Abstract
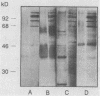
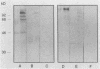
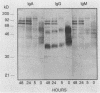
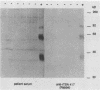
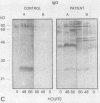
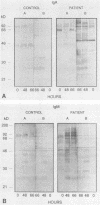
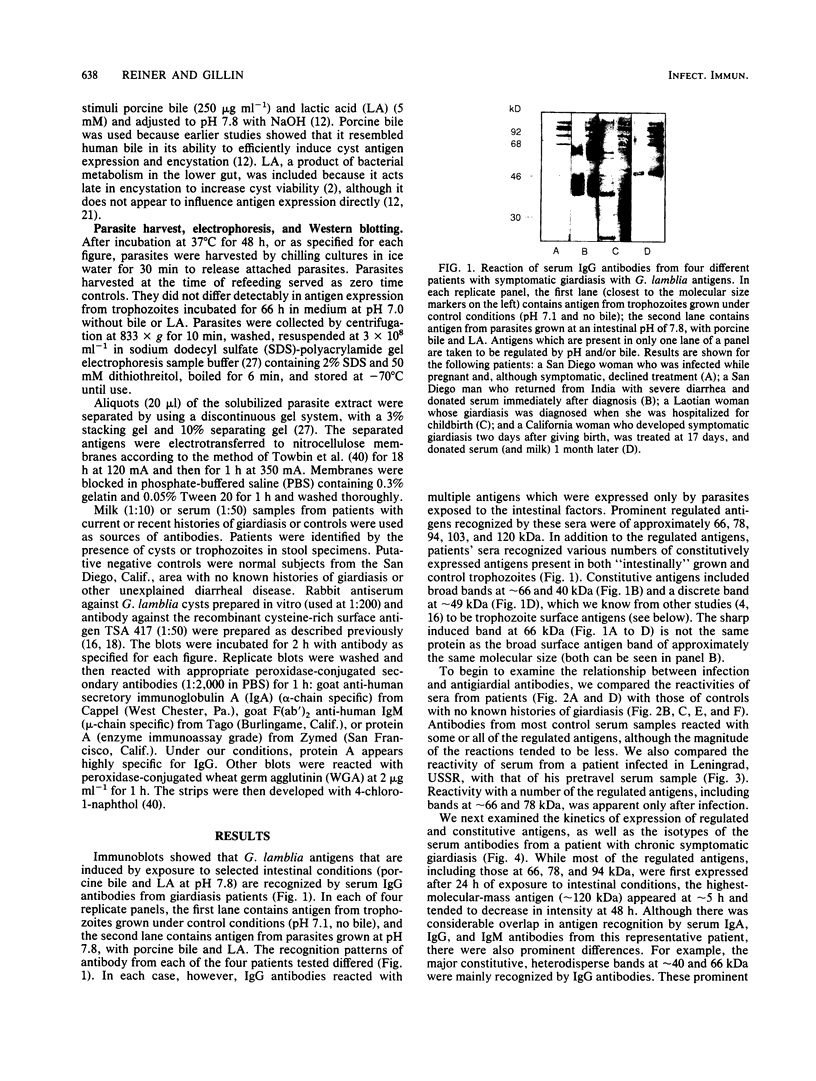
The variability in duration and severity of infection with Giardia lamblia is likely to be due to trophozoite interactions with immune and nonimmune components of the small intestinal milieu. Despite its potential importance, nothing is known of the isotype or the specificity of the secretory antibody response to G. lamblia. In the present study, we show that serum and secretory antibodies recognize many Giardia antigens whose expression is induced by exposure to selected intestinal conditions. Isotype-specific immunoblots of antigens from trophozoites grown at pH 7.0 without bile or at the intestinal pH of 7.8 with bile were reacted with milk or serum antibodies from subjects with or without histories of giardiasis. While the results were complex, several key observations emerged. Serum and secretory immunoglobulin A (IgA), IgM, and IgG antibodies reacted with many regulated antigens. Antigen recognition patterns varied with isotype and between milk and serum antibodies of the same isotype. Antigen recognition also differed among subjects. Antibodies from virtually every patient recognized some G. lamblia antigens. Furthermore, milk and/or serum samples from putative controls without histories of giardiasis were positive more frequently than would be predicted from published prevalence studies, suggesting either that these antibodies may be cross-reactive or that undiagnosed infections with G. lamblia may be more common than previously thought. Thus, recognition of neoantigens induced by host conditions may be due to conserved or cross-reactive epitopes which could constitute a form of immune evasion by G. lamblia.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boucher S. E., Gillin F. D. Excystation of in vitro-derived Giardia lamblia cysts. Infect Immun. 1990 Nov;58(11):3516–3522. doi: 10.1128/iai.58.11.3516-3522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Char S., Shetty N., Narasimha M., Elliott E., Macaden R., Farthing M. J. Serum antibody response in children with Giardia lamblia infection and identification of an immunodominant 57-kilodalton antigen. Parasite Immunol. 1991 May;13(3):329–337. doi: 10.1111/j.1365-3024.1991.tb00286.x. [DOI] [PubMed] [Google Scholar]
- Das S., Traynor-Kaplan A., Reiner D. S., Meng T. C., Gillin F. D. A surface antigen of Giardia lamblia with a glycosylphosphatidylinositol anchor. J Biol Chem. 1991 Nov 5;266(31):21318–21325. [PubMed] [Google Scholar]
- Diamond L. S., Harlow D. R., Cunnick C. C. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg. 1978;72(4):431–432. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- Douglas H., Reiner D. S., Gillin F. D. A new method for purification of Giardia lamblia cysts. Trans R Soc Trop Med Hyg. 1987;81(2):315–316. doi: 10.1016/0035-9203(87)90250-1. [DOI] [PubMed] [Google Scholar]
- Edson C. M., Farthing M. J., Thorley-Lawson D. A., Keusch G. T. An 88,000-Mr Giardia lamblia surface protein which is immunogenic in humans. Infect Immun. 1986 Dec;54(3):621–625. doi: 10.1128/iai.54.3.621-625.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. F., Pye G., Bramley R., Clark A. G., Dyson T. J., Hardcastle J. D. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut. 1988 Aug;29(8):1035–1041. doi: 10.1136/gut.29.8.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faubert G., Reiner D. S., Gillin F. D. Giardia lamblia: regulation of secretory vesicle formation and loss of ability to reattach during encystation in vitro. Exp Parasitol. 1991 May;72(4):345–354. doi: 10.1016/0014-4894(91)90080-g. [DOI] [PubMed] [Google Scholar]
- Gillin F. D., Boucher S. E., Rossi S. S., Reiner D. S. Giardia lamblia: the roles of bile, lactic acid, and pH in the completion of the life cycle in vitro. Exp Parasitol. 1989 Aug;69(2):164–174. doi: 10.1016/0014-4894(89)90185-9. [DOI] [PubMed] [Google Scholar]
- Gillin F. D., Diamond L. S. Clonal growth of Giardia lamblia trophozoites in a semisolid agarose medium. J Parasitol. 1980 Apr;66(2):350–352. [PubMed] [Google Scholar]
- Gillin F. D., Gault M. J., Hofmann A. F., Gurantz D., Sauch J. F. Biliary lipids support serum-free growth of Giardia lamblia. Infect Immun. 1986 Sep;53(3):641–645. doi: 10.1128/iai.53.3.641-645.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillin F. D., Hagblom P., Harwood J., Aley S. B., Reiner D. S., McCaffery M., So M., Guiney D. G. Isolation and expression of the gene for a major surface protein of Giardia lamblia. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4463–4467. doi: 10.1073/pnas.87.12.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillin F. D., Reiner D. S., Boucher S. E. Small-intestinal factors promote encystation of Giardia lamblia in vitro. Infect Immun. 1988 Mar;56(3):705–707. doi: 10.1128/iai.56.3.705-707.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillin F. D., Reiner D. S., Gault M. J., Douglas H., Das S., Wunderlich A., Sauch J. F. Encystation and expression of cyst antigens by Giardia lamblia in vitro. Science. 1987 Feb 27;235(4792):1040–1043. doi: 10.1126/science.3547646. [DOI] [PubMed] [Google Scholar]
- Hill D. R., Hewlett E. L., Pearson R. D. Lectin binding by Giardia lamblia. Infect Immun. 1981 Dec;34(3):733–738. doi: 10.1128/iai.34.3.733-738.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann A. F. Chemistry and enterohepatic circulation of bile acids. Hepatology. 1984 Sep-Oct;4(5 Suppl):4S–14S. doi: 10.1002/hep.1840040803. [DOI] [PubMed] [Google Scholar]
- Jarroll E. L., Manning P., Lindmark D. G., Coggins J. R., Erlandsen S. L. Giardia cyst wall-specific carbohydrate: evidence for the presence of galactosamine. Mol Biochem Parasitol. 1989 Jan 15;32(2-3):121–131. doi: 10.1016/0166-6851(89)90063-7. [DOI] [PubMed] [Google Scholar]
- Jokipii L., Miettinen A., Jokipii A. M. Antibodies to cysts of Giardia lamblia in primary giardiasis and in the absence of giardiasis. J Clin Microbiol. 1988 Jan;26(1):121–125. doi: 10.1128/jcm.26.1.121-125.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keister D. B. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg. 1983;77(4):487–488. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- Kumkum, Khanna R., Khuller M., Mehta S., Vinayak V. K. Plasma membrane associated antigens of trophozoites of axenic Giardia lamblia. Trans R Soc Trop Med Hyg. 1988;82(3):439–444. doi: 10.1016/0035-9203(88)90157-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nash T. E., Herrington D. A., Levine M. M., Conrad J. T., Merritt J. W., Jr Antigenic variation of Giardia lamblia in experimental human infections. J Immunol. 1990 Jun 1;144(11):4362–4369. [PubMed] [Google Scholar]
- Nash T. E., Herrington D. A., Losonsky G. A., Levine M. M. Experimental human infections with Giardia lamblia. J Infect Dis. 1987 Dec;156(6):974–984. doi: 10.1093/infdis/156.6.974. [DOI] [PubMed] [Google Scholar]
- Nash T. E., McCutchan T., Keister D., Dame J. B., Conrad J. D., Gillin F. D. Restriction-endonuclease analysis of DNA from 15 Giardia isolates obtained from humans and animals. J Infect Dis. 1985 Jul;152(1):64–73. doi: 10.1093/infdis/152.1.64. [DOI] [PubMed] [Google Scholar]
- Nayak N., Ganguly N. K., Walia B. N., Wahi V., Kanwar S. S., Mahajan R. C. Specific secretory IgA in the milk of Giardia lamblia-infected and uninfected women. J Infect Dis. 1987 Apr;155(4):724–727. doi: 10.1093/infdis/155.4.724. [DOI] [PubMed] [Google Scholar]
- Ortega-Barria E., Ward H. D., Evans J. E., Pereira M. E. N-acetyl-D-glucosamine is present in cysts and trophozoites of Giardia lamblia and serves as receptor for wheatgerm agglutinin. Mol Biochem Parasitol. 1990 Dec;43(2):151–165. doi: 10.1016/0166-6851(90)90141-8. [DOI] [PubMed] [Google Scholar]
- Reiner D. S., Douglas H., Gillin F. D. Identification and localization of cyst-specific antigens of Giardia lamblia. Infect Immun. 1989 Mar;57(3):963–968. doi: 10.1128/iai.57.3.963-968.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner D. S., McCaffery M., Gillin F. D. Sorting of cyst wall proteins to a regulated secretory pathway during differentiation of the primitive eukaryote, Giardia lamblia. Eur J Cell Biol. 1990 Oct;53(1):142–153. [PubMed] [Google Scholar]
- Schupp D. G., Januschka M. M., Sherlock L. A., Stibbs H. H., Meyer E. A., Bemrick W. J., Erlandsen S. L. Production of viable Giardia cysts in vitro: determination by fluorogenic dye staining, excystation, and animal infectivity in the mouse and Mongolian gerbil. Gastroenterology. 1988 Jul;95(1):1–10. doi: 10.1016/0016-5085(88)90283-1. [DOI] [PubMed] [Google Scholar]
- Smith P. D., Gillin F. D., Brown W. R., Nash T. E. IgG antibody to Giardia lamblia detected by enzyme-linked immunosorbent assay. Gastroenterology. 1981 Jun;80(6):1476–1480. [PubMed] [Google Scholar]
- Smith P. D., Gillin F. D., Spira W. M., Nash T. E. Chronic giardiasis: studies on drug sensitivity, toxin production, and host immune response. Gastroenterology. 1982 Oct;83(4):797–803. [PubMed] [Google Scholar]
- Smithson K. W., Millar D. B., Jacobs L. R., Gray G. M. Intestinal diffusion barrier: unstirred water layer or membrane surface mucous coat? Science. 1981 Dec 11;214(4526):1241–1244. doi: 10.1126/science.7302593. [DOI] [PubMed] [Google Scholar]
- Taylor G. D., Wenman W. M. Human immune response to Giardia lamblia infection. J Infect Dis. 1987 Jan;155(1):137–140. doi: 10.1093/infdis/155.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward H. D., Alroy J., Lev B. I., Keusch G. T., Pereira M. E. Biology of Giardia lamblia. Detection of N-acetyl-D-glucosamine as the only surface saccharide moiety and identification of two distinct subsets of trophozoites by lectin binding. J Exp Med. 1988 Jan 1;167(1):73–88. doi: 10.1084/jem.167.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward H. D., Alroy J., Lev B. I., Keusch G. T., Pereira M. E. Identification of chitin as a structural component of Giardia cysts. Infect Immun. 1985 Sep;49(3):629–634. doi: 10.1128/iai.49.3.629-634.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward H. D., Kane A. V., Ortega-Barria E., Keusch G. T., Pereira M. E. Identification of developmentally regulated Giardia lamblia cyst antigens using GCSA-1, a cyst-specific monoclonal antibody. Mol Microbiol. 1990 Dec;4(12):2095–2102. doi: 10.1111/j.1365-2958.1990.tb00570.x. [DOI] [PubMed] [Google Scholar]
- Wolfe M. S. Giardiasis. N Engl J Med. 1978 Feb 9;298(6):319–321. doi: 10.1056/NEJM197802092980606. [DOI] [PubMed] [Google Scholar]
- Zenian A. J., Gillin F. D. Intestinal mucus protects Giardia lamblia from killing by human milk. J Protozool. 1987 Feb;34(1):22–26. doi: 10.1111/j.1550-7408.1987.tb03124.x. [DOI] [PubMed] [Google Scholar]
- Zenian A., Gillin F. D. Interactions of Giardia lamblia with human intestinal mucus: enhancement of trophozoite attachment to glass. J Protozool. 1985 Nov;32(4):664–668. doi: 10.1111/j.1550-7408.1985.tb03098.x. [DOI] [PubMed] [Google Scholar]