Abstract
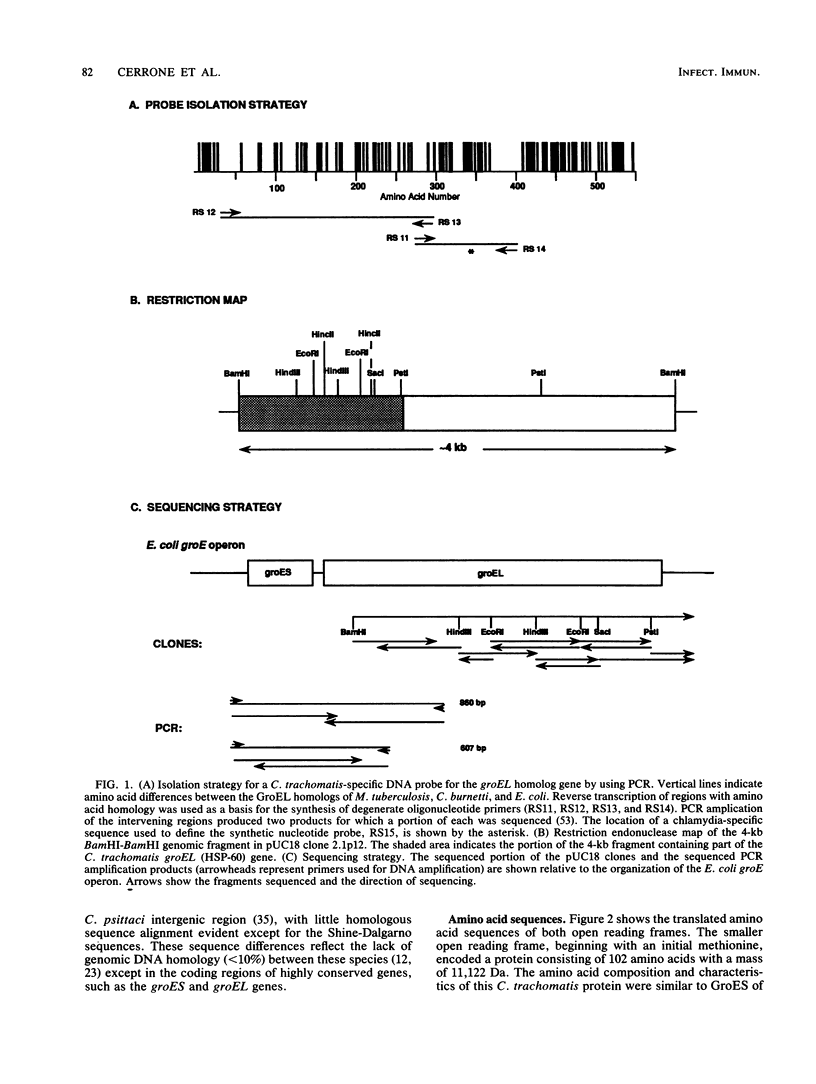
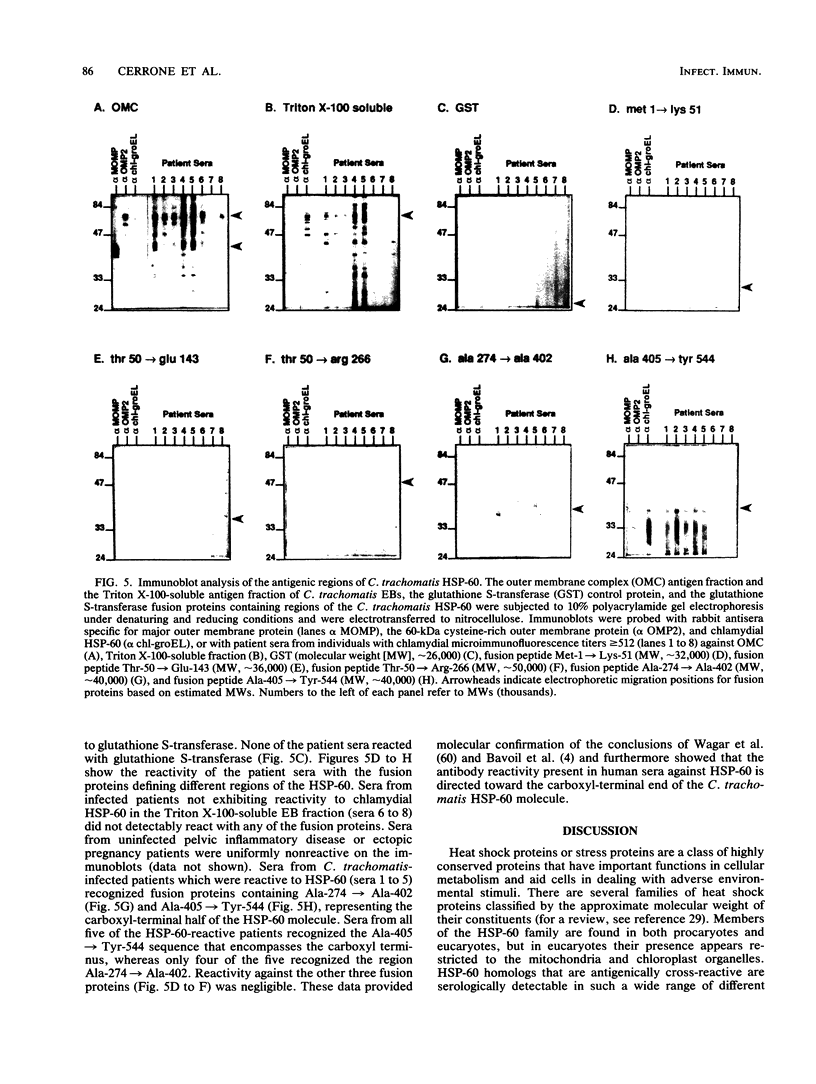
We isolated and sequenced the gene for the chlamydial heat shock protein 60 (HSP-60) from a Chlamydia trachomatis genomic library by molecular genetic methods. The DNA sequence derived revealed an operon-like gene structure with two open reading frames encoding an 11,122- and a 57,956-Da protein. The translated amino acid sequence of the larger open reading frame showed a high degree of homology with known sequences for HSP-60 from several bacterial species as well as with plant and human sequences. By using the determined nucleotide sequence, fragments of the gene were cloned into the plasmid vector pGEX for expression as fusion proteins consisting of glutathione S-transferase and peptide portions of the chlamydial HSP-60. HSP-60 antigenic identity was confirmed by an immunoblot with anti-HSP-60 rabbit serum. Sera from patients that exhibited both high antichlamydial titers and reactivity to chlamydial HSP-60 showed reactivity on immunoblots to two fusion proteins that represented portions of the carboxyl-terminal half of the molecule, whereas fusion proteins defining the amino-terminal half were nonreactive. No reactivity with the fusion proteins was seen with sera from patients that had been previously screened as nonreactive to native chlamydial HSP-60 but which had high antichlamydial titers. Sera from noninfected control subjects also exhibited no reactivity. Definition of recognized HSP-60 epitopes may provide a predictive screen for those patients with C. trachomatis infections who may develop damaging sequelae, as well as providing tools for the study of immunopathogenic mechanisms of Chlamydia-induced disease.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. E., Stephens R. S. Identification by sequence analysis of two-site posttranslational processing of the cysteine-rich outer membrane protein 2 of Chlamydia trachomatis serovar L2. J Bacteriol. 1989 Jan;171(1):285–291. doi: 10.1128/jb.171.1.285-291.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. C., van Schooten W. C., Barry M. E., Janson A. A., Buchanan T. M., de Vries R. R. A Mycobacterium leprae-specific human T cell epitope cross-reactive with an HLA-DR2 peptide. Science. 1988 Oct 14;242(4876):259–261. doi: 10.1126/science.2459778. [DOI] [PubMed] [Google Scholar]
- Baird P. N., Hall L. M., Coates A. R. A major antigen from Mycobacterium tuberculosis which is homologous to the heat shock proteins groES from E. coli and the htpA gene product of Coxiella burneti. Nucleic Acids Res. 1988 Sep 26;16(18):9047–9047. doi: 10.1093/nar/16.18.9047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavoil P., Stephens R. S., Falkow S. A soluble 60 kiloDalton antigen of Chlamydia spp. is a homologue of Escherichia coli GroEL. Mol Microbiol. 1990 Mar;4(3):461–469. doi: 10.1111/j.1365-2958.1990.tb00612.x. [DOI] [PubMed] [Google Scholar]
- Bochkareva E. S., Lissin N. M., Girshovich A. S. Transient association of newly synthesized unfolded proteins with the heat-shock GroEL protein. Nature. 1988 Nov 17;336(6196):254–257. doi: 10.1038/336254a0. [DOI] [PubMed] [Google Scholar]
- Brunham R. C., Maclean I. W., Binns B., Peeling R. W. Chlamydia trachomatis: its role in tubal infertility. J Infect Dis. 1985 Dec;152(6):1275–1282. doi: 10.1093/infdis/152.6.1275. [DOI] [PubMed] [Google Scholar]
- Cheng M. Y., Hartl F. U., Martin J., Pollock R. A., Kalousek F., Neupert W., Hallberg E. M., Hallberg R. L., Horwich A. L. Mitochondrial heat-shock protein hsp60 is essential for assembly of proteins imported into yeast mitochondria. Nature. 1989 Feb 16;337(6208):620–625. doi: 10.1038/337620a0. [DOI] [PubMed] [Google Scholar]
- Ford D. K., Reid G. D., Magge S., Schumacher H. R. Synovial lymphocyte response to chlamydial stimulation associated with intrasynovial chlamydial antigen in a patient with "rheumatoid arthritis". Arthritis Rheum. 1988 Jul;31(7):914–917. doi: 10.1002/art.1780310714. [DOI] [PubMed] [Google Scholar]
- Gerloff R. K., Ritter D. B., Watson R. O. Studies on thermal denaturation of DNA from various chlamydiae. J Infect Dis. 1970 Jan;121(1):65–69. doi: 10.1093/infdis/121.1.65. [DOI] [PubMed] [Google Scholar]
- Ghinsberg R. C., Zigner D., Shoshani-Perlmutter R., Elyan M., Nitzan Y. Chlamydia trachomatis as a possible agent of human autoimmune diseases. Microbiologica. 1989 Oct;12(4):291–296. [PubMed] [Google Scholar]
- Goloubinoff P., Gatenby A. A., Lorimer G. H. GroE heat-shock proteins promote assembly of foreign prokaryotic ribulose bisphosphate carboxylase oligomers in Escherichia coli. Nature. 1989 Jan 5;337(6202):44–47. doi: 10.1038/337044a0. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Wang S. P., Yeh L. J., Kuo C. C. Importance of reinfection in the pathogenesis of trachoma. Rev Infect Dis. 1985 Nov-Dec;7(6):717–725. doi: 10.1093/clinids/7.6.717. [DOI] [PubMed] [Google Scholar]
- Hemmingsen S. M., Woolford C., van der Vies S. M., Tilly K., Dennis D. T., Georgopoulos C. P., Hendrix R. W., Ellis R. J. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988 May 26;333(6171):330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- Holoshitz J., Koning F., Coligan J. E., De Bruyn J., Strober S. Isolation of CD4- CD8- mycobacteria-reactive T lymphocyte clones from rheumatoid arthritis synovial fluid. Nature. 1989 May 18;339(6221):226–229. doi: 10.1038/339226a0. [DOI] [PubMed] [Google Scholar]
- Jindal S., Dudani A. K., Singh B., Harley C. B., Gupta R. S. Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65-kilodalton mycobacterial antigen. Mol Cell Biol. 1989 May;9(5):2279–2283. doi: 10.1128/mcb.9.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karn J., Brenner S., Barnett L., Cesareni G. Novel bacteriophage lambda cloning vector. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5172–5176. doi: 10.1073/pnas.77.9.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H. Heat shock proteins and the immune response. Immunol Today. 1990 Apr;11(4):129–136. doi: 10.1016/0167-5699(90)90050-j. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Väth U., Thole J. E., Van Embden J. D., Emmrich F. Enumeration of T cells reactive with Mycobacterium tuberculosis organisms and specific for the recombinant mycobacterial 64-kDa protein. Eur J Immunol. 1987 Mar;17(3):351–357. doi: 10.1002/eji.1830170308. [DOI] [PubMed] [Google Scholar]
- Keat A. C., Thomas B. J., Taylor-Robinson D., Pegrum G. D., Maini R. N., Scott J. T. Evidence of Chlamydia trachomatis infection in sexually acquired reactive arthritis. Ann Rheum Dis. 1980 Oct;39(5):431–437. doi: 10.1136/ard.39.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. T., Weiss E. Lack of deoxyribonucleic acid homology between species of the genus Chlamydia. J Bacteriol. 1968 Oct;96(4):1421–1423. doi: 10.1128/jb.96.4.1421-1423.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T., Wand-Württenberger A., DeBruyn J., Munk M. E., Schoel B., Kaufmann S. H. T cells against a bacterial heat shock protein recognize stressed macrophages. Science. 1989 Sep 8;245(4922):1112–1115. doi: 10.1126/science.2788923. [DOI] [PubMed] [Google Scholar]
- Kousa M., Saikku P., Richmond S., Lassus A. Frequent association of chlamydial infection with Reiter's syndrome. Sex Transm Dis. 1978 Apr-Jun;5(2):57–61. doi: 10.1097/00007435-197804000-00004. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Bal V., Mendez-Samperio P., Mehlert A., So A., Rothbard J., Jindal S., Young R. A., Young D. B. Stress proteins may provide a link between the immune response to infection and autoimmunity. Int Immunol. 1989;1(2):191–196. doi: 10.1093/intimm/1.2.191. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Menozzi F. D., Menozzi-Dejaiffe C., Nano F. E. Molecular cloning of a gene encoding a Chlamydia psittaci 57-kDa protein that shares antigenic determinants with ca. 60-kDa proteins present in many gram-negative bacteria. FEMS Microbiol Lett. 1989 Mar;49(1):59–63. doi: 10.1016/0378-1097(89)90342-x. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Morrison R. P., Belland R. J., Lyng K., Caldwell H. D. Chlamydial disease pathogenesis. The 57-kD chlamydial hypersensitivity antigen is a stress response protein. J Exp Med. 1989 Oct 1;170(4):1271–1283. doi: 10.1084/jem.170.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison R. P., Lyng K., Caldwell H. D. Chlamydial disease pathogenesis. Ocular hypersensitivity elicited by a genus-specific 57-kD protein. J Exp Med. 1989 Mar 1;169(3):663–675. doi: 10.1084/jem.169.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison R. P., Su H., Lyng K., Yuan Y. The Chlamydia trachomatis hyp operon is homologous to the groE stress response operon of Escherichia coli. Infect Immun. 1990 Aug;58(8):2701–2705. doi: 10.1128/iai.58.8.2701-2705.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munk M. E., Schoel B., Modrow S., Karr R. W., Young R. A., Kaufmann S. H. T lymphocytes from healthy individuals with specificity to self-epitopes shared by the mycobacterial and human 65-kilodalton heat shock protein. J Immunol. 1989 Nov 1;143(9):2844–2849. [PubMed] [Google Scholar]
- Newhall W. J., Batteiger B., Jones R. B. Analysis of the human serological response to proteins of Chlamydia trachomatis. Infect Immun. 1982 Dec;38(3):1181–1189. doi: 10.1128/iai.38.3.1181-1189.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T. Termination of transcription and its regulation in the tryptophan operon of E. coli. Cell. 1981 Apr;24(1):10–23. doi: 10.1016/0092-8674(81)90496-7. [DOI] [PubMed] [Google Scholar]
- Rajasekar R., Sim G. K., Augustin A. Self heat shock and gamma delta T-cell reactivity. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1767–1771. doi: 10.1073/pnas.87.5.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading D. S., Hallberg R. L., Myers A. M. Characterization of the yeast HSP60 gene coding for a mitochondrial assembly factor. Nature. 1989 Feb 16;337(6208):655–659. doi: 10.1038/337655a0. [DOI] [PubMed] [Google Scholar]
- Res P. C., Schaar C. G., Breedveld F. C., van Eden W., van Embden J. D., Cohen I. R., de Vries R. R. Synovial fluid T cell reactivity against 65 kD heat shock protein of mycobacteria in early chronic arthritis. Lancet. 1988 Aug 27;2(8609):478–480. doi: 10.1016/s0140-6736(88)90123-7. [DOI] [PubMed] [Google Scholar]
- Rothman J. E. Polypeptide chain binding proteins: catalysts of protein folding and related processes in cells. Cell. 1989 Nov 17;59(4):591–601. doi: 10.1016/0092-8674(89)90005-6. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987 Mar;169(3):1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Vodkin M. H., Williams J. C. The Mycobacterium tuberculosis 65-kilodalton antigen is a heat shock protein which corresponds to common antigen and to the Escherichia coli GroEL protein. Infect Immun. 1988 Feb;56(2):446–451. doi: 10.1128/iai.56.2.446-451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Sompolinsky D., Hertz J. B., Høiby N., Jensen K., Mansa B., Pedersen V. B., Samra Z. An antigen common to a wide range of bacteria. 2. A biochemical study of a "common antigen" from Pseudomonas aeruginosa. Acta Pathol Microbiol Scand B. 1980 Oct;88(5):253–260. doi: 10.1111/j.1699-0463.1980.tb02637.x. [DOI] [PubMed] [Google Scholar]
- Sompolinsky D., Hertz J. B., Høiby N., Jensen K., Mansa B., Samra Z. An antigen common to a wide range of bacteria. I. The isolation of a 'common antigen' from Pseudomonas aeruginosa. Acta Pathol Microbiol Scand B. 1980 Jun;88(3):143–149. doi: 10.1111/j.1699-0463.1980.tb02620.x. [DOI] [PubMed] [Google Scholar]
- Synovial fluid T cells and 65 kD heat-shock protein. Lancet. 1988 Oct 8;2(8615):856–857. [PubMed] [Google Scholar]
- Taylor H. R., Johnson S. L., Schachter J., Caldwell H. D., Prendergast R. A. Pathogenesis of trachoma: the stimulus for inflammation. J Immunol. 1987 May 1;138(9):3023–3027. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoulfa G., Rook G. A., Bahr G. M., Sattar M. A., Behbehani K., Young D. B., Mehlert A., Van-Embden J. D., Hay F. C., Isenberg D. A. Elevated IgG antibody levels to the mycobacterial 65-kDa heat shock protein are characteristic of patients with rheumatoid arthritis. Scand J Immunol. 1989 Nov;30(5):519–527. doi: 10.1111/j.1365-3083.1989.tb02459.x. [DOI] [PubMed] [Google Scholar]
- Vilppula A. H., Yli-Kerttula U. I., Ahlroos A. K., Terho P. E. Chlamydial isolations and serology in Reiter's syndrome. Scand J Rheumatol. 1981;10(3):181–185. doi: 10.3109/03009748109095295. [DOI] [PubMed] [Google Scholar]
- Vodkin M. H., Williams J. C. A heat shock operon in Coxiella burnetti produces a major antigen homologous to a protein in both mycobacteria and Escherichia coli. J Bacteriol. 1988 Mar;170(3):1227–1234. doi: 10.1128/jb.170.3.1227-1234.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagar E. A., Schachter J., Bavoil P., Stephens R. S. Differential human serologic response to two 60,000 molecular weight Chlamydia trachomatis antigens. J Infect Dis. 1990 Oct;162(4):922–927. doi: 10.1093/infdis/162.4.922. [DOI] [PubMed] [Google Scholar]
- Waldinger D., Eckerskorn C., Lottspeich F., Cleve H. Amino-acid sequence homology of a polymorphic cellular protein from human lymphocytes and the chaperonins from Escherichia coli (groEL) and chloroplasts (Rubisco-binding protein). Biol Chem Hoppe Seyler. 1988 Oct;369(10):1185–1189. doi: 10.1515/bchm3.1988.369.2.1185. [DOI] [PubMed] [Google Scholar]
- Watkins N. G., Hadlow W. J., Moos A. B., Caldwell H. D. Ocular delayed hypersensitivity: a pathogenetic mechanism of chlamydial-conjunctivitis in guinea pigs. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7480–7484. doi: 10.1073/pnas.83.19.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Elliott T. J. Stress proteins, infection, and immune surveillance. Cell. 1989 Oct 6;59(1):5–8. doi: 10.1016/0092-8674(89)90861-1. [DOI] [PubMed] [Google Scholar]
- van Eden W., Thole J. E., van der Zee R., Noordzij A., van Embden J. D., Hensen E. J., Cohen I. R. Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature. 1988 Jan 14;331(6152):171–173. doi: 10.1038/331171a0. [DOI] [PubMed] [Google Scholar]



