Abstract
Enteric pathogens have developed several resistance mechanisms to survive the antimicrobial action of bile. We investigated the transcriptional profile of Vibrio cholerae O1 El Tor strain C6706 under virulence gene-inducing conditions in the presence and absence of bile. Microarray analysis revealed that the expression of 119 genes was affected by bile. The mRNA levels of genes encoding proteins involved in transport were increased in the presence of bile, whereas the mRNA levels of genes encoding proteins involved in pathogenesis and chemotaxis were decreased. This study identified genes encoding transcriptional regulators from the TetR family (vexR and breR) and multidrug efflux pumps from the resistance-nodulation-cell division superfamily (vexB and vexD [herein renamed breB]) that were induced in response to bile. Further analysis regarding vexAB and breAB expression in the presence of various antimicrobial compounds established that vexAB was induced in the presence of bile, sodium dodecyl sulfate, or novobiocin and that the induction of breAB was specific to bile. BreR is a direct repressor of the breAB promoter and is able to regulate its own expression, as demonstrated by transcriptional and electrophoretic mobility shift assays (EMSA). The expression of breR and breAB is induced in the presence of the bile salts cholate, deoxycholate, and chenodeoxycholate, and EMSA showed that deoxycholate is able to abolish the formation of BreR-PbreR complexes. We propose that deoxycholate is able to interact with BreR and induce a conformational change that interferes with the DNA binding ability of BreR, resulting in breAB and breR expression. These results provide new insight into a transcriptional regulator and a transport system that likely play essential roles in the ability of V. cholerae to resist the action of bile in the host.
Pathogenic bacteria continuously monitor environmental parameters (pH, osmolarity, and temperature) to adapt to their host and regulate virulence gene expression. The human gastrointestinal tract can be considered to be an extreme environment for enteric bacteria where they need to adjust to stressful conditions, including exposure to bile (2). Bile is a hepatic secretion that is stored in the gallbladder and is released into the duodenum. It is composed of bile salts, cholesterol, phospholipids, antibodies, pigments, and metals, among other components (20). Bile has the essential role of emulsifying and absorbing lipids from the diet. Moreover, bile aids in the elimination of toxins and the excretion of metabolic products and has bactericidal properties (20).
Enteric pathogens have developed several resistance mechanisms to survive the antimicrobial action of bile (2, 14). The main mechanism of bile resistance in gram-negative bacteria is mediated by the expression of multidrug resistance (MDR) efflux pumps that actively extrude bile out of the cell (14). MDR transporters belonging to the resistance-nodulation-cell division (RND) superfamily are distinct from other transporters because they transport a broad variety of compounds, such as antibiotics, dyes, and detergents, out of the cell (30). They are localized in the inner membrane and associate with a periplasmic membrane fusion protein (MFP) and an outer membrane protein to generate a three-component multidrug efflux system spanning the cytoplasmic and outer membranes that can pump toxic compounds out of the cell (30). Pioneering studies with Escherichia coli have shown that AcrA (an MFP), AcrB (an RND pump), and TolC (an outer membrane protein) make up one such three-component multidrug efflux system that can pump different substrates such as novobiocin, erythromycin, sodium dodecyl sulfate (SDS), cholate, taurodeoxycholate, and decanoate out of the cell (10, 36, 52). The AcrAB-like efflux system of Salmonella enterica serovar Typhimurium (29) and the CmeABC efflux system of Campylobacter jejuni are other known three-component systems for which bile salts, among other compounds, are substrates (33, 34). Since MDR transporters have broad specificities and use proton motive force, their overproduction can cause the excretion of intrinsic metabolites and the loss of membrane potential, processes that would be detrimental to the survival of the bacterial cell (13, 30). Therefore, the expression of the majority of the MDR transporters is tightly controlled (13). The TetR family members AcrR and CmeR, for example, are the transcriptional repressors of acrAB and cmeABC, respectively (31, 35).
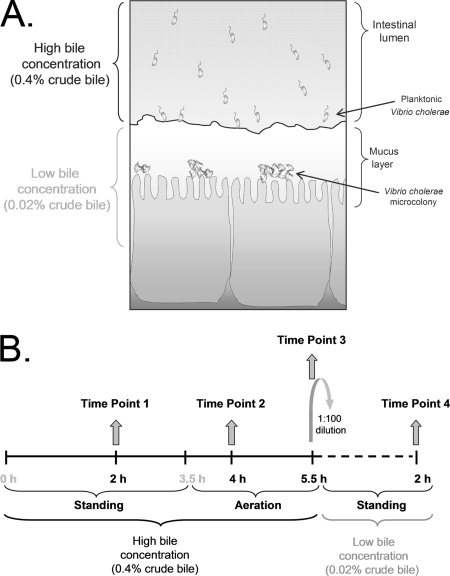
Vibrio cholerae is a gram-negative, curved, rod-shaped enteric bacterium that is the causative agent of the severe diarrheal disease cholera. This pathogen has developed several mechanisms to mediate protection against the action of bile: (i) it increases motility in the presence of bile, which is hypothesized to be important for the bacterium to swim away from high concentrations of bile in the lumen, penetrate the mucus layer, and gain access to the underlying epithelial cells for colonization (15, 48) (see Fig. 1A); (ii) it induces the formation of a biofilm, in which the cells are more resistant than nonbiofilm cells to the bactericidal effect of bile (22); (iii) it enhances the expression of ompU (encoding a small-pore porin) and decreases the expression of ompT (encoding a large-pore porin), reducing bile uptake (4, 43, 44); and (iv) it induces the expression of genes that encode proteins involved in efflux, such as AcrA (7) and TolC (3), and efflux pumps, such as VceB (8), VexB, and VexD (4).
FIG. 1.
(A) Model illustrating the effect of bile on V. cholerae colonization based on a hypothesis proposed by Schuhmacher and Klose (48). When V. cholerae is within the lumen of the intestine, the high bile concentration inhibits the transcription of the virulence genes and induces motility and/or chemotaxis to mobilize the bacterium into the mucus layer. Upon migration through the mucus layer, where the bile concentration is low, motility and/or chemotaxis is inhibited and virulence gene expression is induced, facilitating the colonization of the epithelial cells by V. cholerae. (B) Schematic representation of the microarray experimental design.
The regulation of V. cholerae genes encoding efflux system components has been described previously only for the vceCAB operon. This operon is negatively regulated by the protein encoded by vceR, which is located upstream from vceCAB in a divergent orientation (8). The regulators for the vexAB or the vexCD operon, if any, are still unknown.
To further investigate the response of V. cholerae to bile and begin to define the bile regulon, we performed a microarray study to investigate the global response of V. cholerae to crude bile and determined that the expression of 119 genes was affected in the presence of bile. In particular, we report that vexB, vexCD (herein renamed breAB), and two genes encoding regulators belonging to the TetR family were upregulated in the presence of bile. When vexAB and breAB expression in response to different antibiotics and detergents was analyzed, vexAB was induced by exposure to bile, SDS, or novobiocin whereas the induction of breAB expression was specific for bile. Given its specificity, we further characterized the regulatory mechanism of breAB. We identified BreR as the negative regulator of breAB and established that BreR is also able to regulate its own expression. Using electrophoretic mobility shift assays (EMSA), we demonstrated the direct binding of BreR to the breAB and breR promoters. Furthermore, we showed that breAB and breR expression was induced in the presence of cholate, deoxycholate, or chenodeoxycholate and that deoxycholate was able to disrupt BreR binding to the breR promoter. These findings support the hypothesis that bile plays an important role as a host signal for V. cholerae.
MATERIALS AND METHODS
Bacterial strains, plasmids, primers, antimicrobial compounds, and growth conditions.
The V. cholerae and E. coli strains and the plasmids and primers used in this study are listed in Tables 1 and 2. The strains were grown in Luria-Bertani (LB) (38) or AKI (23) medium. Antibiotics (Sigma) were used at the following concentrations (except for induction experiments): ampicillin, 100 μg/ml; kanamycin, 45 μg/ml; polymyxin B, 50 U/ml; streptomycin, 100 μg/ml or 1 mg/ml (for allelic exchange experiments); and tetracycline, 15 μg/ml. For PvexRAB-lacZ, PbreAB-lacZ, and PbreR-lacZ induction experiments, strains were grown in subinhibitory concentrations of erythromycin, polymyxin B, novobiocin, sodium choleate (crude bile), sodium taurodeoxycholate, sodium cholate hydrate, sodium glycochenodeoxycholate, sodium deoxycholate, taurocholic acid sodium salt hydrate, sodium chenodeoxycholate, sodium glycocholate hydrate, sodium glycodeoxycholate, SDS, and Triton X-100 (all from Sigma) as noted below in the description of the β-galactosidase assay methods. Antibiotic stocks were prepared according to the instructions of the antibiotic manufacturer (Sigma), while detergent stocks were prepared fresh in LB medium and filter sterilized. For allelic exchange experiments, LB agar contained 40 μg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal; Gold Biotechnology Inc.)/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or description | Source or reference |
|---|---|---|
| Strains | ||
| V. cholerae | ||
| C6706 str2 | El Tor; Smr | Laboratory collection |
| KSK262 | C6706 str2 ΔlacZ3 | 28 |
| FCM266 | KSK262 PvexRAB-lacZ1 | This work |
| FCM164 | KSK262 PbreAB-lacZ1 (PvexCD-lacZ1) | This work |
| FCM225 | FCM164 ΔvexR1 | This work |
| FCM168 | FCM164 ΔbreR1 | This work |
| FCM226 | FCM164 ΔvexR1 ΔbreR1 | This work |
| FCM211 | FCM168 pFC33 | This work |
| FCM213 | FCM168 pKAS178 | This work |
| FCM158 | KSK262 PbreR-lacZ1 | This work |
| FCM191 | FCM158 ΔbreR1 | This work |
| FCM214 | FCM191 pFC33 | This work |
| FCM216 | FCM191 pKAS178 | This work |
| E. coli | ||
| Origami(DE3) | Δ(ara-leu)7697 araD139 ΔlacX74 galE galK rpsL ΔphoA PvuII phoR F′ [laclacIqpro] gor-522::Tn10 (Tc) trxB::kan (DE3) | Novagen |
| FCM184 | Origami(DE3) pFC25 Tetr Ampr Kanr | This work |
| Plasmids | ||
| pKAS154 | pKAS32 derivative; Kanr | 27 |
| pKAS178 | pBAD33 derivative; Kanr | 24 |
| pGKK344 | ΔlacZ3 in pKAS154 | This work |
| pGKK346 | lacZ in pGKK344 | This work |
| pFC36 | PvexRAB-lacZ1 in pGKK346 | This work |
| pFC28 | PbreAB-lacZ1 (PvexCD-lacZ1) in pGKK346 | This work |
| pFC27 | PbreR-lacZ1 in pGKK346 | This work |
| pFC32 | pKAS154 ΔvexR1 | This work |
| pFC3 | pKAS154 ΔbreR1 | This work |
| pFC33 | BreR-His6 construct in pKAS178 | This work |
| pFC25 | BreR-His6 construct in pBAD22 | This work |
TABLE 2.
Primers used in this study
| Primer name | Nucleotide sequence (5′ to 3′) |
|---|---|
| AAP | GGCCACGCGTCGACTAGTACGGGIIGGGIIGGGIIG |
| AP… | GGCCACGCGTCGACTAGTAC(T)17 |
| AUAP | GGCCACGCGTCGACTAGTAC |
| CHR2 | GATCGGAATTCGACCGCCACCAAACACTAAG |
| CHR4 | GATCGGCGGCCGCTCTAGAATGCTAACGATTTTTAGAAC |
| FC13F | GATCGGGATCCCGTAAGCAATCTCGCTACTG |
| FC13R | GATCGGCGGCCGCAGAAGGATTCATAGTGTGTTG |
| FC18F | GATCGGAATTCACCATGAAACTCAGTGAGCAAAA |
| FC18R′ | GATCGTCTAGACTAGTGGTGGTGGTGGTGGTGAGGCTTTAGTTCT |
| FC25 | CGATAATCTGAGTGTCTGCT |
| FC24 | AGCAGACACTCAGATTATCG |
| FC32 | ATGGCATTCAGGGATCAGAA |
| FC33 | TTCTGATCCCTGAATGCCAT |
| FC42R | GATCGGAATTCAGCGAGTTACCAATTGGGTTTCG |
| FC49 | TGGGAGCCACTTCTTTCTCAATCAG |
| FC50 | CGCAGTCAGCGCCGTATCTTGAG |
| FC51 | GCTGGATCGCGCACAAATTC |
| FC57 | GATCGGCGGCCGCTTCAGACATCACATTTCTCTG |
| FC62 | GATCGTCTAGAGTAACGCGAGCTGATCTTGGT |
| FC63 | GATCGTCTAGATTCAGACATCACATTTCTCTG |
| FC72 | GGTCGTAGCGATTGGTGACTTTATCCAG |
| FC73 | AAATGGCTTTGGCGCGGCGGTACTCTTGTT |
| FC74 | TTCCAGCGCCGTTTTGGCATCTCG |
| GAL1 | GATCGGCGGCCGCGCCAGAGAGCCTTAAGGCTC |
| GAL2 | GATCGGGATCCACGCCAACGAGGTAAAAACG |
| TetB | GATCGGGATCCTTTCACGGCGAGCAATGGTGG |
| TetE | GATCGGAATTCGTAACGCGAGCTGATCTTGGT |
| TetN2 | GATCGGCGGCCGCACAACACTAATTTGGAGTTCGC |
| TR3B | GATCGGGATCCTAATCGCGGCAACCCAGCCAA |
| TR3E | GATCGGAATTCCGATTGAATCGACGTTGATCC |
| TR3N1 | GATCGGCGGCCGCGATCCATATTCGCTGCATGGA |
| TR3N2 | GATCGGCGGCCGCAAAGCCTTAGAGGCTAACGGAT |
RNA isolation, microarray analyses, and statistical evaluations.
Three independent experiments were performed for the microarray analysis. For each experiment, RNA was obtained at four different time points and was prepared for hybridization; therefore, a total of three slides per time point were analyzed. The growth conditions were as follows: V. cholerae C6706 str2 was grown for 15 h in LB medium at 30°C with aeration. The cultures were subsequently diluted 100-fold in AKI medium with or without 0.4% crude bile (high bile concentration) and were grown at 37°C for 3.5 h under stationary conditions and then for 2 h with aeration to induce virulence gene expression (23). Next, the cultures were diluted 100-fold in AKI medium with or without 0.02% crude bile (low bile concentration) and were grown at 37°C for 2 h under stationary conditions. Samples were obtained at four different time points: 2, 4, and 5.5 h for the high-bile-concentration cultures and 2 h for the low-bile-concentration cultures (Fig. 1B). RNA isolation, cDNA probe labeling, microarray hybridization, data detection, and statistical analyses were carried out as described previously (24).
Construction of in-frame deletion strains.
Deletions were achieved by the PCR amplification of ∼500-bp C6706 str2 DNA fragments flanking the gene of interest while retaining several codons from the 5′ and 3′ ends of the gene fused in frame. The fragments were ligated into pKAS154 (27), and the genes of interest were deleted from the V. cholerae chromosome by allelic exchange (49). vexR was deleted using primers TetB with TetN2 and FC57 with TetE, and breR was deleted using primers TR3B with TR3N2 and TR3N1 with TR3E. The accuracy of all the constructs was confirmed by DNA sequencing.
Construction of PvexRAB-lacZ, PbreAB-lacZ, and PbreR-lacZ fusions.
The ΔlacZ plasmid pGKK344 was constructed by PCR amplification of two ∼600-bp fragments flanking the lacZ gene from C6706 str2 by using primers CHR2 with CHR4 and GAL1 with GAL2. The fragments were joined at a NotI site and ligated into pKAS154 by using the EcoRI and BamHI sites. The pGKK344 plasmid was linearized with NotI, and a promotorless lacZ gene from pVC200 (40) was ligated into pGKK344, generating pGKK346. After being screened for the correct orientation of lacZ, pGKK346 was linearized with XbaI between the chromate homology fragment and the promotorless lacZ gene. Approximately 500 bp of the vexRAB, breAB, or breR promoter region was amplified by PCR using FC62 with FC63, TR3E with TR3N1, or FC13F with FC13R, respectively. The resulting fragments from the vexRAB, breAB, and breR promoters were digested and ligated into the linearized pGKK346 plasmid, generating pFC36, pFC28, and pFC27, respectively. The lacZ fusions were transferred into the chromosome of a V. cholerae ΔlacZ strain by allelic exchange (49) between the chr and gal loci. The accuracy of all the constructs was confirmed by DNA sequencing.
β-Galactosidase assays.
Different V. cholerae strains harboring the PvexRAB-lacZ, PbreAB-lacZ, or PbreR-lacZ transcriptional fusion were grown for 15 h in LB medium at 37°C with aeration. The cultures were then diluted 100-fold in LB medium with or without one of the following compounds: crude bile (0.4%), taurodeoxycholate (300 μM), cholate (300 μM), glycochenodeoxycholate (300 μM), deoxycholate (300 μM), taurocholate (300 μM), chenodeoxycholate (300 μM), glycocholate (300 μM), glycodeoxycholate (300 μM), SDS (300 μM), Triton X-100 (150 μg/ml), erythromycin (0.1 μM), novobiocin (0.1 μM), or polymyxin B (5 U/ml). The cultures were grown at 37°C with aeration until the optical density at 600 nm (OD600) reached 0.8 to 1.0. β-Galactosidase assays were carried out as described previously (38). Prism software was used for all statistics. P values were calculated using a nonparametric Student two-tailed t test. P values of <0.05 were considered statistically significant throughout.
Construction of expression plasmid.
The expression plasmid generated for this study is listed in Table 1. A His6 tag was fused to the C terminus of BreR by amplifying the breR gene from C6706 str2 with primers FC18F and FC18R′. The resulting fragment was ligated into pBAD22 (16), generating pFC25. E. coli was transformed with the pFC25 plasmid by electroporation for BreR purification. The accuracy of the construct was confirmed by DNA sequencing.
Identification of the breAB and breR transcriptional start sites.
The C6706 str2 strain was grown for 15 h in LB medium at 37°C with aeration. The culture was then diluted 100-fold in LB medium in the presence of crude bile and was grown at 37°C with aeration until the OD600 of the culture had reached 0.8 to 1.0. Total RNA was isolated as described previously (24) and subjected to 5′ rapid amplification of cDNA ends (5′ RACE) (11) according to the protocols of the 5′ RACE kit manufacturer (Invitrogen). Briefly, first-strand cDNA synthesis was carried out using 1 μg of RNA, reverse transcriptase, and either the breA-specific primer FC72 or the breR-specific primer FC49. The cDNA was purified using a PCR purification kit (Qiagen), and poly(dC) or poly(dA) tails were added to the 3′ ends using terminal deoxynucleotidyltransferase. Prior to nested amplifications, second-strand cDNA synthesis was necessary for the poly(dA)-tailed cDNA and was carried out using the 3′ RACE adapter primer. PCR amplification of the cDNA was carried out using the 5′ RACE abridged anchor primer with the first nested primer FC73 (breA) for the poly(dC)-tailed cDNA or the abridged universal amplification primer (AUAP) with the first nested primer FC50 (breR) for the poly(dA)-tailed cDNA. A dilution of the PCR mixture was subjected to reamplification using the AUAP with the second nested primer FC74 (breA) for the poly(dC)-tailed cDNA or the AUAP with the second nested primer FC51 (breR) for the poly(dA)-tailed cDNA. The DNA products were then run on an agarose gel, gel extracted (Qiagen), and sequenced.
Purification of BreR-His6.
E. coli Origami(DE3) (Novagen) carrying plasmid pFC25 was grown overnight at 37°C with aeration. The culture was diluted 100-fold in LB medium containing kanamycin, tetracycline, and ampicillin, grown to an OD600 of 0.6 at 37°C, and induced with 0.1% arabinose, with incubation for an additional 2 h. The cells were harvested by centrifugation and resuspended in buffer A (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, 1 mM phenylmethylsulfonyl fluoride, pH 7.0). The suspension was sonicated and centrifuged at 14,000 rpm in a microcentrifuge at 4°C for 20 min. The supernatant was then collected. A column containing Talon metal affinity resin (Clontech) was preequilibrated with buffer B (50 mM NaH2PO4, 300 mM NaCl, pH 7.0). The column was loaded with the supernatant containing BreR-His6 and washed with buffer C (50 mM NaH2PO4, 300 mM NaCl, 20 mM imidazole, pH 7.0), and BreR-His6 was eluted with buffer D (50 mM NaH2PO4, 300 mM NaCl, 250 mM imidazole, pH 7.0). Fractions were collected and analyzed by SDS-polyacrylamide gel electrophoresis (PAGE). Those containing BreR-His6 were pooled and dialyzed overnight in binding buffer [20 mM HEPES (pH 7.6), 1 mM EDTA, 10 mM (NH4)2SO4, 5 mM dithiothreitol, 0.2% Tween 20, 30 mM KCl] (31). BreR-His6 purity was estimated by SDS-PAGE to be ∼90% (data not shown). Glycerol was added to purified BreR-His6 to a 10% (vol/vol) final concentration before storage at −80°C.
EMSA.
The fragments designated AB1 (the nucleotide sequence between −95 and +132 of the breAB promoter region), AB2 (−382 to −76 of the breAB promoter region), and AB2s (−382 to −95 of the breAB promoter region) were amplified from pFC28 using FC24 with FC13R, FC13F with FC25, and FC13F with FC42R, respectively. The fragments R1 (−102 to +131 of the breR promoter region) and R2 (−370 to −83 of the breR promoter region) were amplified from pFC27 using TR3N1 with FC33 and FC32 with TR3E, respectively. The penicillin V amidase (PVA) gene fragment (+18 to +191 of the pva promoter) was obtained by PCR as described previously (25). The fragments were gel purified and 3′ end labeled with digoxigenin (DIG) as described previously (26). Reactions for binding BreR-His6 with the different fragments and the electrophoresis of these samples in a 6% polyacrylamide gel were carried out as described previously (31). For the BreR binding inhibition experiment, 5, 10, 20, 40, or 80 mM deoxycholate, 10 mM glycocholate, or 10 mM glycodeoxycholate was incubated with BreR for 15 min at 37°C prior to the addition of the R1 fragment. The DNA was transferred, probed, and detected as described previously (24).
Susceptibility tests.
The minimal bactericidal concentration (MBC) of crude bile was determined using a modified microtiter dilution method as described previously (31). Briefly, wild-type, ΔbreAB, and ΔbreR strains were grown in LB medium with aeration at 30°C for 15 h. The cultures were then diluted in LB to obtain stocks with a cell density of ∼5 × 105 CFU/ml. A microtiter plate was used for each antimicrobial compound, which was diluted in a 1.5-fold series. Each well was inoculated, in duplicate, with 10 μl of each strain stock. The final volume for each well was 100 μl, and the final bacterial density was ∼5 × 104 CFU/ml. The microtiter plates were incubated at 37°C with aeration (180 rpm) for 6 h. Finally, 10 μl of culture from each well was spotted onto LB agar by using a multichannel pipette, and the agar was incubated overnight at 30°C to determine the MBC.
Microarray data accession number.
The microarray data discussed herein have been deposited in the NCBI Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO series accession number GSE6468.
RESULTS
Global gene expression in V. cholerae in response to crude bile.
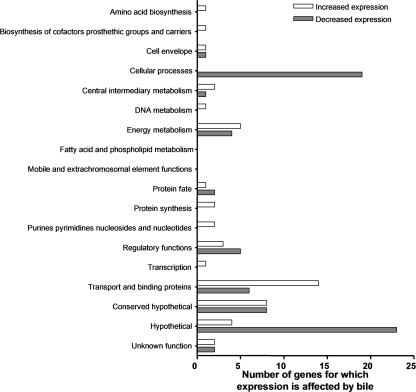
Studies with V. cholerae have shown that in the presence of bile the expression of virulence genes is repressed and motility and/or chemotaxis is increased (15, 48). From these studies, Schuhmacher and Klose formulated a model hypothesizing the effect of bile on colonization: they propose that bile is at a higher concentration in the intestinal lumen than in the mucus layer (48). When the bacterium is within the lumen, bile inhibits the transcription of the virulence genes and induces motility and/or chemotaxis to mobilize the bacterium into the mucus layer. Upon migration through this layer, there is a derepression of virulence gene expression and the colonization of the epithelial cells takes place (Fig. 1A). We investigated the coordinated response of V. cholerae gene expression to the presence of crude bile by using a microarray analysis. The proposed in vivo conditions were simulated by growing V. cholerae O1 El Tor strain C6706 str2 under virulence gene-inducing conditions (in AKI medium) in vitro in the presence of a high bile concentration (0.4% crude bile) for 5.5 h and then diluting the cultures (100-fold) in AKI medium in the presence of a low bile concentration (0.02% crude bile) for 2 h (Fig. 1). Since the effect of bile on V. cholerae has been determined previously using 0.4% crude bile (15, 21, 22, 48), a concentration within the estimated range of concentrations of individual bile salts in the intestine (0.2 to 2%) (20), we utilized this concentration as the high bile concentration, whereas 0.02% crude bile was employed as the low bile concentration because it was 10-fold lower than the lowest individual bile salt concentration in the intestine (0.2%) (20). Samples were obtained at 2, 4, and 5.5 h from the high-bile cultures and at 2 h from the low-bile cultures (Fig. 1B). The same scheme was followed for the reference cultures grown without bile. RNA was extracted, reverse transcription was performed, and cDNA from control and bile-treated samples was used for microarray analysis. A ≥2-fold change in the mRNA level in high-bile cultures at two or more of the three time points and the reverse trend in the low-bile cultures were used as the criteria to indicate genes for which expression was affected by bile. By these criteria, a total of 119 genes were found to be differentially expressed in the presence and absence of bile. Forty-eight genes showed an increase in the RNA level, while 71 genes showed a decrease. Figure 2 shows the distribution of the corresponding gene products within cluster of orthologous group classifications (from the TIGR genome database). The majority of the genes that showed an increase in the RNA level and encode products with assigned functions belong to the group encoding transport and binding proteins (14 genes), whereas the majority of those that displayed a decrease in the RNA level encode products that belong to the cellular processes category (19 genes in total, 8 being part of the pathogenesis subset and 11 belonging to the chemotaxis and motility subset) (Fig. 2; see also Tables S1 and S2 in the supplemental material). The pathogenesis genes tcpA, ctxA, and ctxB showed a decrease in the mRNA level in the presence of the high bile concentration, consistent with previous findings (15, 48), and reversed this decrease in the presence of the low bile concentration, consistent with the hypothesis of Schuhmacher and Klose (48). The same pattern was observed for the following genes: tcpQ, tcpD, tcpS, pspA, and hlyA (see Table S2 in the supplemental material). Studies with the OmpU and OmpT porins have demonstrated previously that ompU transcription is stimulated in the presence of crude bile or deoxycholate (44) and that the expression of ompT is repressed (4). Neither ompU nor ompT mRNA was identified as being affected by bile by our criteria. However, the ompT mRNA level in the presence of the high bile concentration was decreased 24-fold compared to the baseline level at the 5.5-h time point, and this trend was reverted in the presence of the low bile concentration, although we did not detect changes in ompU mRNA levels at any of the different time points. None of the genes involved in biofilm formation in the presence of bile (vps genes and vpsR) (22) were identified using our criteria. This outcome was anticipated, though, since our experimental conditions did not promote biofilm formation. The vexB and vexD genes, encoding efflux components that have been shown previously to play a role in resistance to deoxycholate and other compounds (4), and the vexC gene, encoding a putative MFP, showed an increase in the mRNA level in the presence of a high bile concentration and reversed this increase under the low-bile conditions (see Table S1 in the supplemental material). Using our criteria, we did not identify the vceA, vceB, or vexA gene, encoding an MFP, a multidrug efflux pump, and a putative MFP, respectively, as being affected by bile, even though these genes encode proteins involved in bile resistance (4, 8). However, vceA and vexA showed three- and twofold increases in the mRNA level, respectively, only in the presence of the high bile concentration at the 5.5-h time point, and this trend was reverted in the presence of the low bile concentration. In addition, three genes, vexR, VC1746, and VCA0933, classified as having regulatory functions, showed an increase in the mRNA level in the presence of the high bile concentration and a reversion of this trend in the presence of the low bile concentration (see Table S1 in the supplemental material). Since the aim of this study was to identify genes for which expression is affected by bile and genes encoding proteins involved in the regulation of these genes in response to bile, we further explored the expression of vexAB and vexCD to determine their response specificities.
FIG. 2.
Distribution of V. cholerae El Tor genes differentially expressed in response to crude bile as classified within clusters of orthologous groups assigned by the TIGR database.
The expression of vexAB can be induced by structurally and chemically unrelated compounds, whereas vexCD expression is induced exclusively by bile.
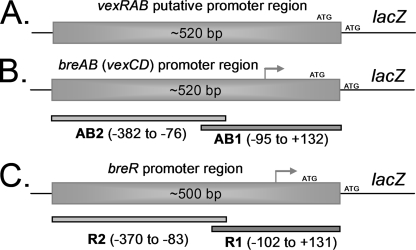
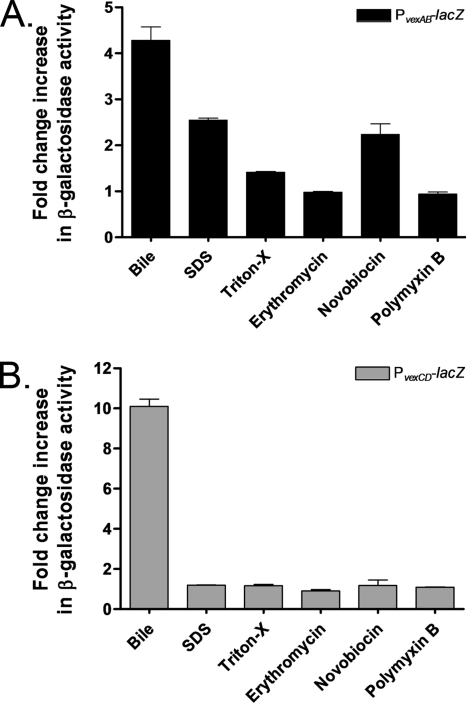
The vexB and vexD genes encode efflux pumps belonging to the RND superfamily, while the vexA and vexC genes each encode an MFP (4). In E. coli (36), C. jejuni (32), Neisseria gonorrhoeae (46), Pseudomonas aeruginosa (37), and Pseudomonas putida (50), the expression of genes that encode RND efflux pumps is induced by the different substrates extruded by the products of these genes. Bina et al. demonstrated that bile increases the mRNA levels of the vexB and vexD transcripts (4). Additionally, after a thorough study testing several antibiotics, detergents, and metals, it was shown previously that a vexB mutant is susceptible to structurally dissimilar compounds: SDS, Triton X-100, erythromycin, novobiocin, polymyxin B, and deoxycholate. This study also showed that a vexD mutant is susceptible only to deoxycholate (4). Therefore, we used these compounds to further determine the specific transcriptional induction of the efflux pump-encoding genes. The expression of vexAB and vexCD was examined using strains containing lacZ fusions integrated elsewhere in the chromosome so as not to interfere with the resistance to the compound being tested. A promotorless lacZ gene was fused to either ∼520 bp of DNA upstream of vexR codon 3 or ∼520 bp of DNA upstream of vexC codon 4, generating the PvexRAB-lacZ or PvexCD-lacZ fusion, respectively (Fig. 3A and B). The specific β-galactosidase activities from strains grown in the presence or absence of subinhibitory concentrations of the compounds mentioned above were measured. Exposure to crude bile, SDS, and novobiocin induced the expression of vexAB between 2.2- and 4.3-fold (Fig. 4A), whereas vexCD expression was induced exclusively by bile, with a 10-fold increase (Fig. 4B). Similar induction results were obtained with strains harboring lacZ fusions to the wild-type promoter of the vexRAB or vexCD operon constructed by the insertion/deletion of lacZ at the vexR or vexC locus, respectively (data not shown). Overall, these results suggest that the vexCD operon responds specifically to bile. Therefore, we renamed this operon breAB for bile response genes and continued to characterize its expression and identify regulatory proteins associated with it.
FIG. 3.
Diagrams showing the promoter regions and fragments employed to generate lacZ transcriptional fusions and DIG-dUTP-labeled fragments. (A) Fragment of ∼520 bp from the upstream region of the putative vexAB ATG start codon. (B) breAB (vexCD) promoter region. Fragments AB1 and AB2 were used for EMSA. (C) breR promoter region. The R1 and R2 fragments were used for EMSA. The breAB and breR transcriptional start sites are indicated by gray arrows.
FIG. 4.
Induction of PvexRAB-lacZ (A) and PvexCD-lacZ (PbreAB-lacZ) (B) expression by different compounds. β-Galactosidase expression was measured by growing the strains in the absence or presence of subinhibitory concentrations of crude bile (0.4%), SDS (300 μM), Triton X-100 (150 μg/ml), erythromycin (0.1 μM), novobiocin (0.1 μM), or polymyxin B (5 U/ml) in LB at 37°C until the OD600 of the cultures reached 0.8 to 1.0. The amount of change (n-fold) in β-galactosidase activity was calculated by dividing the level of β-galactosidase activity obtained in the presence of each compound by the activity obtained in the absence of the compound. The results shown are from three independent experiments. Error bars indicate standard deviations.
BreR represses the expression of the breAB operon.
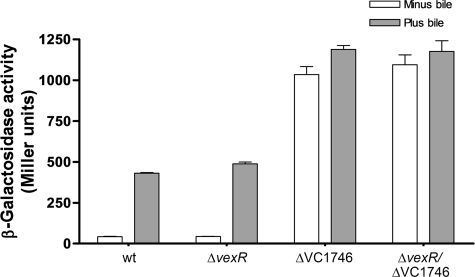
Genes encoding efflux pumps from the RND family are usually regulated by TetR family members (30). From the microarray data, we identified two genes, vexR and VC1746 (see Table S1 in the supplemental material), that encode regulators belonging to the TetR family as determined by BLASTP. We hypothesized that vexR and/or VC1746 may regulate breAB expression in response to bile. In order to test this hypothesis, we deleted vexR, VC1746, or both genes in the strain harboring the PbreAB-lacZ fusion at the lacZ locus. The results showed that the vexR deletion did not affect lacZ expression, either in the absence or the presence of 0.4% crude bile (Fig. 5). In contrast, the deletion of VC1746 increased lacZ expression 24.5- and 2.8-fold in the absence and in the presence of crude bile, respectively, compared to that in the wild type (Fig. 5). These results indicated that VC1746 deletion caused the derepression of PbreAB-lacZ expression and the loss of the majority of bile responsiveness, suggesting that VC1746 encodes a bile-responsive repressor. Finally, the vexR VC1746 double mutant showed an induction pattern that was the same as that associated with the VC1746 single deletion (Fig. 5). Since the results (including those discussed below) indicated that VC1746 encodes a transcriptional repressor of the breAB operon, VC1746 was named breR. Further analysis using the TIGR database showed that the protein encoded by breR is composed of 209 amino acids and has a predicted helix-turn-helix DNA binding motif near its N terminus, characteristic of transcriptional regulators in the TetR family (45).
FIG. 5.
Induction of PbreAB-lacZ expression in various strain backgrounds by crude bile. β-Galactosidase expression was measured by growing the strains in the absence or presence of 0.4% crude bile in LB at 37°C until the OD600 of the cultures reached 0.8 to 1.0. The results shown are from three independent experiments. Error bars indicate standard deviations.
BreR binds to the breAB promoter region.
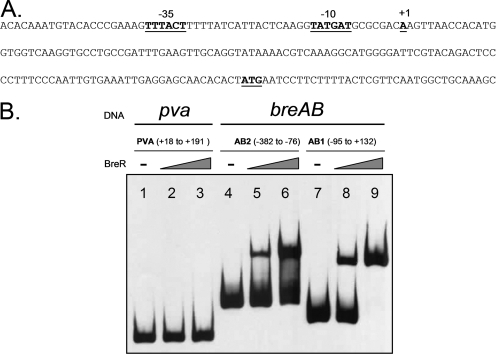
To determine if BreR directly interacts with the breAB promoter region, we initially determined the breAB transcriptional start site. 5′ RACE (11) determined that the +1 nucleotide of the breAB promoter is an A located 121 bp upstream of the predicted breA ATG start codon (Fig. 6A). Further examination of the upstream region revealed the presence of putative −35 (TTTACT) and −10 (TATGAT) regions (Fig. 6A) separated by 18 bp. The −35 sequence has two mismatches from the consensus sequence TTGACA, and the −10 sequence has only one mismatch from the consensus sequence TATAAT.
FIG. 6.
Specific binding of BreR to the breAB promoter region. (A) Nucleotide sequence of the breAB promoter. The position of the transcriptional start site for breAB was determined by 5′ RACE. The transcriptional start site (+1), ATG start codon, and putative −35 and −10 regions are boldfaced and underlined. (B) EMSA was performed with the control DNA fragment from the pva promoter (25) (lanes 1 to 3) or the breAB promoter fragments AB1 and AB2 (lanes 4 to 9). DIG-dUTP-labeled DNA (10 ng) was incubated with 0 ng (lanes 1, 4, and 7), 50 ng (lanes 2, 5, and 8), or 250 ng (lanes 3, 6, and 9) of BreR-His6 prior to electrophoresis. −, no BreR.
Subsequently, EMSA was performed using a purified BreR-His6 fusion protein. BreR-His6 was overexpressed from pFC25 in E. coli Origami(DE3) (Novagen) and purified by nickel-nitrilotriacetic acid affinity column chromatography to approximately 90% purity as judged by SDS-PAGE on a gel stained with Coomassie blue. The apparent molecular mass of purified BreR-His6 is ∼23 kDa, consistent with the predicted size (data not shown). The breAB promoter region utilized for the lacZ transcriptional fusions was divided into two slightly overlapping fragments, an ∼230-bp fragment (the nucleotide sequence between −95 and +132) named AB1 and an ∼300-bp fragment (−382 to −76) named AB2 (Fig. 3B). In addition, an ∼175-bp fragment from the unrelated PVA gene (pva) promoter (the PVA fragment) (25) was utilized as a negative control. BreR-His6 was incubated with the AB1, AB2, or PVA fragment in the presence of poly(dI-dC) and, surprisingly, caused a mobility shift of both the AB1 and AB2 fragments at low (50-ng) and high (250-ng) protein levels (Fig. 6B, lanes 5, 6, 8, and 9), whereas no shift of the PVA control fragment was observed (Fig. 6B, lanes 2 and 3). These data provide evidence for the direct binding of BreR to the breAB promoter region. Furthermore, they suggest that BreR binds the breAB promoter at two independent sites. However, the AB1 and AB2 fragments overlap by 20 bp, and this may account for BreR's binding of both fragments. Therefore, an ∼280-bp fragment (−382 to −95) named AB2s was designed (see Fig. S1A in the supplemental material). The AB1, AB2s, and PVA fragments were incubated with BreR-His6, and a mobility shift was observed with both the AB1 and AB2s fragments (see Fig. S1B in the supplemental material), thus demonstrating the ability of BreR to bind to the breAB promoter at two independent sites, one distal from the +1 site (within AB2 or AB2s) and one proximal (within AB1).
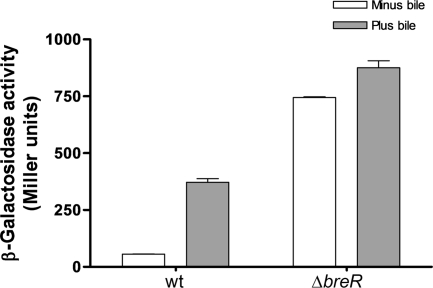
Expression of breR is induced in response to bile in a BreR-dependent manner.
To analyze the regulation of breR expression, we used a PbreR-lacZ transcriptional fusion. We designed a reporter strain containing the wild-type breR operon and ∼500 bp of DNA upstream of breR codon 30 fused to a promotorless lacZ gene and integrated into the V. cholerae chromosome at the lacZ locus (Fig. 3C). The transcription of the PbreR-lacZ fusion was increased 6.6-fold when the strain was grown in the presence of crude bile (Fig. 7). Similar induction was obtained with strains harboring lacZ fusions to the wild-type promoter of the breR operon constructed by the insertion/deletion of lacZ at the breR locus (data not shown).
FIG. 7.
Induction of PbreR-lacZ expression by crude bile and breR autoregulation. β-Galactosidase activity was measured by growing the strains in the absence or presence of 0.4% crude bile in LB at 37°C until the OD600 of the cultures reached 0.8 to 1.0. The results shown are from three independent experiments. Error bars indicate standard deviations. wt, wild type.
Several transcriptional regulators from the TetR family, such as TetR, AcrR, UidR, and MexR, negatively regulate their own expression (5, 18, 35, 41). To investigate if breR is subject to autoregulation, we generated a breR deletion derivative of the reporter strain harboring the PbreR-lacZ fusion at the lacZ locus. The deletion of breR led to 13- and 2.4-fold increases in lacZ expression in the absence and presence of crude bile, respectively, compared to the expression in the wild-type background (Fig. 7).
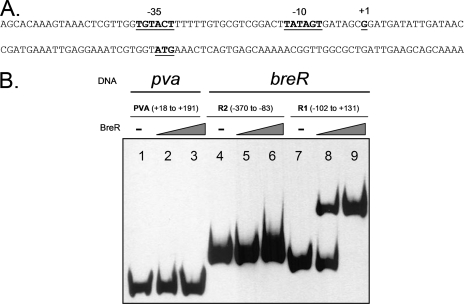
BreR binds to the breR promoter region.
To study the potential interaction of BreR with the breR promoter, the transcriptional start site at the breR promoter was first identified. By using 5′ RACE, it was determined that the +1 nucleotide of the breR promoter is a G located 40 bp upstream of the predicted ATG start codon (Fig. 8A). The inspection of the upstream region revealed a putative −35 (TGTACT) region with three mismatches from the consensus sequence TTGACA and a putative −10 (TATAGT) region with only one mismatch from the consensus sequence TATAAT. The putative −35 and −10 sequences (Fig. 8A) are separated by 17 bp.
FIG. 8.
BreR interaction with the breR promoter region. (A) The breR promoter nucleotide sequence is shown. 5′ RACE was utilized to determine the position of the transcriptional start site for breR. The transcriptional start site (+1), ATG start codon, and putative −35 and −10 regions are boldfaced and underlined. (B) EMSA was performed with the control DNA fragment from the pva promoter (25) (lanes 1 to 3) or the breR promoter fragments R1 and R2 (lanes 4 to 9). In the DNA binding assay, the DIG-dUTP-labeled DNA (10 ng) was incubated with 0 ng (lanes 1, 4, and 7), 50 ng (lanes 2, 5, and 8), or 250 ng (lanes 3, 6, and 9) of BreR-His6.
Consequently, EMSA was performed utilizing BreR-His6 and two fragments: a ∼230-bp fragment (−102 to +131) named R1 and a ∼290-bp fragment (−370 to −83) named R2 obtained from the division of the breR promoter region used for the lacZ fusions (Fig. 3C). The results showed that after the incubation of BreR-His6 with the R1, R2, or PVA (negative control) fragment, BreR bound only to the R1 fragment (Fig. 8B, lanes 8 and 9) and not to the R2 or PVA fragment (Fig. 8B, lanes 2, 3, 5, and 6). Given that no supershift was observed with the R1 fragment in the presence of BreR, it is highly probable that BreR binds at only one site at this promoter. From these data, we can conclude that BreR binds differently at the breR promoter and the breAB promoter (Fig. 7), utilizing a single binding region for the former and two for the latter.
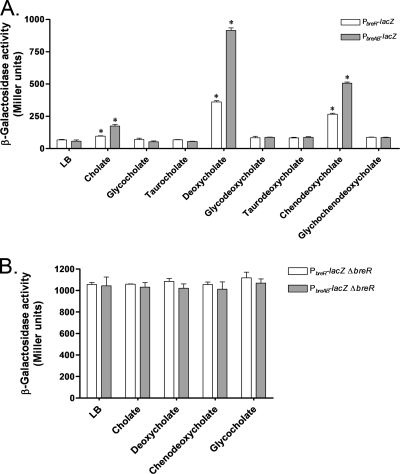
Cholate, deoxycholate, and chenodeoxycholate are inducers of breAB and breR expression.
Since bile salts are abundant components of crude bile (2, 20), we tested individual bile salts to determine if any could induce breAB and/or breR expression. The reporter strains harboring the PbreAB-lacZ or the PbreR-lacZ fusion at the lacZ locus were grown in the presence of a subinhibitory concentration (300 μM) of eight different bile salts. The expression of PbreAB-lacZ and PbreR-lacZ was significantly induced (P < 0.05) in the presence of deoxycholate (17- and 5-fold, respectively) and chenodeoxycholate (10- and 4-fold, respectively) (Fig. 9A), whereas cholate produced lower-level inductions of the expression of breAB (3-fold) and breR (1.4-fold) (Fig. 9A). These results demonstrate that the induction of breAB and breR expression can be accomplished by the specific bile salts cholate, deoxycholate, and chenodeoxycholate and that the hierarchy of stronger to weaker inducers is as follows: deoxycholate > chenodeoxycholate > cholate. To determine if the response to individual bile salts was also mediated through BreR, we examined the expression of the breAB and breR promoters in a ΔbreR background with and without subinhibitory concentrations of cholate, deoxycholate, chenodeoxycholate, and glycocholate (negative control). The deletion of breR led to constitutive expression of PbreAB-lacZ and PbreR-lacZ (Fig. 9B) in the absence and presence of the different compounds tested.
FIG. 9.
Influence of different bile salts on the expression of PbreAB-lacZ and PbreR-lacZ as determined by β-galactosidase assays. Strains carrying the PbreR-lacZ or the PbreAB-lacZ fusion were grown in LB in the absence or presence of a subinhibitory concentration (300 μM) of different bile salts at 37°C until the OD600 of the cultures reached 0.8 to 1.0. The results shown are from three independent experiments. Error bars indicate standard deviations. Asterisks indicate statistically significant differences from the LB control. (A) Induction of PbreAB-lacZ and PbreR-lacZ fusions in the presence of different bile salts. (B) Expression of PbreAB-lacZ and PbreR-lacZ in a ΔbreR background in response to different bile salts.
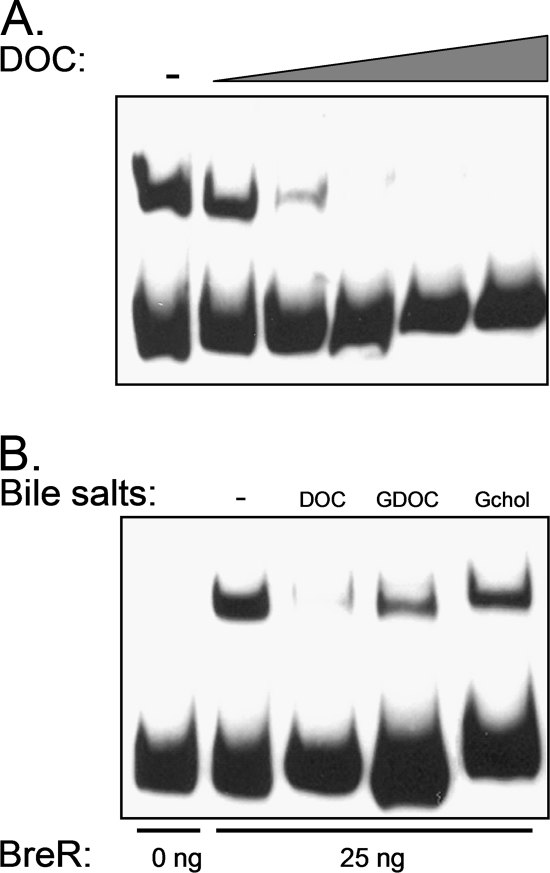
Deoxycholate inhibits the binding of BreR to the breR promoter.
It has been shown previously that the tetracycline repressor (TetR) binds to the tetA operator in the absence of tetracycline, repressing the expression of tetA, which encodes an efflux pump. When tetracycline enters the cytoplasm, it binds to TetR, inducing a conformational change that dissociates TetR from the tetA operator, allowing the production of TetA and the active efflux of tetracycline (18, 19). This general regulatory mechanism has also been described previously for several members of the TetR family (6, 9, 12, 31, 39, 47, 51). Given that cholate, deoxycholate, and chenodeoxycholate induce breAB and breR expression, we hypothesized that these bile salts may interact with BreR. To test this possibility, we performed EMSA with the R1 fragment and BreR in the presence of deoxycholate because this bile salt gives the most robust induction of PbreR-lacZ expression (Fig. 9A). In addition, we tested BreR binding in the presence of glycocholate, a noninducing bile salt, and glycodeoxycholate, a noninducing bile salt with structural similarity to deoxycholate. Initially, we determined the concentration at which deoxycholate abolished the formation of the BreR-R1 complex (Fig. 10A). The result showed that in the presence of ≥10 mM deoxycholate, BreR was unable to bind to R1. We selected 10 mM deoxycholate as the concentration to be used in our binding assay given that it was the lowest concentration at which we observed the inhibition of binding. In addition, we used 10 mM glycodeoxycholate and glycocholate as control bile salts. Figure 10B shows that 10 mM deoxycholate abolished the formation of the BreR-R1 complex but that the same concentration of glycodeoxycholate or glycocholate did not disrupt this interaction. These results suggest that deoxycholate is able to specifically prevent the formation of the BreR-R1 complex.
FIG. 10.
Effect of deoxycholate on the DNA binding activity of BreR. (A) Titration of deoxycholate (DOC) to determine the concentration that prevents the formation of BreR-R1 complexes. EMSA was performed with the R1 fragment and 25 ng of BreR-His6. DIG-dUTP-labeled DNA (10 ng) was incubated with increasing concentrations (0, 5, 10, 20, 40, and 80 mM) of DOC prior to electrophoresis. −, no DOC. (B) EMSA analysis showing the disruption of the BreR-DNA complex in the presence of DOC but not glycodeoxycholate (GDOC) or glycocholate (Gchol). EMSA was performed with the R1 fragment and 0 or 25 ng of BreR-His6 (lanes − to Gchol). DIG-dUTP-labeled DNA (10 ng) was incubated with no bile salts (−), 10 mM DOC, 10 mM GDOC, or 10 mM Gchol.
DISCUSSION
In the present study, we investigated the mechanism of transcriptional activation of genes that encode components involved in facilitating the resistance of V. cholerae to bile. We initially used microarray technology to investigate the changes in V. cholerae global gene expression during growth in the presence of different bile concentrations. The results confirmed the repression of the virulence regulon and the induction of the vexB and breAB (vexCD) genes, which encode efflux system components. Furthermore, they revealed the induction of genes associated with transcriptional regulation, vexR and breR (see Table S1 in the supplemental material).
Since it has been shown previously that the expression of genes that encode components of RND efflux pumps are inducible by the various substrates extruded by the pumps (32, 36, 37, 46, 50), we analyzed the expression of vexAB and breAB in the presence of various compounds. In doing so, we established that crude bile, SDS, and novobiocin induced the expression of the vexAB genes but that the induction of the expression of breAB was specific to bile, suggesting that this operon responded exclusively to bile, unlike vexAB, which responded to several molecular signals. We therefore pursued the study of the breAB regulatory mechanism.
Genes encoding components of RND efflux systems are tightly regulated by regulators of the TetR family (30). We showed that BreR, a TetR-like regulator, repressed breAB expression, while VexR, another TetR regulator, did not affect breAB expression. It was also demonstrated that BreR, as other TetR members, was able to repress the expression of its own gene. VexR does not repress or activate the breR promoter to indirectly affect breAB expression since PbreAB-lacZ expression was not affected in the ΔvexR strain. β-Galactosidase assays demonstrated that a PbreAB-lacZ fusion exhibited high-level expression in the presence of crude bile; however, in a ΔbreR strain, the expression was even greater regardless of the presence of bile. Usually, local regulators play a modulating role, while the principal transcriptional expression is controlled by global regulators (13). These data support the hypothesis that BreR functions as a repressor of the breAB operon by acting as a local modulator preventing the excessive production of the BreAB efflux pump and that VexR is neither a global nor a local regulator of the breAB promoter. In addition, they strongly suggest that there is no global activator that regulates the expression of the breAB operon, such as the global activators MarA, SoxS, and Rob that induce the expression of the genes encoding the AcrAB efflux system in E. coli (13). Moreover, since the β-galactosidase assays indicated that the level of expression of breAB was highest in the breR mutant, it is possible that a ΔbreR strain may be more resistant to bile than the wild-type strain. MBC experiments with the wild-type and ΔbreR strains determined that there was a 1.5-fold increase in the resistance of a breR mutant to bile compared to that of the wild type (data not shown), as may be expected.
EMSA confirmed that BreR directly binds to the breAB promoter region at two independent sites, one (AB1 fragment) proximal to and one (AB2 or AB2s fragment) distal from the transcriptional start site. The finding that BreR completely shifted the AB1 fragment at a level (250 ng) that produced only a fractional shift of the AB2 or the AB2s fragment may indicate that the affinity for the distal site is lower. Finally, the results presented here indicate that BreR is able to repress the expression of its corresponding gene and interacts directly with the breR promoter region at a single site.
It is known that a number of regulators belonging to the TetR family act as transcriptional repressors by binding to their own operator sequences in the absence of effector/inducer molecules. Once the effector enters the cell, it will bind to a nonconserved domain on the C terminus of the repressor and cause a conformational change resulting in the dissociation of the repressor from the DNA and the transcription of the negatively regulated genes (45). Figure 9A shows that of all the bile salts tested, cholate, deoxycholate, and chenodeoxycholate, induce the expression of the PbreAB-lacZ and PbreR-lacZ transcriptional fusions, suggesting that these bile salts can serve as an environmental signal(s) necessary for the activation of breAB and breR expression. In addition, MBC tests showed that BreB mediates resistance to these bile salts (data not shown). Previous studies with C. jejuni demonstrated that CmeR, a TetR repressor, binds to the cmeABC promoter and represses its expression (31). When bile is incubated with the CmeR-cmeRAB complex, it interacts with CmeR, causing it to dissociate from the promoter region (32). In V. cholerae, VceR, a TetR family repressor of the vceAB operon, dissociates from its operator sequence in the presence of 77.2 mM deoxycholate (6). The findings of these studies demonstrate that bile or bile salts can act as effectors/inducers of TetR family regulators. We performed EMSA using deoxycholate, glycocholate, or glycodeoxycholate, which demonstrated that deoxycholate, at 10 mM, specifically disrupted the binding of BreR to the breR promoter. Similar results have been observed previously with MarR, an S. enterica serovar Typhimurium transcriptional regulator belonging to the MarR family (42). MarR represses the marRAB operon, which is involved in decreasing OmpF porin levels and increasing AcrAB-TolC levels to reduce the influx and enhance the efflux of antibiotic compounds, respectively (30). Most importantly, it has been shown previously that deoxycholate specifically induces marR expression, and gel shift experiments have demonstrated that this bile salt specifically interacts with MarR, disrupting binding to the marRAB operon (42) in parallel to the interaction with BreR described here.
Based on the results that show (i) breAB and breR induction by cholate, deoxycholate, and chenodeoxycholate and (ii) the inhibition of BreR binding to the breR promoter by deoxycholate, as well as the data in the supporting literature, we propose a model wherein BreR is continuously associated with the breR and breAB promoters, repressing their expression. Once cholate, deoxycholate, and/or chenodeoxycholate enters the cell, it binds to BreR, causing the dissociation of the BreR-DNA complex, resulting in breR and breAB expression.
It has been demonstrated previously that the tetracycline repressor (TetR) binds to the tetA operator in the absence of tetracycline (an effector/inducer molecule), repressing the expression of tetA, which encodes an efflux pump, and that the tetR gene is expressed simultaneously with the tetA gene. This synchronized expression ensures that there is enough repressor available to inactivate the expression of tetA when tetracycline has been completely secreted out of the cell (18). Our results show that breR and breAB share this feature since both PbreR-lacZ and PbreAB-lacZ showed higher levels of expression in the presence of bile, specifically in the presence of cholate, deoxycholate, and chenodeoxycholate, than in the absence of bile.
Finally, the organization of breR with respect to breAB represents a novel arrangement for these systems, since the genes that encode TetR regulators that control the expression of the cognate genes encoding RND efflux systems are localized either in a divergent orientation adjacent to the genes they regulate (1, 17, 31, 35) or in the same operon (39, 53). In contrast, breR is located 8.99 kb upstream, positioned several genes away from the breAB operon. Genes encoding hypothetical proteins, paraquat-inducible protein A and B, and a putative lipoprotein are among the genes between breR and the breAB operon. Interestingly, three of the genes encoding hypothetical proteins were also identified in our microarray study as being induced in the presence of bile (see Table S1 in the supplemental material). These genes are currently under investigation.
The findings reported here demonstrate that BreR is the transcriptional repressor of the breAB efflux system operon and that this repression is probably accomplished by binding at two independent binding sites in the breAB promoter. In addition, BreR negatively regulates its own expression by binding to one site at the breR promoter. The mechanism of BreR repression at these promoters is currently under investigation. Lastly, we propose that BreR requires an effector/inducer molecule(s) to dissociate from the breAB and/or breR promoter and that the effector/inducer molecule(s) may be cholate, deoxycholate, and/or chenodeoxycholate.
Supplementary Material
Acknowledgments
We thank Karen Skorupski for helpful discussions and critical reading of the manuscript and Gabriela Kovacikova for technical assistance.
This work was supported by NIH grant AI039654 and NSF grant OCN-0120677.
Footnotes
Published ahead of print on 5 September 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Aires, J. R., T. Kohler, H. Nikaido, and P. Plesiat. 1999. Involvement of an active efflux system in the natural resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob. Agents Chemother. 432624-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Begley, M., C. G. Gahan, and C. Hill. 2005. The interaction between bacteria and bile. FEMS Microbiol. Rev. 29625-651. [DOI] [PubMed] [Google Scholar]
- 3.Bina, J. E., and J. J. Mekalanos. 2001. Vibrio cholerae tolC is required for bile resistance and colonization. Infect. Immun. 694681-4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bina, J. E., D. Provenzano, C. Wang, X. R. Bina, and J. J. Mekalanos. 2006. Characterization of the Vibrio cholerae vexAB and vexCD efflux systems. Arch. Microbiol. 186171-181. [DOI] [PubMed] [Google Scholar]
- 5.Blanco, C., M. Mata-Gilsinger, and P. Ritzenthaler. 1985. The use of gene fusions to study the expression of uidR, a negative regulatory gene of Escherichia coli K-12. Gene 36159-167. [DOI] [PubMed] [Google Scholar]
- 6.Borges-Walmsley, M. I., D. Du, K. S. McKeegan, G. J. Sharples, and A. R. Walmsley. 2005. VceR regulates the vceCAB drug efflux pump operon of Vibrio cholerae by alternating between mutually exclusive conformations that bind either drugs or promoter DNA. J. Mol. Biol. 349387-400. [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee, A., S. Chaudhuri, G. Saha, S. Gupta, and R. Chowdhury. 2004. Effect of bile on the cell surface permeability barrier and efflux system of Vibrio cholerae. J. Bacteriol. 1866809-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colmer, J. A., J. A. Fralick, and A. N. Hamood. 1998. Isolation and characterization of a putative multidrug resistance pump from Vibrio cholerae. Mol. Microbiol. 2763-72. [DOI] [PubMed] [Google Scholar]
- 9.Folcher, M., R. P. Morris, G. Dale, K. Salah-Bey-Hocini, P. H. Viollier, and C. J. Thompson. 2001. A transcriptional regulator of a pristinamycin resistance gene in Streptomyces coelicolor. J. Biol. Chem. 2761479-1485. [DOI] [PubMed] [Google Scholar]
- 10.Fralick, J. A. 1996. Evidence that TolC is required for functioning of the Mar/AcrAB efflux pump of Escherichia coli. J. Bacteriol. 1785803-5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frohman, M. A., M. K. Dush, and G. R. Martin. 1988. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc. Natl. Acad. Sci. USA 858998-9002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grkovic, S., M. H. Brown, N. J. Roberts, I. T. Paulsen, and R. A. Skurray. 1998. QacR is a repressor protein that regulates expression of the Staphylococcus aureus multidrug efflux pump QacA. J. Biol. Chem. 27318665-18673. [DOI] [PubMed] [Google Scholar]
- 13.Grkovic, S., M. H. Brown, and R. A. Skurray. 2002. Regulation of bacterial drug export systems. Microbiol. Mol. Biol. Rev. 66671-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunn, J. S. 2000. Mechanisms of bacterial resistance and response to bile. Microbes Infect. 2907-913. [DOI] [PubMed] [Google Scholar]
- 15.Gupta, S., and R. Chowdhury. 1997. Bile affects production of virulence factors and motility of Vibrio cholerae. Infect. Immun. 651131-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 1774121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagman, K. E., and W. M. Shafer. 1995. Transcriptional control of the mtr efflux system of Neisseria gonorrhoeae. J. Bacteriol. 1774162-4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hillen, W., C. Gatz, L. Altschmied, K. Schollmeier, and I. Meier. 1983. Control of expression of the Tn10-encoded tetracycline resistance genes. Equilibrium and kinetic investigation of the regulatory reactions. J. Mol. Biol. 169707-721. [DOI] [PubMed] [Google Scholar]
- 19.Hinrichs, W., C. Kisker, M. Duvel, A. Muller, K. Tovar, W. Hillen, and W. Saenger. 1994. Structure of the Tet repressor-tetracycline complex and regulation of antibiotic resistance. Science 264418-420. [DOI] [PubMed] [Google Scholar]
- 20.Hofmann, A. F. 1998. Bile secretion and the enterohepatic circulation of bile salts, 6th ed. W. B. Saunders, Philadelphia, PA.
- 21.Hung, D. T., E. A. Shakhnovich, E. Pierson, and J. J. Mekalanos. 2005. Small-molecule inhibitor of Vibrio cholerae virulence and intestinal colonization. Science 310670-674. [DOI] [PubMed] [Google Scholar]
- 22.Hung, D. T., J. Zhu, D. Sturtevant, and J. J. Mekalanos. 2006. Bile acids stimulate biofilm formation in Vibrio cholerae. Mol. Microbiol. 59193-201. [DOI] [PubMed] [Google Scholar]
- 23.Iwanaga, M., K. Yamamoto, N. Higa, Y. Ichinose, N. Nakasone, and M. Tanabe. 1986. Culture conditions for stimulating cholera toxin production by Vibrio cholerae O1 El Tor. Microbiol. Immunol. 301075-1083. [DOI] [PubMed] [Google Scholar]
- 24.Kovacikova, G., W. Lin, and K. Skorupski. 2005. Dual regulation of genes involved in acetoin biosynthesis and motility/biofilm formation by the virulence activator AphA and the acetate-responsive LysR-type regulator AlsR in Vibrio cholerae. Mol. Microbiol. 57420-433. [DOI] [PubMed] [Google Scholar]
- 25.Kovacikova, G., W. Lin, and K. Skorupski. 2003. The virulence activator AphA links quorum sensing to pathogenesis and physiology in Vibrio cholerae by repressing the expression of a penicillin amidase gene on the small chromosome. J. Bacteriol. 1854825-4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovacikova, G., and K. Skorupski. 2001. Overlapping binding sites for the virulence gene regulators AphA, AphB and cAMP-CRP at the Vibrio cholerae tcpPH promoter. Mol. Microbiol. 41393-407. [DOI] [PubMed] [Google Scholar]
- 27.Kovacikova, G., and K. Skorupski. 2002. Regulation of virulence gene expression in Vibrio cholerae by quorum sensing: HapR functions at the aphA promoter. Mol. Microbiol. 461135-1147. [DOI] [PubMed] [Google Scholar]
- 28.Kovacikova, G., and K. Skorupski. 1999. A Vibrio cholerae LysR homolog, AphB, cooperates with AphA at the tcpPH promoter to activate expression of the ToxR virulence cascade. J. Bacteriol. 1814250-4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lacroix, F. J., A. Cloeckaert, O. Grepinet, C. Pinault, M. Y. Popoff, H. Waxin, and P. Pardon. 1996. Salmonella typhimurium acrB-like gene: identification and role in resistance to biliary salts and detergents and in murine infection. FEMS Microbiol. Lett. 135161-167. [DOI] [PubMed] [Google Scholar]
- 30.Li, X. Z., and H. Nikaido. 2004. Efflux-mediated drug resistance in bacteria. Drugs 64159-204. [DOI] [PubMed] [Google Scholar]
- 31.Lin, J., M. Akiba, O. Sahin, and Q. Zhang. 2005. CmeR functions as a transcriptional repressor for the multidrug efflux pump CmeABC in Campylobacter jejuni. Antimicrob. Agents Chemother. 491067-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin, J., C. Cagliero, B. Guo, Y. W. Barton, M. C. Maurel, S. Payot, and Q. Zhang. 2005. Bile salts modulate expression of the CmeABC multidrug efflux pump in Campylobacter jejuni. J. Bacteriol. 1877417-7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin, J., L. O. Michel, and Q. Zhang. 2002. CmeABC functions as a multidrug efflux system in Campylobacter jejuni. Antimicrob. Agents Chemother. 462124-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin, J., O. Sahin, L. O. Michel, and Q. Zhang. 2003. Critical role of multidrug efflux pump CmeABC in bile resistance and in vivo colonization of Campylobacter jejuni. Infect. Immun. 714250-4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma, D., M. Alberti, C. Lynch, H. Nikaido, and J. E. Hearst. 1996. The local repressor AcrR plays a modulating role in the regulation of acrAB genes of Escherichia coli by global stress signals. Mol. Microbiol. 19101-112. [DOI] [PubMed] [Google Scholar]
- 36.Ma, D., D. N. Cook, M. Alberti, N. G. Pon, H. Nikaido, and J. E. Hearst. 1995. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol. Microbiol. 1645-55. [DOI] [PubMed] [Google Scholar]
- 37.Masuda, N., E. Sakagawa, S. Ohya, N. Gotoh, H. Tsujimoto, and T. Nishino. 2000. Contribution of the MexX-MexY-OprM efflux system to intrinsic resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 442242-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 39.Namwat, W., C. K. Lee, H. Kinoshita, Y. Yamada, and T. Nihira. 2001. Identification of the varR gene as a transcriptional regulator of virginiamycin S resistance in Streptomyces virginiae. J. Bacteriol. 1832025-2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parsot, C., and J. J. Mekalanos. 1990. Expression of ToxR, the transcriptional activator of the virulence factors in Vibrio cholerae, is modulated by the heat shock response. Proc. Natl. Acad. Sci. USA 879898-9902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poole, K., K. Tetro, Q. Zhao, S. Neshat, D. E. Heinrichs, and N. Bianco. 1996. Expression of the multidrug resistance operon mexA-mexB-oprM in Pseudomonas aeruginosa: mexR encodes a regulator of operon expression. Antimicrob. Agents Chemother. 402021-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prouty, A. M., I. E. Brodsky, S. Falkow, and J. S. Gunn. 2004. Bile-salt-mediated induction of antimicrobial and bile resistance in Salmonella typhimurium. Microbiology 150775-783. [DOI] [PubMed] [Google Scholar]
- 43.Provenzano, D., and K. E. Klose. 2000. Altered expression of the ToxR-regulated porins OmpU and OmpT diminishes Vibrio cholerae bile resistance, virulence factor expression, and intestinal colonization. Proc. Natl. Acad. Sci. USA 9710220-10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Provenzano, D., D. A. Schuhmacher, J. L. Barker, and K. E. Klose. 2000. The virulence regulatory protein ToxR mediates enhanced bile resistance in Vibrio cholerae and other pathogenic Vibrio species. Infect. Immun. 681491-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramos, J. L., M. Martinez-Bueno, A. J. Molina-Henares, W. Teran, K. Watanabe, X. Zhang, M. T. Gallegos, R. Brennan, and R. Tobes. 2005. The TetR family of transcriptional repressors. Microbiol. Mol. Biol. Rev. 69326-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rouquette, C., J. B. Harmon, and W. M. Shafer. 1999. Induction of the mtrCDE-encoded efflux pump system of Neisseria gonorrhoeae requires MtrA, an AraC-like protein. Mol. Microbiol. 33651-658. [DOI] [PubMed] [Google Scholar]
- 47.Sandu, C., C. B. Chiribau, and R. Brandsch. 2003. Characterization of HdnoR, the transcriptional repressor of the 6-hydroxy-d-nicotine oxidase gene of Arthrobacter nicotinovorans pAO1, and its DNA-binding activity in response to l- and d-nicotine derivatives. J. Biol. Chem. 27851307-51315. [DOI] [PubMed] [Google Scholar]
- 48.Schuhmacher, D. A., and K. E. Klose. 1999. Environmental signals modulate ToxT-dependent virulence factor expression in Vibrio cholerae. J. Bacteriol. 1811508-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skorupski, K., and R. K. Taylor. 1996. Positive selection vectors for allelic exchange. Gene 16947-52. [DOI] [PubMed] [Google Scholar]
- 50.Teran, W., A. Felipe, A. Segura, A. Rojas, J. L. Ramos, and M. T. Gallegos. 2003. Antibiotic-dependent induction of Pseudomonas putida DOT-T1E TtgABC efflux pump is mediated by the drug binding repressor TtgR. Antimicrob. Agents Chemother. 473067-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teran, W., T. Krell, J. L. Ramos, and M. T. Gallegos. 2006. Effector-repressor interactions, binding of a single effector molecule to the operator-bound TtgR homodimer mediates derepression. J. Biol. Chem. 2817102-7109. [DOI] [PubMed] [Google Scholar]
- 52.Touze, T., J. Eswaran, E. Bokma, E. Koronakis, C. Hughes, and V. Koronakis. 2004. Interactions underlying assembly of the Escherichia coli AcrAB-TolC multidrug efflux system. Mol. Microbiol. 53697-706. [DOI] [PubMed] [Google Scholar]
- 53.Yoshida, K., Y. H. Ohki, M. Murata, M. Kinehara, H. Matsuoka, T. Satomura, R. Ohki, M. Kumano, K. Yamane, and Y. Fujita. 2004. Bacillus subtilis LmrA is a repressor of the lmrAB and yxaGH operons: identification of its binding site and functional analysis of lmrB and yxaGH. J. Bacteriol. 1865640-5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.