Abstract
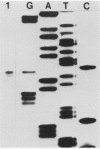
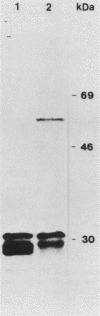
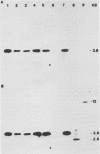
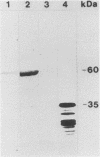
The entire nucleotide sequence of an open reading frame located immediately downstream of the listeriolysin gene from a virulent Listeria monocytogenes serotype 1/2a strain was determined. The product of the open reading frame was 510 amino acids with a predicted molecular weight of 57,400. The deduced amino acid sequence of this open reading frame is highly similar to that of a family of secreted metalloproteases produced by various members of the genus Bacillus, of which thermolysin is the prototype. Immunoblots performed with specific antisera raised against thermolysin from Bacillus stearothermophilus allowed the detection of a 60-kDa polypeptide, corresponding to the pro-form of the protease, in culture supernatants of L. monocytogenes strains. In maxicell experiments, Escherichia coli recombinants harboring this open reading frame also specifically directed production of a 60-kDa protein. Protease activity was low to undetectable in both Listeria strains and E. coli recombinants. This is due to lack of processing of the inactive pro-form of the protease to its mature active form in both species. We have designated this gene mpl for metalloprotease of L. monocytogenes. The gene was present only in pathogenic L. monocytogenes strains, in which it was physically linked to the listeriolysin gene.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baskerville A., Conlan J. W., Ashworth L. A., Dowsett A. B. Pulmonary damage caused by a protease from Legionella pneumophila. Br J Exp Pathol. 1986 Aug;67(4):527–536. [PMC free article] [PubMed] [Google Scholar]
- Bever R. A., Iglewski B. H. Molecular characterization and nucleotide sequence of the Pseudomonas aeruginosa elastase structural gene. J Bacteriol. 1988 Sep;170(9):4309–4314. doi: 10.1128/jb.170.9.4309-4314.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black W. J., Quinn F. D., Tompkins L. S. Legionella pneumophila zinc metalloprotease is structurally and functionally homologous to Pseudomonas aeruginosa elastase. J Bacteriol. 1990 May;172(5):2608–2613. doi: 10.1128/jb.172.5.2608-2613.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button L. L., McMaster W. R. Molecular cloning of the major surface antigen of leishmania. J Exp Med. 1988 Feb 1;167(2):724–729. doi: 10.1084/jem.167.2.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty T., Goebel W. Recent developments in the study of virulence in Listeria monocytogenes. Curr Top Microbiol Immunol. 1988;138:41–58. [PubMed] [Google Scholar]
- Chakraborty T., Huhle B., Bergbauer H., Goebel W. Cloning, expression, and mapping of the Aeromonas hydrophila aerolysin gene determinant in Escherichia coli K-12. J Bacteriol. 1986 Jul;167(1):368–374. doi: 10.1128/jb.167.1.368-374.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart P., Mengaud J. Listeria monocytogenes. A model system for the molecular study of intracellular parasitism. Mol Biol Med. 1989 Oct;6(5):463–474. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domann E., Chakraborty T. Nucleotide sequence of the listeriolysin gene from a Listeria monocytogenes serotype 1/2a strain. Nucleic Acids Res. 1989 Aug 11;17(15):6406–6406. doi: 10.1093/nar/17.15.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fleming D. W., Cochi S. L., MacDonald K. L., Brondum J., Hayes P. S., Plikaytis B. D., Holmes M. B., Audurier A., Broome C. V., Reingold A. L. Pasteurized milk as a vehicle of infection in an outbreak of listeriosis. N Engl J Med. 1985 Feb 14;312(7):404–407. doi: 10.1056/NEJM198502143120704. [DOI] [PubMed] [Google Scholar]
- Jongeneel C. V., Bouvier J., Bairoch A. A unique signature identifies a family of zinc-dependent metallopeptidases. FEBS Lett. 1989 Jan 2;242(2):211–214. doi: 10.1016/0014-5793(89)80471-5. [DOI] [PubMed] [Google Scholar]
- Kubo M., Imanaka T. Cloning and nucleotide sequence of the highly thermostable neutral protease gene from Bacillus stearothermophilus. J Gen Microbiol. 1988 Jul;134(7):1883–1892. doi: 10.1099/00221287-134-7-1883. [DOI] [PubMed] [Google Scholar]
- Leimeister-Wächter M., Chakraborty T. Detection of listeriolysin, the thiol-dependent hemolysin in Listeria monocytogenes, Listeria ivanovii, and Listeria seeligeri. Infect Immun. 1989 Aug;57(8):2350–2357. doi: 10.1128/iai.57.8.2350-2357.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- McClure W. R. Mechanism and control of transcription initiation in prokaryotes. Annu Rev Biochem. 1985;54:171–204. doi: 10.1146/annurev.bi.54.070185.001131. [DOI] [PubMed] [Google Scholar]
- Mengaud J., Vicente M. F., Cossart P. Transcriptional mapping and nucleotide sequence of the Listeria monocytogenes hlyA region reveal structural features that may be involved in regulation. Infect Immun. 1989 Dec;57(12):3695–3701. doi: 10.1128/iai.57.12.3695-3701.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahama K., Yoshimura K., Marumoto R., Kikuchi M., Lee I. S., Hase T., Matsubara H. Cloning and sequencing of Serratia protease gene. Nucleic Acids Res. 1986 Jul 25;14(14):5843–5855. doi: 10.1093/nar/14.14.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T. Transcription termination and the regulation of gene expression. Annu Rev Biochem. 1986;55:339–372. doi: 10.1146/annurev.bi.55.070186.002011. [DOI] [PubMed] [Google Scholar]
- Sidler W., Niederer E., Suter F., Zuber H. The primary structure of Bacillus cereus neutral proteinase and comparison with thermolysin and Bacillus subtilis neutral proteinase. Biol Chem Hoppe Seyler. 1986 Jul;367(7):643–657. doi: 10.1515/bchm3.1986.367.2.643. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Takagi M., Imanaka T., Aiba S. Nucleotide sequence and promoter region for the neutral protease gene from Bacillus stearothermophilus. J Bacteriol. 1985 Sep;163(3):824–831. doi: 10.1128/jb.163.3.824-831.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Burg B., Eijsink V. G., Stulp B. K., Venema G. One-step affinity purification of Bacillus neutral proteases using bacitracin-silica. J Biochem Biophys Methods. 1989 May;18(3):209–219. doi: 10.1016/0165-022x(89)90005-5. [DOI] [PubMed] [Google Scholar]
- Vasantha N., Thompson L. D., Rhodes C., Banner C., Nagle J., Filpula D. Genes for alkaline protease and neutral protease from Bacillus amyloliquefaciens contain a large open reading frame between the regions coding for signal sequence and mature protein. J Bacteriol. 1984 Sep;159(3):811–819. doi: 10.1128/jb.159.3.811-819.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt P., Schorn T., Frei U. Ofloxacin in the treatment of urinary tract infection in renal transplant recipients. Infection. 1988 May-Jun;16(3):175–178. doi: 10.1007/BF01644096. [DOI] [PubMed] [Google Scholar]
- Watkins J., Sleath K. P. Isolation and enumeration of Listeria monocytogenes from Sewage, Sewage Sludge and River Water. J Appl Bacteriol. 1981 Feb;50(1):1–9. doi: 10.1111/j.1365-2672.1981.tb00865.x. [DOI] [PubMed] [Google Scholar]
- Wretlind B., Pavlovskis O. R. Pseudomonas aeruginosa elastase and its role in pseudomonas infections. Rev Infect Dis. 1983 Nov-Dec;5 (Suppl 5):S998–1004. doi: 10.1093/clinids/5.supplement_5.s998. [DOI] [PubMed] [Google Scholar]