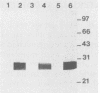
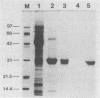
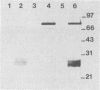
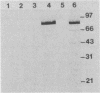
Abstract
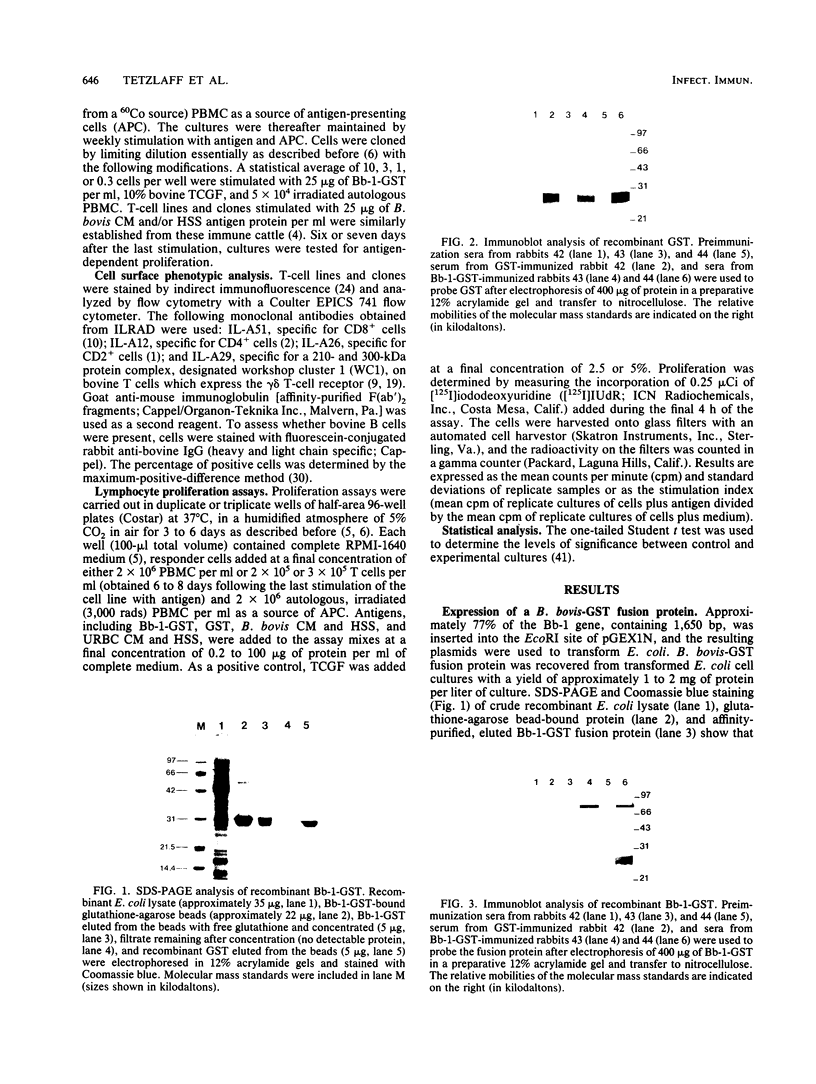
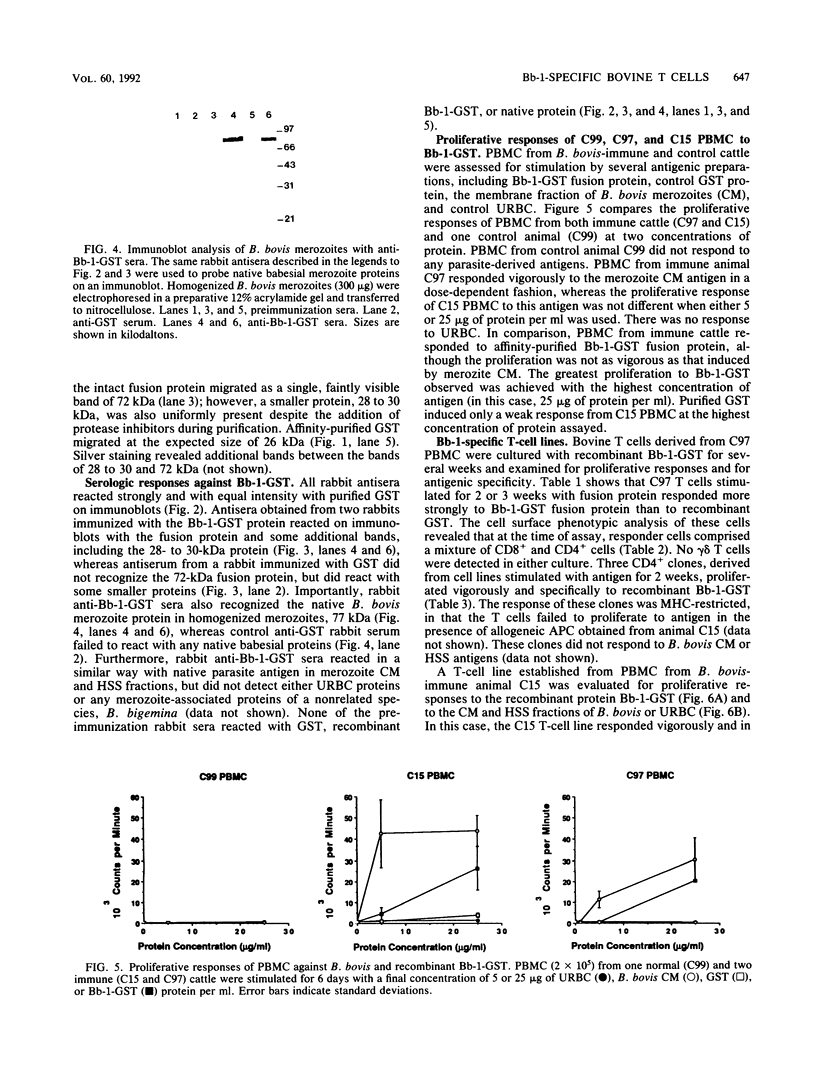
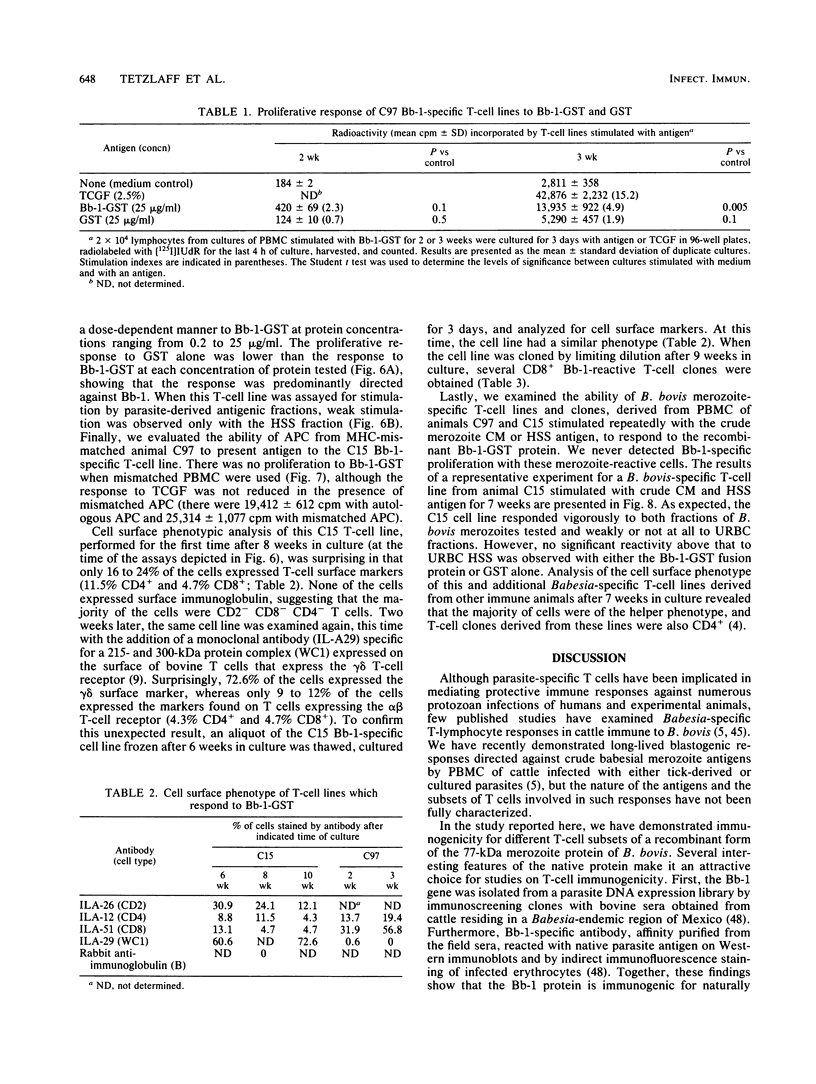
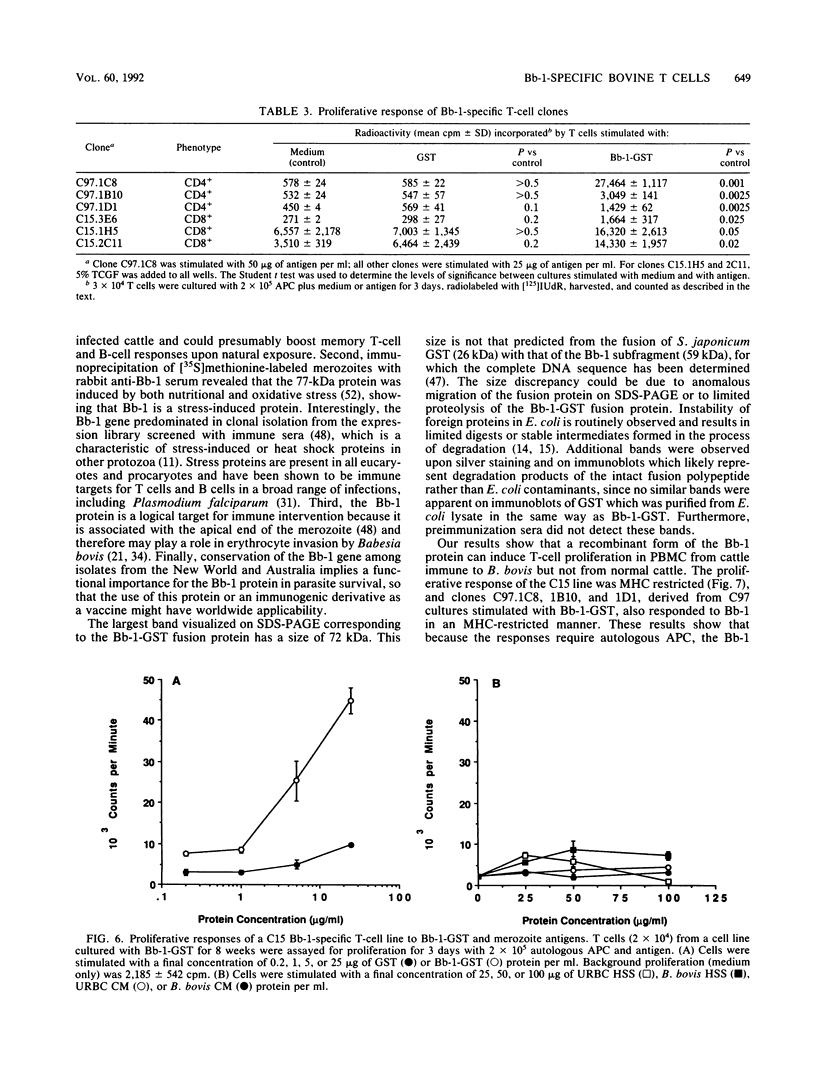
A major portion of a Babesia bovis-specific gene encoding a 77-kDa merozoite protein (Bb-1) produced during natural infection in cattle and in microaerophilous culture was subcloned into the pGEX1N expression vector. Recombinant Bb-1 protein fused to glutathione S-transferase (Bb-1-GST) was used to examine cellular immune responses in B. bovis-immune cattle. Sera from rabbits immunized with Bb-1-GST reacted with fusion protein and with the native antigen present in crude B. bovis but not with B. bigemina merozoites. Bb-1-GST but not GST induced strong proliferation of T lymphocytes from these immune cattle, and Bb-1-reactive T-cell lines which consisted of a mixed population of either CD4+ and CD8+ cells or CD4+, CD8+, and "null" (gamma delta T) cells were established by in vitro stimulation of peripheral blood mononuclear cells with the recombinant fusion protein. Three CD4+ CD8- and three CD4- CD8+ Bb-1-specific T-cell clones were identified after limiting-dilution cloning of the cell lines. The studies described here demonstrate that the 77-kDa protein of B. bovis contains T-cell epitopes capable of eliciting proliferation of two types of T cells in immune cattle, an important consideration for the design of a recombinant subunit vaccine.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin C. L., Machugh N. D., Ellis J. A., Naessens J., Newson J., Morrison W. I. Monoclonal antibodies which react with bovine T-lymphocyte antigens and induce blastogenesis: tissue distribution and functional characteristics of the target antigens. Immunology. 1988 Mar;63(3):439–446. [PMC free article] [PubMed] [Google Scholar]
- Brown W. C., Grab D. J. Biological and biochemical characterization of bovine interleukin 2. Studies with cloned bovine T cells. J Immunol. 1985 Nov;135(5):3184–3190. [PubMed] [Google Scholar]
- Brown W. C., Logan K. S., Wagner G. G., Tetzlaff C. L. Cell-mediated immune responses to Babesia bovis merozoite antigens in cattle following infection with tick-derived or cultured parasites. Infect Immun. 1991 Jul;59(7):2418–2426. doi: 10.1128/iai.59.7.2418-2426.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. C., Lonsdale-Eccles J. D., DeMartini J. C., Grab D. J. Recognition of soluble Theileria parva antigen by bovine helper T cell clones: characterization and partial purification of the antigen. J Immunol. 1990 Jan 1;144(1):271–277. [PubMed] [Google Scholar]
- Brown W. C., Sugimoto C., Conrad P. A., Grab D. J. Differential response of bovine T-cell lines to membrane and soluble antigens of Theileria parva schizont-infected cells. Parasite Immunol. 1989 Nov;11(6):567–583. doi: 10.1111/j.1365-3024.1989.tb00921.x. [DOI] [PubMed] [Google Scholar]
- Clevers H., MacHugh N. D., Bensaid A., Dunlap S., Baldwin C. L., Kaushal A., Iams K., Howard C. J., Morrison W. I. Identification of a bovine surface antigen uniquely expressed on CD4-CD8- T cell receptor gamma/delta+ T lymphocytes. Eur J Immunol. 1990 Apr;20(4):809–817. doi: 10.1002/eji.1830200415. [DOI] [PubMed] [Google Scholar]
- Ellis J. A., Baldwin C. L., MacHugh N. D., Bensaid A., Teale A. J., Goddeeris B. M., Morrison W. I. Characterization by a monoclonal antibody and functional analysis of a subset of bovine T lymphocytes that express BoT8, a molecule analogous to human CD8. Immunology. 1986 Jul;58(3):351–358. [PMC free article] [PubMed] [Google Scholar]
- Engman D. M., Dragon E. A., Donelson J. E. Human humoral immunity to hsp70 during Trypanosoma cruzi infection. J Immunol. 1990 May 15;144(10):3987–3991. [PubMed] [Google Scholar]
- Fikrig E., Barthold S. W., Kantor F. S., Flavell R. A. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science. 1990 Oct 26;250(4980):553–556. doi: 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- Goff W. L., Davis W. C., Palmer G. H., McElwain T. F., Johnson W. C., Bailey J. F., McGuire T. C. Identification of Babesia bovis merozoite surface antigens by using immune bovine sera and monoclonal antibodies. Infect Immun. 1988 Sep;56(9):2363–2368. doi: 10.1128/iai.56.9.2363-2368.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L. Degradation of abnormal proteins in Escherichia coli (protein breakdown-protein structure-mistranslation-amino acid analogs-puromycin). Proc Natl Acad Sci U S A. 1972 Feb;69(2):422–426. doi: 10.1073/pnas.69.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Good M. F., Miller L. H. T-cell antigens and epitopes in malaria vaccine design. Curr Top Microbiol Immunol. 1990;155:65–78. doi: 10.1007/978-3-642-74983-4_5. [DOI] [PubMed] [Google Scholar]
- Herman A., Kappler J. W., Marrack P., Pullen A. M. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annu Rev Immunol. 1991;9:745–772. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- Hines S. A., McElwain T. F., Buening G. M., Palmer G. H. Molecular characterization of Babesia bovis merozoite surface proteins bearing epitopes immunodominant in protected cattle. Mol Biochem Parasitol. 1989 Nov;37(1):1–9. doi: 10.1016/0166-6851(89)90096-0. [DOI] [PubMed] [Google Scholar]
- Howard C. J., Morrison W. I., Bensaid A., Davis W., Eskra L., Gerdes J., Hadam M., Hurley D., Leibold W., Letesson J. J. Summary of workshop findings for leukocyte antigens of cattle. Vet Immunol Immunopathol. 1991 Jan;27(1-3):21–27. doi: 10.1016/0165-2427(91)90072-k. [DOI] [PubMed] [Google Scholar]
- Howard R. F., Stanley H. A., Campbell G. H., Reese R. T. Proteins responsible for a punctate fluorescence pattern in Plasmodium falciparum merozoites. Am J Trop Med Hyg. 1984 Nov;33(6):1055–1059. doi: 10.4269/ajtmh.1984.33.1055. [DOI] [PubMed] [Google Scholar]
- Johnson K. S., Harrison G. B., Lightowlers M. W., O'Hoy K. L., Cougle W. G., Dempster R. P., Lawrence S. B., Vinton J. G., Heath D. D., Rickard M. D. Vaccination against ovine cysticercosis using a defined recombinant antigen. Nature. 1989 Apr 13;338(6216):585–587. doi: 10.1038/338585a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lalor P. A., Morrison W. I., Goddeeris B. M., Jack R. M., Black S. J. Monoclonal antibodies identify phenotypically and functionally distinct cell types in the bovine lymphoid system. Vet Immunol Immunopathol. 1986 Sep;13(1-2):121–140. doi: 10.1016/0165-2427(86)90054-1. [DOI] [PubMed] [Google Scholar]
- Levy M. G., Ristic M. Babesia bovis: continuous cultivation in a microaerophilous stationary phase culture. Science. 1980 Mar 14;207(4436):1218–1220. doi: 10.1126/science.7355284. [DOI] [PubMed] [Google Scholar]
- McElwain T. F., Palmer G. H., Goff W. L., McGuire T. C. Identification of Babesia bigemina and Babesia bovis merozoite proteins with isolate- and species-common epitopes recognized by antibodies in bovine immune sera. Infect Immun. 1988 Jun;56(6):1658–1660. doi: 10.1128/iai.56.6.1658-1660.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison W. I., Goddeeris B. M., Brown W. C., Baldwin C. L., Teale A. J. Theileria parva in cattle: characterization of infected lymphocytes and the immune responses they provoke. Vet Immunol Immunopathol. 1989 Feb;20(3):213–237. doi: 10.1016/0165-2427(89)90003-2. [DOI] [PubMed] [Google Scholar]
- O'Brien R. L., Happ M. P., Dallas A., Palmer E., Kubo R., Born W. K. Stimulation of a major subset of lymphocytes expressing T cell receptor gamma delta by an antigen derived from Mycobacterium tuberculosis. Cell. 1989 May 19;57(4):667–674. doi: 10.1016/0092-8674(89)90135-9. [DOI] [PubMed] [Google Scholar]
- Overton W. R. Modified histogram subtraction technique for analysis of flow cytometry data. Cytometry. 1988 Nov;9(6):619–626. doi: 10.1002/cyto.990090617. [DOI] [PubMed] [Google Scholar]
- Polla B. S. Heat shock proteins in host-parasite interactions. Immunol Today. 1991 Mar;12(3):A38–A41. doi: 10.1016/S0167-5699(05)80011-8. [DOI] [PubMed] [Google Scholar]
- Reduker D. W., Jasmer D. P., Goff W. L., Perryman L. E., Davis W. C., McGuire T. C. A recombinant surface protein of Babesia bovis elicits bovine antibodies that react with live merozoites. Mol Biochem Parasitol. 1989 Jul;35(3):239–247. doi: 10.1016/0166-6851(89)90210-7. [DOI] [PubMed] [Google Scholar]
- Rudzinska M. A., Trager W., Lewengrub S. J., Gubert E. An electron microscopic study of Babesia microti invading erythrocytes. Cell Tissue Res. 1976 Jun 28;169(3):323–334. doi: 10.1007/BF00219605. [DOI] [PubMed] [Google Scholar]
- Ruebush M. J., Hanson W. L. Thymus dependence of resistance to infection with Babesia microti of human origin in mice. Am J Trop Med Hyg. 1980 Jul;29(4):507–515. doi: 10.4269/ajtmh.1980.29.507. [DOI] [PubMed] [Google Scholar]
- Schofield L., Villaquiran J., Ferreira A., Schellekens H., Nussenzweig R., Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987 Dec 17;330(6149):664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- Scott P., Caspar P., Sher A. Protection against Leishmania major in BALB/c mice by adoptive transfer of a T cell clone recognizing a low molecular weight antigen released by promastigotes. J Immunol. 1990 Feb 1;144(3):1075–1079. [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Suarez C. E., Palmer G. H., Jasmer D. P., Hines S. A., Perryman L. E., McElwain T. F. Characterization of the gene encoding a 60-kilodalton Babesia bovis merozoite protein with conserved and surface exposed epitopes. Mol Biochem Parasitol. 1991 May;46(1):45–52. doi: 10.1016/0166-6851(91)90197-e. [DOI] [PubMed] [Google Scholar]
- Templeton J. W., Tipton R. C., Garber T., Bondioli K., Kraemer D. C. Expression and genetic segregation of parental BoLA serotypes in bovine embryos. Anim Genet. 1987;18(4):317–322. doi: 10.1111/j.1365-2052.1987.tb00775.x. [DOI] [PubMed] [Google Scholar]
- Timms P. Development of babesial vaccines. Trans R Soc Trop Med Hyg. 1989;83 (Suppl):73–79. doi: 10.1016/0035-9203(89)90608-1. [DOI] [PubMed] [Google Scholar]
- Timms P., Stewart N. P., Rodwell B. J., Barry D. N. Immune responses of cattle following vaccination with living and non-living Babesia bovis antigens. Vet Parasitol. 1984 Nov;16(3-4):243–251. doi: 10.1016/0304-4017(84)90042-6. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp C. A., Wagner G. G., Rice-Ficht A. C. Babesia bovis: gene isolation and characterization using a mung bean nuclease-derived expression library. Exp Parasitol. 1989 Oct;69(3):211–225. doi: 10.1016/0014-4894(89)90068-4. [DOI] [PubMed] [Google Scholar]
- Wright I. G., Goodger B. V., Clark I. A. Immunopathophysiology of Babesia bovis and Plasmodium falciparum infections. Parasitol Today. 1988 Aug;4(8):214–218. doi: 10.1016/0169-4758(88)90161-5. [DOI] [PubMed] [Google Scholar]
- Wright I. G., Mirre G. B., Rode-Bramanis K., Chamberlain M., Goodger B. V., Waltisbuhl D. J. Protective vaccination against virulent Babesia bovis with a low-molecular-weight antigen. Infect Immun. 1985 Apr;48(1):109–113. doi: 10.1128/iai.48.1.109-113.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright I. G., White M., Tracey-Patte P. D., Donaldson R. A., Goodger B. V., Waltisbuhl D. J., Mahoney D. F. Babesia bovis: isolation of a protective antigen by using monoclonal antibodies. Infect Immun. 1983 Jul;41(1):244–250. doi: 10.1128/iai.41.1.244-250.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]