1000 plants in various experiments). These precociously flowering plants were removed and the remainder were still vegetative at 3 months.
For flower induction, the plants were always 5 weeks old when exposed to one or up to five LDs. The LD light extension was for a duration of 16 h from incandescent lamps at 10 μmol m−2 s−1 or from fluorescent lamps at either 10 μmol m−2 s−1 or 100 μmol m−2 s−1. In one experiment (Fig. 7), light intensity in 8 h SDs was increased ∼3-fold (to 270 μmol m−2 s−1 or 360 μmol m−2 s−1) for a total of 12 d; each SD was terminated by a 10 min exposure to incandescent lamps.
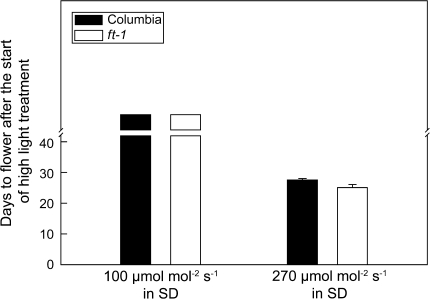
Fig. 7.
An increase in light intensity in SD causes flowering equally well for Columbia and ft-1. Columbia (solid bar) and ft-1 (open bar) were held for 12 d in an 8 h SD terminated daily by a 10 min exposure to low intensity FR-rich light from incandescent lamps. Half the plants were exposed to the normal SD light intensity of 100 μmol m−2 s−1 and half to an intensity of 270 μmol m−2 s−1. None of the plants held at 100 μmol m−2 s−1 had flowered when the experiment was terminated after 42 d (77 d after germination). Values are means ±SE (n=18).
The spectral output of the fluorescent lamps used here is enriched in yellow/orange and R wavebands, and the incandescent bulbs provide predominantly FR light (Supplementary Fig. S1 available at JXB online). The R:FR ratio of 0.8 in the FR-rich light provides spectral conditions close to direct sunlight (R:FR 1.1–1.25) especially as there is a substantial further enrichment for FR at twilight (Smith, 1982). Exposure to an LD from R-rich fluorescent lamps (R:FR 4.5) is often used but does not match natural conditions as closely as the FR-rich LD treatment. Compared with these fluorescent and incandescent lamps, there are matching responses of growth and flowering of Arabidopsis to light from narrow-band R and FR sources (Bagnall and King, 2001; Hisamatsu et al., 2005). Therefore, subsequently, the LDs here from fluorescent lamps is referred to as a R-rich LDs (LD-R) at a high light intensity (100 μmol m−2 s−1) and as a low light R-rich LDs (LD-lR) at a 10-fold lower intensity An LD from incandescent lamps at low light is referred to as an FR-rich LD (LD-FR).
Flowering began 10–20 d after an LD (flower buds were visible ∼2–3 d after bolt appearance) and was recorded both as percentage flowering and as days from the start of the LD until petal appearance. The findings of all experiments presented here have been confirmed in repeat studies.
For genetic studies of CO/FT in Columbia, the co mutant was from the SAIL T-DNA collection and carries a T-DNA insertion at bp 342 after the ATG (Laubinger et al., 2006). It causes late flowering in LD as with other co mutants (Putterill et al., 1995), produces very low levels of CO (Dr I Searle, Max Planck Institute, Koeln, Germany, personal communication), and lacks detectable FT expression in LD (T Hisamatsu, data not shown). The ft-1 mutant was a third backcross line in Columbia crossed in from Landsberg erecta. Eighth backcross material of this ft-1 line became available at the end of this study and it responded in a similar way to the third backcross material.
To block photosynthesis, 16 plants per treatment were enclosed in 7. 0 l clear plastic boxes which were flushed with CO2-free or normal air at 2 l min−1. Scrubbing of CO2 was through a 1.0 l plastic cylinder filled with indicating soda lime. All CO2 was quickly removed (<4 min), as measured with a gas analysis system. At the flow rates used, the soda lime column remained effective for up to 48 h. To balance the humidity of the air streams, both the CO2-free and the normal air were aspirated in sealed 2.0 l water columns before entering the boxes.
Statistical analysis
Some statistical analysis involved analysis of variance (ANOVA) and calculation of LSD0.05. Otherwise errors are shown as means ±SE. In many instances, the error was smaller than the symbol and is not visible in the figures. Unless indicated otherwise, 16 plants per treatment were assessed for calculating percentagr flowering and flowering time. All experiments reported here have been repeated at least once.
Quantitative real-time PCR analysis of gene expression
For studies of gene expression, the youngest fully expanded leaves were harvested from ≥16 plants. Where harvests were during the dark period, a green safe light was used. Total RNA was extracted using an RNeasy Plant Mini Kit (Qiagen, Clifton Hills, Victoria, Australia) and treated with RNase-free DNase (Qiagen) according to the manufacturer's instructions An aliquot of 1 or 2 μg of total RNA was reverse-transcribed using Super Script II (Invitrogen, Mt Waverley, Victoria, Australia) according to the manufacturer's instructions. The cDNA was diluted 5- or 25-fold, and 4 μl was used in a 10 μl Q-PCR with SYBR Green JumpStart Taq ReadyMix (Sigma Aldrich, Castle Hill, NSW, Australia) performed on a Rotor-Gene 2000 Real-Time Cycler (Corbett Research, Sydney, Australia). The Q-PCR assays were repeated three times and, for any claimed treatment effects, the result was confirmed in at least one further independent experiment. All samples were normalized using the ‘Comparative Quantification’ analysis method (Rotogene-5 software, Corbett Research), and RNA expression is compared directly after normalization against an ACTIN2 loading standard.
Primer pairs previously characterized were: CO, FT, and SOC1 (Halliday et al., 2003) and ACTIN2 (Hisamatsu et al., 2005). The internal standard, ACTIN2 (At3g18780), was constant in the samples assayed. Further details of these assays are given in Hisamatsu et al. (2005). The means presented are averages from three technical replicates, and all experiments have been repeated.
Measurement of leaf and shoot apex sucrose content
As shown in Supplementary Fig. S2 at JXB online, the ‘shoot apex’ used for the sucrose assays refers to a tissue piece no bigger than 250 μm diameter after dissection and which weighed <1 μg dry weight. It included the true shoot apex, some basal pith tissue, and up to two leaf primordia as large as the apex itself (Supplementary Fig. S2 at JXB online). This ‘shoot apex’ is smaller by several orders of magnitude than the 3 mm tissues pieces harvested as shoot tips but incorrectly described in the literature as ‘the shoot apex’. Dissecting this minute shoot apex was not difficult and the GCMS-SIM assays of sucrose showed high reproducibility due to the inclusion of a [13C]sucrose internal standard. Sensitivity of the microbalance was a limitation so four to five apices were combined in each assay and sufficient apices were collected for 5 replicate assays. The methods for sucrose extraction and quantification by GCMS-SIM are given in detail in King and Ben-Tal (2001).
Results
A sensitive LD flowering response
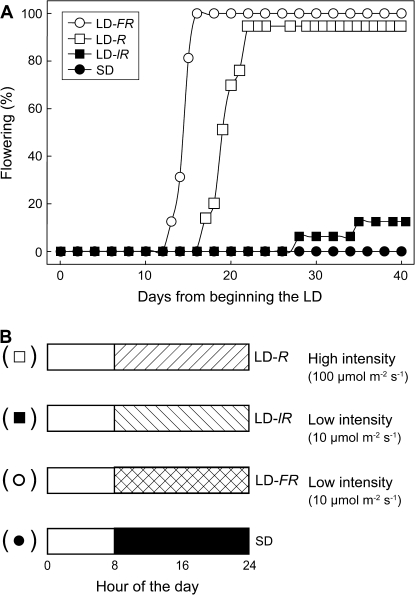
Arabidopsis (ecotype Columbia) flowered rapidly on exposure to a single 16 h LD light extension either at high light intensity (100 μmol m−2 s−1) from R-rich (LD-R) fluorescent lamps or at a low intensity (10 μmol m−2 s−1) from FR-rich (LD-FR) incandescent lamps (Fig. 1A). These plants were 5 weeks old when exposed to this single LD, and flower buds were visible 2 weeks later. Untreated control plants in SD were still vegetative 7 weeks later (i.e. 3 months after germination). Obligate and precise LD flowering in response to a single LD confirms earlier reports for Arabidopsis after its exposure to an R-rich LD (Corbesier et al., 1996) or to an FR-rich LD (Gocal et al., 2001).
Fig. 1.
A rapid LD flowering response of Arabidopsis, ecotype Columbia. Effect of a single LD on flowering of plants of Columbia (% plants flowering; A). The four daylength treatments imposed here and in further studies are shown schematically in (B). Prior to the single LD exposure, all plants were grown in 8 h SD for 5 weeks. After the LD, they were returned to SDs for daily recording of flowering. At 40 d (75 d from germination) there was little or no flowering of plants in SDs or after a single low intensity R-rich LD. There were 16 replicate plants for flowering assays. The shaded areas show the ‘overnight’ 16 h light or dark exposure.
In Fig. 1B the conditions for a single cycle floral induction by an R- (LD-R) or FR-rich LD (LD-FR) are shown schematically along with the SD control. Plants exposed to a low intensity (10 μmol m−2 s−1) R-rich LD (LD-lR) were essentially vegetative (Fig. 1A), and this provided an additional ‘control’ treatment.
Based on published light response curves for photosynthesis of Arabidopsis (Walters et al., 1999), only the higher intensity R-rich LD would contribute photosynthetically. Thus, the restricted flowering in the R-rich low light LD-lR treatment (Fig. 1A) could suggest photosynthetic regulation of flowering in a high light intensity LD. The spectral contrast between flowering at a low light intensity after exposure to a single FR-rich LD but not after an R-rich LD, indicates an additional LD light response and one which, for these incandescent lamps, would involve PHYB, as shown previously (Bagnall and King, 2001).
Referencing time of the day to the time from the daily light-on signal (hour 0) is consistent with other studies. However, the response to a single LD cycle is sometimes referred back to the end of the 8 h SD as this adds focus to the rapidity of the response to the LD.
Following the single LD there was a rapid response at the shoot tip; its expression of SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 (SOC1) began to increase by hour 20 (Supplementary Fig. S3 at JXB online) and the size of the apex had increased dramatically by hour 24–48 (Gocal et al., 2001). Based on this evidence of early change at the shoot tip/apex, the subsequent analysis of the timing of leaf blade gene expression has focused on changes during the first LD.
An FT mutant blocks LD flowering
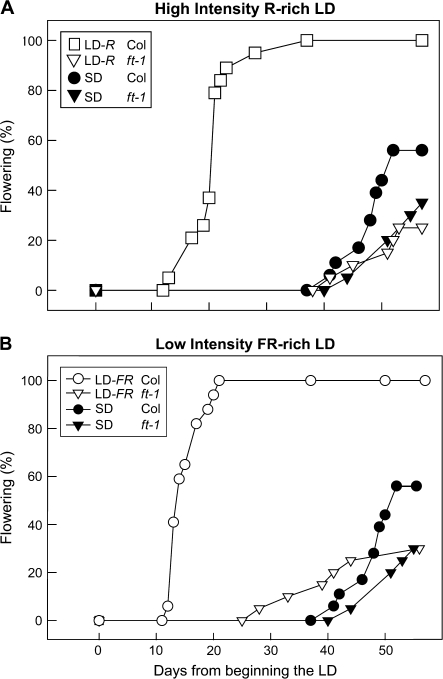
Flowering of Arabidopsis is inhibited in ft mutants (Koorneef et al., 1991; Kardailsky et al., 1999; Kobayashi et al., 1999; Yoo et al., 2005) and, here, ft-1 in Columbia completely inhibited the flowering of plants exposed to high intensity R-rich LD (Fig. 2). The point mutation in ft-1 is near to the C-terminal group (Kardailsky et al., 1999) and there is detectable mRNA production (Yoo et al., 2005); nevertheless, its protein product is apparently sufficiently defective for ft-1 to block LD flowering.
Fig. 2.
The ft-1 mutant blocks LD flowering. Plants of Columbia or ft-1 were held in SD (filled symbols) or exposed to five LDs (open symbols) from high intensity R-rich fluorescent lamps (A) or from low intensity FR-rich lamps (B). These daylength treatments are described in Fig. 1.
The lack of effect of ft-1 on the late onset of flowering in SDs not only shows the specificity of FT for LDs but, more importantly, that FT accounts for all the flowering response to high intensity R-rich LD (LD-R). In contrast, flowering in FR-rich LD (LD-FR) was inhibited in ft-1 (Fig. 2B) but it was not blocked completely, and the same result was found in a repeat experiment with ft-1 backcrossed eight times into Columbia (not shown). Apparently, floral signalling in an R-rich LD involves FT alone but in an FR-rich LD there may be an additional signalling component, and this possibility is examined in the companion paper (Hisamatsu and King, 2008).
CO regulates FT (Valverde et al., 2004; see review in Turck et al., 2008) and it was found that a co mutant in Columbia delayed flowering in LD-R and LD-FR (Supplementary Fig. S4 at JXB online) and blocked FT expression (not shown). However, this co mutant was not as effective as ft-1.
LDs and FT expression
Within 4–8 h of commencing a single florally inductive LD (hour 12–16), FT expression in the leaf blade increased rapidly and dramatically (Fig. 3A, B). Normalization to the highest value emphasizes the timing of the LD increase in FT in any one LD light exposure. When all four daylength conditions were included in a repeat experiment (Fig. 3C), their normalization to the FR-rich LD allows comparison across light treatments. An early and substantial increase in FT expression was only evident in the two florally effective LDs (LD-R and LD-FR). There was little or no increase in SDs or in a low intensity R-rich LD (LD-lR). Thus, as for flowering (Fig. 1), there is separate spectral and light intensity specificity for LD up-regulation of FT expression. There is no obvious explanation for the delayed response of FT to an FR-rich LD relative to an R-rich LD.
Fig. 3.
An LD rapidly up-regulates FT expression. FT expression was assayed in the leaf blade over the first day of LD exposure and during a repeat of this LD. Compared with the SD control, both a high light R-rich LD (A) and an FR-rich LD (B) dramatically increased FT expression. In a repeat experiment, shown in (C), FT expression was assayed for all four daylength treatments described in Fig. 1. The dashed lines and schematic underneath the figures show the daily timing of light and of dark. Gene expression is normalized to the maximum value taken as 1.0. All values are means ±SE (n=3). The error bars are generally not evident because they were smaller than the symbols.
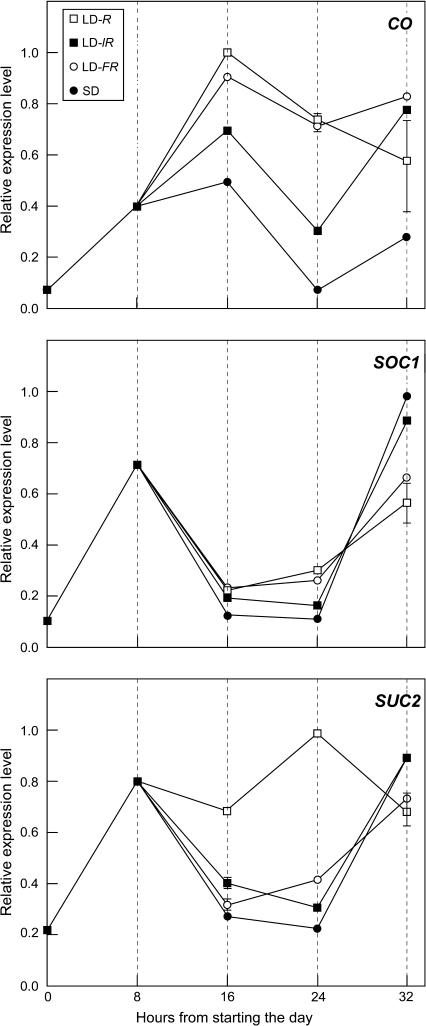
In parallel with increase in FT expression, a florally effective R- or FR-rich LD increased CO expression (Fig. 4). In a low intensity R light LD (LD-lR) there was some increase in CO expression, but this was apparently not sufficient to affect FT expression which was weak (Fig. 3C) and matched by poor or nil flowering (Fig. 1A). A repeat experiment with more frequent sampling confirmed the FR-rich LD increase in CO expression (Supplementary Fig. S5 at JXB online).
Fig. 4.
Effects of LD on leaf blade gene expression. Expression in the leaf blade is shown for three genes CO, SOC1, and SUC2. FT expression in this experiment is shown in Fig. 3C. Other conditions were as for Figs 1 and 3.
None of the LD treatments increased SOC1 expression in the leaf blade relative to plants in SD (Fig. 4), which confirms the findings of Wigge et al. (2005). In contrast, LDs increased expression of SUC2, a photosynthetically regulated gene, but only in a high light, R-rich LD (Fig. 4). This finding emphasizes the potential for a photosynthetic input in this high light LD.
Taken together, these studies (Figs 1–3) show that activation of FT in LDs accounts for flowering. In addition, they highlight inputs by two photoresponses, a low intensity (non-photosynthetic) response in a low light FR-rich LD and a photosynthetic input in a high intensity R-rich LD.
Photosynthetic regulation of FT and flowering
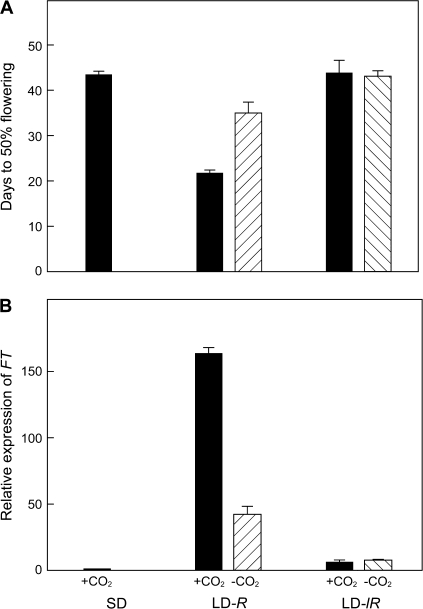
Photosynthetic regulation of FT in a high intensity R-rich LD is implied by the evidence that flowering and FT expression were both restricted by a 10-fold reduction in the LD light intensity (Figs 1, 3). To confirm this role for photosynthesis, plants were exposed to CO2-free air to block photosynthesis during the 16 h high light LD exposure (LD-R). Flowering was delayed by the removal of atmospheric CO2 for the single 16 h ‘overnight’ period of high light intensity (Fig. 5A) and, in parallel, the normal FT increase was restricted (Fig. 5B). The same treatment had no effect on plants exposed to this LD at a 10-fold lower intensity (LD-lR); their flowering was late and FT expression was low (Fig. 5). Two further studies confirmed the strong delay of flowering when CO2-free conditions were imposed for the entire period of a two LD high light period or for the 16 h ‘overnight’ periods of two high light LDs (not shown).
Fig. 5.
Blocking photosynthesis during an LD can inhibit flowering and FT expression. Flowering (A) and FT expression (B) are compared following an exposure to normal (solid bar) or CO2-free air (hatched bar) during a 16 h high light LD (LD-R, 100 μmol m−2 s−1) or to an LD at a 10-fold lower intensity from the same fluorescent lamps (LD-lR, 10 μmol m−2 s−1).
A role for photosynthesis in a high light LD is also supported by the measurements of sucrose content of the leaf and shoot apex. At a high light intensity, the LD caused an early doubling of leaf blade sucrose (<8 h after starting the LD, Fig. 6A), but there was little or no increase for plants in 16 h of darkness (SD) or exposed to a low intensity LD (LD-lR or LD-FR). At the shoot apex, sucrose increased in parallel with its increase in the LD leaf (Fig. 6B, C). Furthermore, exposure to CO2-free air during a 16 h high light LD extension blocked sucrose increase in the leaf blade and shoot apex (Table 1).
Fig. 6.
Sucrose content of the leaf and shoot apex increases within hours in a high intensity R-rich LD from fluorescent lamps. Daily change in sucrose content of the leaf (A); or shoot apex (B, C) for plants in SD or exposed to an LD at a high intensity (LD-R; 100 μmol m−2 s−1), at a low intensity (LD-lR; 10 μmol m−2 s−1), or to an FR-rich LD (LD-FR; 10 μmol m−2 s−1). The hatched bars indicate the timing of exposure to the various LD light extensions, the white bars indicate the daily 8 h light period of the SD, and the black bars indicate darkness. Each value is the mean of five replicates. Errors are shown as the LSD (P?=0.05) for all the data in each experiment. Other conditions are as for Fig. 1.
Table 1.
Effect of daylength and photosynthetic input on leaf and shoot apex sucrose content (mg g−1 dry weight)
| SD | LD-R |
||
| Normal air | CO2-free air | ||
| Columbia leaf | 1.7±0.1 | 4.1±0.1 | 1.6±0.2 |
| co mutant leaf | 1.9±0.1 | 5.2±0.4 | |
| co mutant shoot apex | 3.6±0.5 | 5.7±0.7 | 0.8±0.2 |
An 8 h SD light period was followed by a 16 h overnight dark period (SD) or a 16 h LD exposure to light from fluorescent lamps (LD-R) at a light intensity of 100 μmol m−2 s−1 in either normal or CO2-free air. The values are means and SE of five replicate assays for apices and three replicates for the leaf for the Columbia ecotype of Arabidopsis and a co mutant of Columbia.
LD increases in expression in the leaf blade of photosynthetically regulated genes (Fig. 4; Supplementary Fig. S6 at JXB online) also support this claim of a photosynthetic role for a high light LD. For example, up-regulation of SUC2, a gene which regulates sucrose transport, was only evident in a high light LD (Fig. 4). Comparable high light LD increases are shown in Supplementary Fig. S6 at JXB online, not only for SUC2, but also for genes involved in sucrose accumulation (SUS1), its perception (AKIN1 a SNF1-like kinase), carbon interconversions (e.g. ADPG and INVERTASE), and other aspects of photosynthetic carbon fixation (SUC3).
In addition to the finding that ft-1 completely blocked the high light LD response (Fig. 2), there was no flowering when only sucrose was allowed to increase. First, when co was used to block the FT increase, a high light LD was ineffective for flowering but the LD still increased the sucrose content of the leaf and shoot apex (Table 1). Secondly, in SD with its low FT expression (Fig. 3), Columbia did not flower when the light intensity in a single SD was increased to 360 μmol m−2 s −1 (not shown). Assuming a linear increase in photosynthesis with light intensity (Walters et al., 1999), the daily photosynthetic gain in an 8 h SD at a light intensity of 360 μmol m−2 s−1 exceeds that for a 24 h LD exposure at 100 μmol m−2 s−1.
Taken together, these findings imply that photosynthesis in the LD leaf regulates flowering by up-regulating FT expression. Secondly, because increasing photosynthesis for one SD was not sufficient to trigger flowering, LD specification to allow photosynthetic up-regulation of FT must be signalled via an additional photoresponse..
Can photosynthetic sucrose act directly in SD floral induction?
When grown in SD, Arabidopsis eventually flowers (>7 weeks later Fig. 2) and there is an associated increase in shoot tip sucrose (Ericksson et al., 2006). Here, 5-week-old plants of Columbia flowered rapidly in 8 h SDs (2–3 weeks) when the light intensity was increased for 12 d to 270 μmol m−2 s−1 (Fig. 7). These findings were confirmed in a repeat experiment involving a 12 d exposure in SD at 360 μmol m−2 s−1 (not shown). A single high light SD exposure did not induce flowering (as noted above) and SD plants held at 100 μmol m−2 s−1 were still vegetative when the experiment was terminated at 42 d (Fig. 7). Furthermore, SD flowering was independent of FT because high light stimulated flowering equally well for ft-1 and Columbia (Fig. 7).
Compared with the FT-dependent flowering response to a single high light LD, the FT-independent SD response required more cycles of high light (12 d) and the response was slower (e.g. 20.0 ± 1.4 d to flower in Fig. 1 versus 29.0 ± 1.4 d in Fig. 7). Such differences strengthen the claim that high light in LD acts via FT as a dominant LD floral signal.
Discussion
Information on LD photoresponse(s) in the leaf blade is essential for any understanding of floral signals transported from the leaf to the shoot apex. Here, with Arabidopsis, its rapid and obligate flowering after a single LD has allowed identification of two LD photoresponses which act by up-regulating FT expression. Phytochrome is effective in an FR-rich LD at a low light intensity from incandescent lamps. In contrast, in a high light intensity LD from R-rich fluorescent lamps, photosynthesis is important and this novel observation has implications for studies with mutants and for understanding the role of FT in natural conditions.
There are at least two photoresponses controlling FT and flowering in LDs
Published studies of LD up-regulation of FT expression have introduced roles for both R and FR light but, sometimes, without resolving which photoreceptors were active. However, when light action involves phytochrome, it is only FR light which enhances FT expression (Cerdän and Chory, 2003; Halliday et al., 2003; Valverde et al., 2004) and flowering (Goto et al., 1991; Reed et al., 1994; Bagnall and King, 2001, and references therein). Here, use of an additional non-florally inductive, low intensity R-rich LD separates the FR-rich phytochrome response from an intensity-dependent, R-rich photosynthetic input. The evidence provided of contributions by two photoresponses resolves the paradox that both R and FR light promote FT expression and flowering.
All the response to R-light LD is blocked in the ft-1 mutant but, interestingly, flowering is not blocked completely by ft-1 in a low intensity FR-rich LD (Fig. 2B). This result implies additional, albeit weak, signalling in an FR-rich LD. Gene redundancy involving the FT-related gene, TSF, seems unlikely. The tsf mutant delays flowering (Yamaguchi et al., 2005), but this would not explain the difference in response to ft-1 between an FR- and an R-rich LD (Fig. 2). An alternative involves the known phytochrome up-regulation of gibberellin (GA) biosynthesis in shoots exposed to an FR-rich LD (Hisamatsu et al., 2005), and this explanation is examined in the companion paper (Hisamatsu and King, 2008).
Photosynthetic regulation of FT and flowering in LDs
In a leaf exposed to a high light, R-rich LD (LD-R), photosynthesis is important for FT-dependent floral signalling because: (i) this LD rapidly increases FT expression in the leaf blade (in 4–8 h; hours 12–16); (ii) ft-1 completely blocks this LD-R flowering; (iii) this LD increases leaf sucrose and expression of genes regulating sucrose synthesis; (iv) exposure to this LD in CO2-free air restricts increases in both FT and sucrose and delays flowering; and (v) a 10-fold reduction in light intensity during this LD restricts FT expression, prevents any sucrose increase, and delays flowering.
Support for a florigenic effect of photosynthetically derived sucrose acting in the leaf via FT is provided by the failure of applied sucrose to reverse late flowering of ft-1 despite its effectiveness with five other late flowering mutants (Roldán et al., 1999). Nevertheless, how sucrose might act to up-regulate FT is unclear in the study of Roldán et al. (1999). They grew their plants in total darkness, so it is likely that sucrose was acting as an energy source. Similarly, the action of sucrose as an energy source for FT expression fits with applied sucrose reversing late flowering in lines with restricted carbon metabolism (Yu et al., 2000; see review in Bernier and Perilleux, 2005).
It is not clear why an increase in photosynthesis regulates FT expression in one LD but not in one SD at a much higher light intensity. Speculatively, in LDs, there must be an additional photoresponse potentiating the FT response which is then amplified by photosynthetic input. Blue light acting via the cryptochromes in a fluorescent light LD might determine daylength specificity, although it would not be part of the response to light intensity because there is little or no intensity dependence for blue light activation of CO expression and, it is assumed, of FT (Imaizumi et al., 2003). A more complex alternative which allows for ‘gating’ of the FT response by an endogenous rhythm introduces R light regulation of the rhythm phase, as has been reported for a number of plant responses including rhythmic CO2 fixation in Lemna perpusilla Torr.(Hillman, 1971) and for a flowering rhythm in the SD plant Chenopodium rubrum L. (King and Cumming, 1972).
The possibility of parallel inputs to FT by photosynthesis, by FR-rich light, and by a potential third blue light input introduces unexpected complexity, particularly to studies with mutants. For example, delayed flowering of a Columbia phyA mutant could be the result of reduced photosynthetic input to FT since this mutant has half the wild-type leaf area and, in addition, a reduced photosynthetic pigment content (Walters et al., 1999; Bagnall and King, 2001). Consistent with a photosynthetic effect in phyA, higher light intensities reverse its late flowering. Furthermore, in the same studies, phyA did not delay flowering in non-photosynthetic, low light, FR-rich LD conditions (Bagnall and King, 2001). A more complex model may also be required to explain the effects of GIGANTEA (GI) on flowering. It acts as an upstream regulator of CO and FT via a link to a circadian rhythm (Mizoguchi et al., 2005) but also regulates sucrose interconversion to starch (Eimert et al., 1995), and its protein interacts with SPINDLY (SPY) to modulate GA actions on flowering (Tseng et al., 2004).
FT-independent flowering in high light SDs
Arabidopsis flowers early when a cell wall invertase is overexpressed near the shoot apex to enhance sucrose unloading there (Heyer et al., 2004). Also, sucrose increases at the shoot apex when Arabidopsis flowers in a photosynthetic LD (Fig. 6, and see Corbesier et al., 1998) and, in an SD, there is a dramatic increase in shoot tip sucrose content when Columbia eventually flowers (Ericksson et al., 2006). Direct regulation of flowering by transported sucrose is also favoured by the evidence presented of FT-independent flowering after 12 d exposure to a high light intensity in SDs (Fig. 7). Potentially, such promotion of flowering by sucrose would involve activation of LEAFY at the shoot apex, a possibility raised by Blázquez et al. (1998) from their studies of sucrose/GA-regulated increases in LEAFY::GUS expression.
On the other hand, FT-independent flowering in SDs is far weaker than FT-dependent flowering in a single LD. SD flowering required many more cycles of high light (12 cycles at 270 μmol m−2 s−1) and with a far greater light integral (Fig. 1, versus Fig. 7), as also reported previously for two other LD plants, Sinapis alba L. (Bodson et al., 1977) and Fuchsia hybrida (King and Ben-Tal, 2001). A simple photosynthetic regulation is unlikely, especially because when the co mutant was used to block FT increase and flowering (Supplementary Fig. S4 at JXB online), shoot apex sucrose still increased (Table 1). Perhaps, if maintained for 12 d this same sucrose increase would be sufficient for flowering, but there does not appear to be any evidence to support or deny such a speculation.
FT regulation in natural conditions
There have been no studies of flowering of Arabidopsis which combine the effects of seasonal light intensity and daylength. However, in the LD species L. temulentum there is strong additivity between photosynthetic input and the LD response (King and Evans, 1991). Thus, in late spring and summer, ‘photosynthetic’ amplification could become important for LD, FT-regulated flowering but photosynthesis may not be as dominant as the direct FT response to daylength, stop. This claim is consistent with the quantitative relationship to expression of an FT homologue associated with latitudinal adaptation for autumn bud set in Populus tricocarpa (Böhlenius et al., 2006).
In SD species, seasonal photosynthetic differences should be less relevant as FT expression and flowering increase in response to exposure to a prolonged daily dark period, as in rice (Izawa et al., 2002) and Pharbitis nil Chois (Hayama et al., 2007). Interestingly, an SD dark period leads to loss of spectrophotometrically detectable PHY Pfr in P. nil after 1–2 h (King et al., 1978). Thus, a reduction in the level of Pfr, either in darkness in SD species or with an FR exposure in LD species, leads to FT up-regulation. There is complexity yet to be explained for P. nil where FT expression is not related simply to that of CO (Hayama et al., 2007). It is also unclear why an SD plant does not up-regulate FT in LD, and vice versa for an LD response type.
Supplementary material
Supplementary Figures S1–S6 may be found at JXB online.
Supplementary Material
Acknowledgments
Drs George Coupland and Iain Searle (MPI, Koeln, Germany) are thanked for providing seed of and information on the Columbia co and ft mutants. Drs Liz Dennis, Lloyd Evans, and Masumi Robertson (CSIRO) provided valuable comment on the manuscript.
Glossary
Abbreviations
- CO
CONSTANS
- FR
far red light
- FT
FLOWERING LOCUS T
- GA
gibberellin
- LD
long day conditions
- R
red light
- SD
short day conditions
References
- Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science. 2005;309:1052–1056. doi: 10.1126/science.1115983. [DOI] [PubMed] [Google Scholar]
- An HL, Roussot C, Suarez-Lopèz P, Corbesier L, Vincent C, Pineiro M, Hepworth S, Mouradov A, Justin S, Turnbull C, Coupland G. Constans acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development. 2004;131:3615–3626. doi: 10.1242/dev.01231. [DOI] [PubMed] [Google Scholar]
- Bagnall DJ, King RW. Phytochrome and flowering of Arabidopsis thaliana: photophysiological studies using mutants and transgenic lines. Australian Journal of Plant Physiology. 2001;28:401–408. [Google Scholar]
- Bernier G, Perilleux C. A physiological overview of the genetics of flowering time control. Plant Biotechnology Journal. 2005;3:3–16. doi: 10.1111/j.1467-7652.2004.00114.x. [DOI] [PubMed] [Google Scholar]
- Blázquez MA, Green R, Nilsson O, Sussman MR, Weigel D. Gibberellins promote flowering of Arabidopsis by activating the LEAFY promoter. The Plant Cell. 1998;10:791–800. doi: 10.1105/tpc.10.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodson M, King RW, Evans LT, Bernier G. Role of photosynthesis in flowering of the long-day plant Sinapis alba. Australian Journal of Plant Physiology. 1977;4:467–478. [Google Scholar]
- Böhlenius H, Huang T, Charbonell-Campaa L, Brunner AN, Jansson S, Strauss S, Nielsson O. CO/FT regulatory modules control timing of flowering and seasonal growth cessation in trees. Science. 2006;312:1040–1043. doi: 10.1126/science.1126038. [DOI] [PubMed] [Google Scholar]
- Boss PK, Bastow RM, Mylne JS, Dean C. Multiple pathways in the decision to flower: enabling, promoting, and resetting. The Plant Cell. 2004;16:S18–S31. doi: 10.1105/tpc.015958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdän PD, Chory J. Regulation of flowering time by light quality. Nature. 2003;423:881–885. doi: 10.1038/nature01636. [DOI] [PubMed] [Google Scholar]
- Corbesier L, Gadisseur I, Silvestre G, Jacqmard A, Bernier G. Design in Arabidopsis thaliana of a synchronous system of floral induction by one long day. The Plant Journal. 1996;9:947–952. doi: 10.1046/j.1365-313x.1996.9060947.x. [DOI] [PubMed] [Google Scholar]
- Corbesier L, Lejeune P, Bernier G. The role of carbohydrates in the induction of flowering in Arabidopsis thaliana—comparison between the wild type and a starchless mutant. Planta. 1998;206:131–137. doi: 10.1007/s004250050383. [DOI] [PubMed] [Google Scholar]
- Corbesier L, Vincent C, Jang SH, et al. FT protein movement contributes to long-distance signalling in floral induction of Arabidopsis. Science. 2007;316:1030–1033. doi: 10.1126/science.1141752. [DOI] [PubMed] [Google Scholar]
- Eimert K, Wang SM, Lue WI, Chen J. Monogenic recessive mutations causing both late floral initiation and excess starch accumulation in Arabidopsis. The Plant Cell. 1995;7:1703–1712. doi: 10.1105/tpc.7.10.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M, Mochizuki N, Susuki T, Nagatini A. CRYPTOCHROME2 in vascular bundles regulates flowering in Arabidopsis. The Plant Cell. 2007;19:84–93. doi: 10.1105/tpc.106.048157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson S, Bohlenius H, Moritz T, Nilsson O. GA4 is the active gibberellin in the regulation of LEAFY transcription and Arabidopsis floral initiation. The Plant Cell. 2006;18:2172–2181. doi: 10.1105/tpc.106.042317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocal GFW, Sheldon C, Gubler F, Moritz T, Bagnall D, Song FL, Parish RW, Dennis ES, Weigel D, King RW. GAMYB-like genes and gibberellin signalling in Arabidopsis. Plant Physiology. 2001;127:1682–1693. [PMC free article] [PubMed] [Google Scholar]
- Goto N, Kumagi T, Koornneef M. Flowering responses to light breaks in photomorphogenic mutants of Arabidopsis thaliana, a long day plant. Physiologia Plantarum. 1991;83:209–215. [Google Scholar]
- Hayama R, Agashe B, Luley E, King R, Coupland G. A circadian rhythm set by dusk controls the expression of FT; homologues and short day photoperiodic flowering response in Pharbitis. The Plant Cell. 2007;19:2988–3000. doi: 10.1105/tpc.107.052480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday KJ, Salter MG, Thingnaes E, Whitelam GC. Phytochrome control of flowering is temperature sensitive and correlates with expression of the floral integrator FT. The Plant Journal. 2003;33:875–885. doi: 10.1046/j.1365-313x.2003.01674.x. [DOI] [PubMed] [Google Scholar]
- Heyer AG, Raap M, Schroeer B, Marty B, Willmitzer L. Cell wall invertase expression at the apical meristem alters floral, architectural, and reproductive traits in Arabidopsis thaliana. The Plant Journal. 2004;39:161–169. doi: 10.1111/j.1365-313X.2004.02124.x. [DOI] [PubMed] [Google Scholar]
- Hillman WS. Entrainment of Lemna CO2 output through phytochrome. Plant Physiology. 1971;48:770–774. doi: 10.1104/pp.48.6.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisamatsu T, King RW, Helliwell CA, Koshioka M. The involvement of gibberellin 20-oxidase genes in phytochrome-regulated petiole elongation of Arabidopsis. Plant Physiology. 2005;138:1106–1116. doi: 10.1104/pp.104.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi T, Tran HG, Swartz TE, Briggs WR, Kay SA. FKF1 is essential for photoperiodic-specific light signalling in Arabidopsis. Nature. 2003;426:302–306. doi: 10.1038/nature02090. [DOI] [PubMed] [Google Scholar]
- Imaizumi T, Kay SA. Photoperiodic control of flowering not only by coincidence. Trends in Plant Science. 2006;11:550–558. doi: 10.1016/j.tplants.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Izawa T, Oikawa T, Sugiyama N, Tanisaka T, Yano M, Shimamoto K. Phytochrome mediates the external light signal to repress FT orthologs in photoperiodic flowering of rice. Genes and Development. 2002;16:2006–2020. doi: 10.1101/gad.999202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger KE, Wigge PA. FT protein acts as a long-range signal in Arabidopsis. Current Biology. 2007;17:1050–1054. doi: 10.1016/j.cub.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Kardailsky I, Shukla VK, Ahn JH, Dagenais N, Christensen SK, Nguyen JT, Chory J, Harrison MJ, Weigel D. Activation tagging of the floral inducer FT. Science. 1999;286:1962–1965. doi: 10.1126/science.286.5446.1962. [DOI] [PubMed] [Google Scholar]
- King RW, Ben-Tal Y. A florigenic effect of sucrose in Fuchsia and its inhibition by gibberellin-induced assimilate competition. Plant Physiology. 2001;125:1–9. doi: 10.1104/pp.125.1.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RW, Cumming BG. Role of phytochrome in photoperiodic time measurement and its relation to rhythmic time keeping in the control of flowering in Chenopodium rubrum. Planta. 1972;108:39–57. doi: 10.1007/BF00386505. [DOI] [PubMed] [Google Scholar]
- King RW, Evans LT. Shoot apex sugars in relation to long-day induction of flowering in Lolium temulentum L. Australian Journal of Plant Physiology. 1991;18:121–135. [PMC free article] [PubMed] [Google Scholar]
- King RW, Vince-Prue D, Quail PH. Light requirement, phytochrome and photoperiodic induction of flowering of Pharbitis nil Chois. III. A comparison of spectrophotometric and physiological assay of phytochrome transformation during induction. Planta. 1978;141:15–22. doi: 10.1007/BF00387738. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Weigel D. Move on up, it's time for change—mobile signals controlling photoperiodic-dependent flowering. Genes and Development. 2007;21:2371–2384. doi: 10.1101/gad.1589007. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T. A pair of related genes with antagonistic roles in mediating flowering signals. Science. 1999;286:1960–1962. doi: 10.1126/science.286.5446.1960. [DOI] [PubMed] [Google Scholar]
- Koornneef M, Hanhart CJ, van der Veen JH. A genetic and physiological analysis of flowering mutants in Arabidopsis thaliana. Molecular and General Genetics. 1991;229:57–66. doi: 10.1007/BF00264213. [DOI] [PubMed] [Google Scholar]
- Laubinger S, Marchal V, Gen J, Wenkel S, Adrian J, Jang S-h, Kulajta1 C, Braun H, Coupland G, Hoecker U. Arabidopsis SPA proteins regulate photoperiodic flowering and interact with the floral inducer CONSTANS to regulate its stability. Development. 2006;133:3213–3222. doi: 10.1242/dev.02481. [DOI] [PubMed] [Google Scholar]
- Lin MK, Belanger H, Lee YJ, et al. FLOWERING LOCUS T protein may act as the long-distance florigenic signal in the Cucurbits. The Plant Cell. 2007;19:488–1506. doi: 10.1105/tpc.107.051920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu J, Warthmann N, Kuttner F, Schmid M. Export of FT protein from phloem companion cells is sufficient for floral induction in Arabidopsis. Current Biology. 2007;17:1055–1060. doi: 10.1016/j.cub.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Mizoguchi T, Wright L, Fujiwara S, et al. Distinct roles of GIGANTEA in promoting flowering and regulating circadian rhythms in Arabidopsis. The Plant Cell. 2005;17:2255–2270. doi: 10.1105/tpc.105.033464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putterill J, Robson F, Lee K, Simon R, Coupland G. The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell. 1995;80:847–857. doi: 10.1016/0092-8674(95)90288-0. [DOI] [PubMed] [Google Scholar]
- Reed JW, Nagatini A, Elich TD, Fagan M, Chory J. Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiology. 1994;104:1139–1149. doi: 10.1104/pp.104.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roldán M, Gomez-Mena C, Ruiz-Garcia L, Salinas J, Martinez-Zapater JM. Sucrose availability on the aerial part of the plant promotes morphogenesis and flowering of Arabidopsis in the dark. The Plant Journal. 1999;20:581–590. doi: 10.1046/j.1365-313x.1999.00632.x. [DOI] [PubMed] [Google Scholar]
- Searle I, Coupland G. Induction of flowering by seasonal changes in photoperiod. EMBO Journal. 2004;23:1217–1222. doi: 10.1038/sj.emboj.7600117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. Light quality, photoperception, and plant strategy. Annual Review of Plant Physiology. 1982;33:481–518. [Google Scholar]
- Suarez-Lopez P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G. CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature. 2001;410:1116–1120. doi: 10.1038/35074138. [DOI] [PubMed] [Google Scholar]
- Takada S, Goto K. TERMINAL FLOWER 2, an Arabidopsis homolog of heterochromatin protein1, counteracts the activation of FLOWERING LOCUS T by CONSTANS in the vascular tissues of leaves to regulate flowering time. The Plant Cell. 2003;15:2856–2865. doi: 10.1105/tpc.016345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K. Hd3a protein is a mobile flowering signal in rice. Science. 2007;316:1033–1036. doi: 10.1126/science.1141753. [DOI] [PubMed] [Google Scholar]
- Tseng T-S, Salome PA, McClung CR, Olszewski NE. SPINDLY and GIGANTEA interact and act in Arabidopsis thaliana pathways involved in light responses, flowering, and rhythms in cotyledon movements. The Plant Cell. 2004;16:1550–1563. doi: 10.1105/tpc.019224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turck F, Formara F, Coupland G. Regulation and identity of florigen: FLOWERING LOCUS T moves centre stage. Annual Reviews of Plant Biology. 2008;59:573–594. doi: 10.1146/annurev.arplant.59.032607.092755. [DOI] [PubMed] [Google Scholar]
- Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, Coupland G. Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science. 2004;303:1003–1006. doi: 10.1126/science.1091761. [DOI] [PubMed] [Google Scholar]
- Walters RG, Rogers JJM, Shephard F, Horton P. Acclimation of Arabidopsis thaliana to the light environment: the role of photoreceptors. Planta. 1999;209:517–527. doi: 10.1007/s004250050756. [DOI] [PubMed] [Google Scholar]
- Wigge PA, Kim MC, Jaeger KE, Busch W, Schmid M, Lohmann JU, Weigel D. Integration of spatial and temporal information during floral induction in Arabidopsis. Science. 2005;309:1056–1059. doi: 10.1126/science.1114358. [DOI] [PubMed] [Google Scholar]
- Yamaguchi A, Kobayashi Y, Goto K, Abe M, Araki T. TWIN SISTER OF FT (TSF) acts as a floral pathway integrator redundantly with FT. Plant and Cell Physiology. 2005;46:1175–1189. doi: 10.1093/pcp/pci151. [DOI] [PubMed] [Google Scholar]
- Yoo SK, Chung KS, Kim J, Lee JH, Hong SM, Yoo SJ, Yoo SY, Lee JS, Ahn JH. CONSTANS activates SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 through FLOWERING LOCUS T to promote flowering in Arabidopsis. Plant Physiology. 2005;139:770–778. doi: 10.1104/pp.105.066928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu TS, Lue WL, Wang SM, Chen J. Mutation of Arabidopsis plastid phosphoglucose isomerase affects leaf starch synthesis and floral initiation. Plant Physiology. 2000;123:319–325. doi: 10.1104/pp.123.1.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.