Abstract
Stilbenes are considered the most important phytoalexin group in grapevine (Vitis vinifera) and they are known to contribute to the protection against various pathogens. The main stilbenes in grapevine are resveratrol and its derivatives and, among these, pterostilbene has recently attracted much attention due both to its antifungal and pharmacological properties. Indeed, pterostilbene is 5 to 10 times more fungitoxic than resveratrol in vitro and recent studies have shown that pterostilbene exhibits anticancer, hypolipidemic, and antidiabetic properties. A candidate gene approach was used to identify a grapevine resveratrol O-methyltransferase (ROMT) cDNA and the activity of the corresponding protein was characterized after expression in Escherichia coli. Transient coexpression of ROMT and grapevine stilbene synthase in tobacco (Nicotiana benthamiana) using the agroinfiltration technique resulted in the accumulation of pterostilbene in tobacco tissues. Taken together, these results showed that ROMT was able to catalyze the biosynthesis of pterostilbene from resveratrol both in vitro and in planta. ROMT gene expression in grapevine leaves was induced by different stresses, including downy mildew (Plasmopara viticola) infection, ultraviolet light, and AlCl3 treatment.
Stilbenes represent a small class of plant secondary metabolites derived from the phenylpropanoid pathway. In addition to their participation in both constitutive and inducible defense mechanisms in plants, several stilbenes display important pharmacological properties, and, in this respect, the best-studied stilbene is resveratrol (3,5,4′-trihydroxy-trans-stilbene). Hundreds of studies have shown that resveratrol can prevent or slow the progression of a wide variety of illnesses, including cancer and cardiovascular disease, as well as extend the lifespans of various organisms (Baur and Sinclair, 2006). However, pterostilbene, a methyl ether of resveratrol, has recently attracted much attention as a growing number of reports describe promising pharmacological properties. Pterostilbene (3,5-dimethoxy-4′-hydroxy-trans-stilbene) was first isolated from red sandalwood (Pterocarpus santalinus; Seshadri, 1972) and is one of the active constituents of Pterocarpus marsupium, which is used in traditional medicine for the treatment of diabetes. Indeed, pterostilbene was found to decrease significantly plasma Glc levels in hyperglycemic rats (Manickam et al., 1997). Pterostilbene has also been reported to have hypolipidemic properties comparable to clinically used fibrate lipid-lowering drugs (Rimando et al., 2005). The targets of fibrate drugs are members of the peroxisome proliferator-activated receptor (PPAR) group of transcription factors, which play a major role in regulating lipoprotein metabolism (Kersten, 2008). Activation of PPARα in mice and humans reduces hepatic triglyceride production and promotes plasma triglyceride clearance, leading to a reduction in plasma triglyceride levels. Pterostilbene was found to be very effective as a lipid/lipoprotein-lowering agent in hypercholesterolemic hamsters, probably due to the fact that, like fibrate drugs, pterostilbene was shown to act as a PPARα agonist (Rimando et al., 2005). In addition, pterostilbene exhibits antioxidant and anticancer properties similar to those of resveratrol (Jang et al., 1997). Pterostilbene was found to be very effective in preventing carcinogen-induced preneoplastic lesions in a mouse mammary organ culture model (Rimando et al., 2002) and it showed preventive activity against colon carcinogenesis in rats (Suh et al., 2007).
Pterostilbene accumulates constitutively in various organs from a small number of plant species, including wood from P. santalinus and Vaccinium berries (Seshadri, 1972; Rimando et al., 2004). Pterostilbene has also been identified as a phytoalexin in grapevine (Vitis vinifera). Together with resveratrol and its derivatives piceid and viniferins, pterostilbene accumulates in grapevine leaves infected by Plasmopara viticola (Langcake et al., 1979), and low amounts of pterostilbene are also produced during the preparation of grapevine protoplasts (Commun et al., 2003). This compound was also found in healthy grape berries as a constitutive stilbene (Pezet and Pont, 1988). Like resveratrol, pterostilbene was shown to possess antifungal activity against various grapevine pathogens (Langcake et al., 1979). Indeed, pterostilbene was 5 to 10 times more effective than resveratrol in inhibiting the germination of conidia of Botrytis cinerea and sporangia of P. viticola (Jeandet et al., 2002). Despite its high antifungal activity and its promising pharmacological properties, the biosynthetic pathway that produces pterostilbene has not yet been characterized. There is not even any direct evidence that pterostilbene is derived from resveratrol by methylation and earlier attempts to characterize a putative resveratrol O-methyltransferase (ROMT) that could catalyze the direct conversion of resveratrol into pterostilbene were unsuccessful (Jeandet et al., 2002).
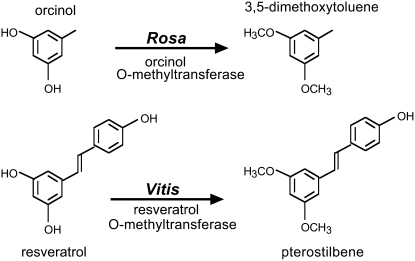
Several O-methyltransferases (OMTs) active with substrates structurally analogous to resveratrol have been characterized. For example, in Scots pine (Pinus sylvestris), pinosylvin (3,5-dihydroxystilbene) can be methylated by a pinosylvin O-methyltransferase (PMT) to pinosylvin 3-O-methyl ether, following ozone or fungal elicitor treatment (Chiron et al., 2000a). In roses (Rosa spp.), orcinol O-methyltransferases (OOMTs) have been shown to catalyze the methylation of orcinol to yield the volatile phenolic ether 3,5-dimethoxytoluene, a major compound of rose scent (Lavid et al., 2002; Scalliet et al., 2002). Starting from these results, we used a candidate gene approach to identify a previously uncharacterized ROMT involved in the biosynthesis of pterostilbene in grapevine. We show that ROMT gene expression in grapevine leaves is induced by fungal infection, UV light, and AlCl3 treatment.
RESULTS
Biosynthesis of Pterostilbene in Grapevine Leaves Infected with P. viticola
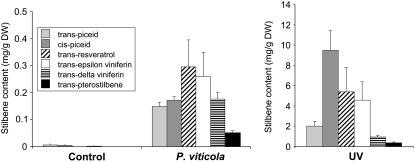
To characterize a putative ROMT from grapevine, the variety Cabernet Sauvignon was chosen because it was shown previously to accumulate pterostilbene upon fungal infection (Langcake et al., 1979). Indeed, inoculation of Cabernet Sauvignon leaves with P. viticola resulted in the accumulation of various stilbenes, including trans-resveratrol, trans- and cis-piceid, viniferins, and pterostilbene (Fig. 1). UV treatment resulted in massive accumulation of stilbenes to 10-fold the levels observed following fungal infection. However, when we specifically quantified pterostilbene, this molecule did not accumulate to as high a relative level in the UV-treated leaves as in the P. viticola-infected leaves. Stilbene quantifications showed leaf-to-leaf variations, probably due to differences in leaf developmental stages because maturity has been shown to influence P. viticola infection (Liu et al., 2003). A number of plant OMTs have been characterized using biochemical approaches (He and Dixon, 1996; Wang et al., 1997; Chiron et al., 2000a; Dudareva et al., 2000; Wu et al., 2004). These strategies typically involved enzyme purification and sequencing of peptides derived from the purified protein. However, this kind of approach can be used only if sufficient enzyme activity is detected in plant extracts. When incubated with resveratrol and S-adenosyl-l-[methyl-14C]Met ([14C]SAM), both P. viticola-infected and UV-treated Cabernet Sauvignon leaf extracts failed to exhibit significant ROMT activity (data not shown). This may be due to low abundance or poor extractability of the ROMT enzyme. Therefore, a candidate gene approach was chosen to search for grapevine ROMT.
Figure 1.
Stilbene contents in control, P. viticola-infected, and UV-treated Cabernet Sauvignon leaves. Stilbenes were analyzed with HPLC-DAD, 72 h posttreatment. Note that, for better readability, the stilbene content scale is different in UV-treated leaves. Standard errors were calculated based on three replicates.
Isolation of a Candidate ROMT cDNA from Grapevine
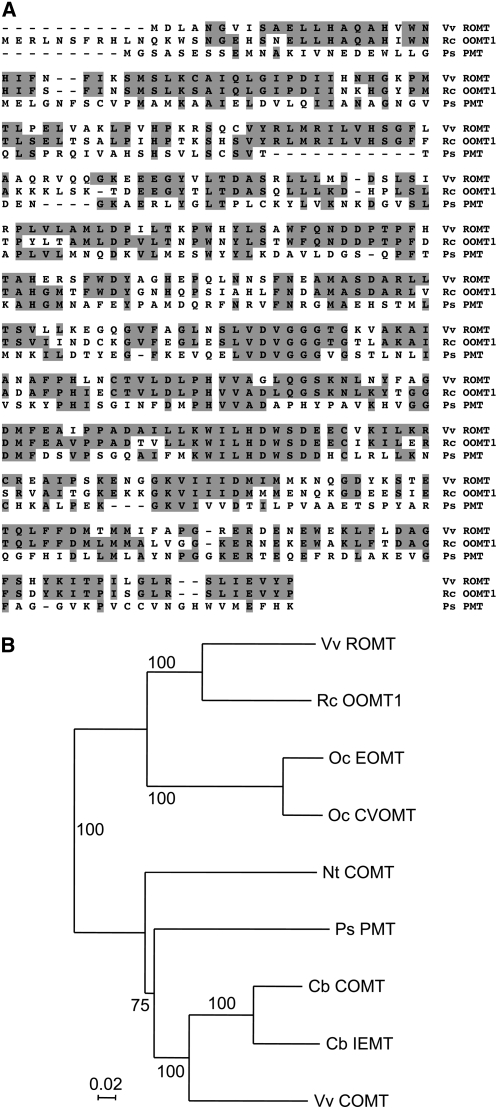
To search for candidate resveratrol OMTs, we first looked for OMTs that have been shown to be active with substrates that are structurally analogous to resveratrol. Such enzymes include PMT from Scots pine and OOMTs from roses. However, PMT was shown to methylate pinosylvin on one hydroxyl group only to yield pinosylvin 3-O-methyl ether (Chiron et al., 2000a). Conversely, OOMTs can methylate both hydroxyl groups of orcinol at positions 3 and 5 to yield the dimethylated molecule 3,5-dimethoxytoluene (Fig. 2; Lavid et al., 2002; Scalliet et al., 2002). In previous studies, we characterized OOMTs from Chinese rose (Rosa chinensis) cv Old Blush (Scalliet et al., 2002, 2006), and preliminary experiments showed that resveratrol could be methylated by purified recombinant OOMT1 from cv Old Blush. Therefore, we searched grapevine public EST collections for OOMT homologs. BLAST searches identified several ESTs with homology to rose OOMTs and their sequences were used to design oligonucleotides to PCR amplify the corresponding full-length coding sequence. Assuming that ROMT is expressed in P. viticola-infected tissues, leaves from Cabernet Sauvignon were infected by immersion in a suspension of P. viticola sporangia (2 × 104 mL−1) and total leaf RNAs were prepared 24 h postinfection. Reverse transcription (RT)-PCR allowed the amplification of a single 1.3-kb DNA fragment (data not shown) that was cloned into pGEM-T Easy and 20 different pGEM clones were sequenced. All clones were very similar, with minor differences probably corresponding to allelic forms. The most represented clone (15 of 20) was selected as a candidate ROMT. This putative ROMT cDNA from Cabernet Sauvignon shared 74% nucleotide sequence identity with rose OOMT1. The predicted amino acid sequence of the candidate ROMT shared 69.5% and 30% identity with rose OOMT1 and PMT from Scots pine, respectively (Fig. 3A). Phylogenetic analysis of selected members of the plant small molecule OMT gene family (Noel et al., 2003) showed that PMT and ROMT belong to distinct clades corresponding to different OMT subfamilies (Fig. 3B).
Figure 2.
Biosynthesis of 3,5-dimethoxytoluene in Rosa and proposed biosynthesis of pterostilbene in grapevine.
Figure 3.
Comparison of grapevine ROMT with other OMTs. A, The predicted amino acid sequence of grapevine ROMT was aligned with Chinese rose cv Old Blush OOMT1 and Scots pine PMT with ClustalW. Residues shaded in gray indicate identical amino acids. B, Phylogenetic tree of selected OMT cDNA sequences: grapevine ROMT (VvROMT, accession no. FM178870), grapevine caffeic acid OMT (VvCOMT, AF239740), Chinese rose OOMT1 (RcOOMT1, AJ439741), basil chavicol OMT (ObCVOMT, AF435007), basil eugenol OMT (ObEOMT, AF435008), Clarkia breweri (iso)eugenol OMT (CbIEMT, U86760), C. breweri caffeic acid OMT (CbCOMT, AF006009), Nicotiana tabacum caffeic acid OMT (NtCOMT, AF484252), and Scots pine pinosylvin OMT (PsPMT; Chiron et al., 2000b). The numbers beside the branches represent bootstrap values based on 500 replicates.
Characterization of Recombinant ROMT
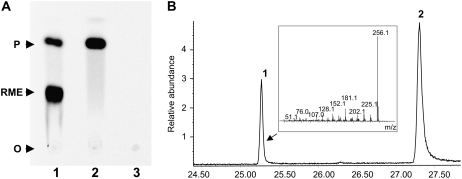
The ROMT coding sequence was then cloned into the Gateway-compatible entry vector pDNOR207, and then transferred into the destination vector pHNGWA (Busso et al., 2005) to express the corresponding protein in Escherichia coli. ROMT was expressed as a NusA fusion protein, purified and cleaved on the resin with thrombin to yield purified recombinant ROMT enzyme. To characterize its putative ROMT activity, purified recombinant ROMT was incubated with resveratrol, resveratrol monomethyl ether (RME; 3-methoxy-4′,5-dihydroxy-trans-stilbene), and pterostilbene in the presence of [14C]SAM. Thin-layer chromatography (TLC) analysis of reaction products showed that, after a short incubation of ROMT with resveratrol and [14C]SAM, two products were produced that comigrated with RME and pterostilbene standards, respectively. ROMT was also able to methylate RME to yield pterostilbene. Conversely, no methylation product of pterostilbene was detected (Fig. 4A). Gas chromatography (GC)-mass spectrometry (MS) analysis following a longer incubation of ROMT with resveratrol and a 2-fold molar amount of SAM identified pterostilbene as the major reaction product (Fig. 4B).
Figure 4.
Analysis of ROMT reaction products. A, TLC analysis of reaction products produced following incubation of recombinant ROMT with 200 μm resveratrol (1), 200 μm RME (2), and 200 μm pterostilbene (3) in the presence of [14C]SAM (50 μm). Reactions were carried out in a total volume of 25 μL with 200 ng of purified protein and were allowed to proceed for 15 min. The positions of the origin (O) and the reaction products RME and pterostilbene (P) are indicated. B, GC-MS analysis of ROMT reaction products. Recombinant ROMT was incubated with resveratrol (500 μm) in the presence of [14C]SAM (1 mm). Peak 1, Pterostilbene; peak 2, resveratrol. A total ion chromatogram is shown and mass spectra of the peaks matched those of the corresponding authentic standards. The reaction was carried out in a total volume of 200 μL with 2 μg of purified protein and was allowed to proceed for 60 min.
ROMT exhibited equivalent Km values for resveratrol and RME (12 and 14 μm, respectively); however, RME was the preferred substrate, with Kcat and specific activity values being significantly higher than for resveratrol. The activity of ROMT was then tested in vitro with a number of potential substrates. ROMT exhibited a remarkable preference for resveratrol and RME over all compounds tested. Indeed, the specific activities of ROMT with orcinol, eugenol, or caffeic acid were a few percent of its specific activity with its preferred substrate RME (Table I).
Table I.
Kinetic parameters of ROMT with potential substrates
Data are expressed as the means of triplicate assays and ses are indicated in parentheses. n.d., Not determined.
| Substrate | Km | Kcat × 10−3 | Kcat/Km | Specific Activity |
|---|---|---|---|---|
| μm | s−1 | m−1 s−1 | pkat mg−1 | |
| Resveratrol | 12 (4) | 62 (3) | 5,160 (254) | 786 (30) |
| RME | 14 (3) | 86 (9) | 6,142 (325) | 1,097 (108) |
| Pterostilbene | n.d. | n.d. | n.d. | 5 (4) |
| Orcinol | n.d. | n.d. | n.d. | 10 (6) |
| Caffeic acid | n.d. | n.d. | n.d. | 58 (7) |
| Eugenol | n.d. | n.d. | n.d. | 75 (8) |
Characterization of ROMT Activity in Planta
Recombinant ROMT exhibited ROMT activity in vitro, catalyzing the biosynthesis of pterostilbene from resveratrol in the presence of SAM. However, predicting the physiological substrate of OMTs from in vitro data only is not trivial (Schröder et al., 2002). In a previous work, we used Agrobacterium-mediated transient transformation of Nicotiana benthamiana to characterize rose OOMT activity (Scalliet et al., 2006). Therefore, we chose the same approach to investigate ROMT activity in planta. For in planta expression, the ROMT coding sequence was transferred in the Gateway-compatible plant transformation vector pB2GW7 (Karimi et al., 2002) to yield the pB2GW7-ROMT construct. To provide ROMT with resveratrol substrate, ROMT was coexpressed with the grapevine stilbene synthase (STS) cDNA VST1 (Melchior and Kindl, 1991). Transgenic expression of VST1 in various plant species, including tobacco (Nicotiana spp.), tomato (Solanum lycopersicum), papaya (Carica papaya), and grapevine, has been shown to result in resveratrol and resveratrol derivative accumulation in transformed plants (Hain et al., 1990; Thomzik et al., 1997; Hipskind and Paiva, 2000; Coutos-Thévenot et al., 2001). As a control, tobacco leaves were infiltrated with Agrobacterium harboring a 35S-GFP construct (Haseloff et al., 1997). No stilbenes were detected in extracts from leaves expressing GFP (Fig. 5A). However, significant amounts of resveratrol and piceid accumulated when STS was expressed (Fig. 5B) and the coexpression of STS and ROMT resulted in a marked decrease in the resveratrol and piceid peaks, together with strong accumulation of pterostilbene (Fig. 5C). The pterostilbene peak was collected and its identity was confirmed by GC-MS analysis (data not shown). Together, these results showed that ROMT from Cabernet Sauvignon possessed ROMT activity catalyzing the biosynthesis of pterostilbene from resveratrol both in vitro and in planta.
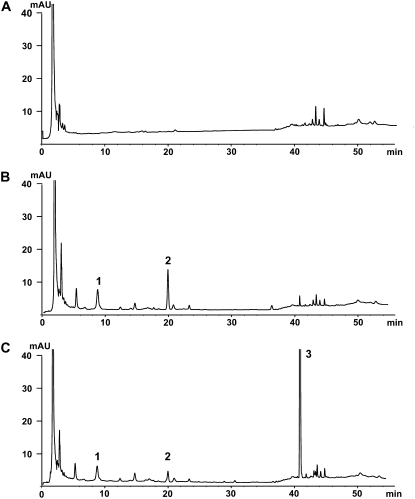
Figure 5.
Characterization of ROMT activity in planta using Agrobacterium-mediated transient transformation. Tobacco leaf sectors (150 mg fresh weight) expressing GFP (A), grapevine STS (B), or coexpressing STS and ROMT (C) were excised 48 h after Agrobacterium-mediated transformation. Stilbene content was analyzed using HPLC-DAD. Peak 1, Piceid; peak 2, resveratrol; peak 3, pterostilbene. Identity of peak 3 was confirmed by GC-MS analysis (data not shown).
Analysis of ROMT Gene Expression
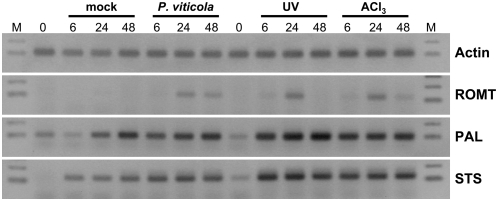
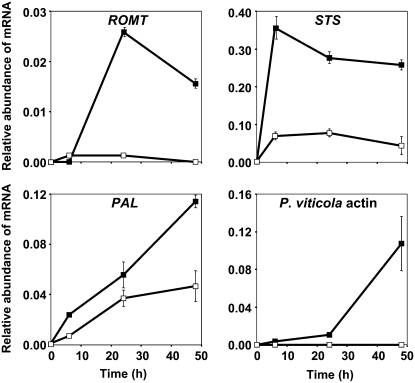
Both biotic and abiotic stresses (UV exposure, AlCl3 treatment) have been shown to induce the accumulation of stilbenes and the expression of genes involved in stilbene biosynthesis in grapevine (Fritzmeier and Kindl, 1981; Adrian et al., 1996; Douillet-Breuil et al., 1999; Kortekamp, 2006). Therefore, we analyzed ROMT gene expression in grapevine leaf discs submitted to abiotic stresses and fungal infection. Equal amounts of total RNA from control (t = 0), mock-infected, P. viticola-infected, UV-treated, and AlCl3-treated grapevine leaf discs were used for semiquantitative RT-PCR analysis of ROMT, PAL (for Phe ammonia-lyase), and STS expression. Total RNAs were prepared 0, 6, 24, and 48 h posttreatment and actin was used as a control (Fig. 6). PAL and STS expression was induced 6 h posttreatment compared to the control (t = 0) in P. viticola-infected and in UV- and AlCl3-treated leaf discs and transcript levels remained high 48 h posttreatment. PAL and STS were also induced in mock-infected discs, presumably as a result of the wounding response following disc cutting. Indeed, wound induction of PAL and STS gene expression has been shown to occur in different plant species (Lawton and Lamb, 1987; Chiron et al., 2000b). Like PAL and STS, ROMT gene expression was induced in P. viticola-infected and UV- and AlCl3-treated leaf discs, reaching a maximum 24 h posttreatment. However, ROMT gene expression was not induced in mock-infected discs, indicating that this gene does not respond to the stress induced by this experimental procedure, which includes wounding stress due to disc cutting. These results were confirmed in an independent experiment using real-time quantitative RT-PCR (Fig. 7). ROMT expression was transiently induced in response to P. viticola infection, with a maximum 24 h postinfection.
Figure 6.
RT-PCR analysis of ROMT expression in grapevine leaf discs submitted to different stresses. Sets of leaf discs were immersed in sterile ultrapure water (mock) or infected by immersion in a suspension of P. viticola sporangia (2 × 104 mL−1) in sterile ultrapure water. UVC irradiation (λ = 254 nm, 90 μW cm−2) was applied for 7 min. AlCl3 solution (1% [w/v]) was applied directly on the discs. Leaf discs were collected at t = 0 (control), 6, 24, and 48 h after treatments and frozen before RNA extraction. Selected genes are actin, ROMT, PAL, and STS. M, DNA Mr marker. The data shown are representative of three independent experiments.
Figure 7.
Quantitative RT-PCR analysis of ROMT expression in grapevine leaf discs infected by P. viticola. Transcript accumulation of ROMT (accession no. FM178870), STS (accession no. X76892), and PAL (accession no. CF511227) genes was monitored in mock-infected leaf discs (white squares) and P. viticola-infected leaf discs (black squares). Pathogen development was evaluated by monitoring P. viticola actin transcript accumulation (P. viticola actin). Analyses were performed by real-time quantitative RT-PCR. Transcript levels of grapevine genes are expressed as relative values normalized to the transcript level of actin gene, used as an internal reference (accession no. AF369525). Absolute copy number of mRNA for each target gene in the t = 0 control sample was 0 (OMT), 24 × 103 (STS), and 571 × 103 (PAL) molecules/μg of plant total RNAs. Results are means of duplicate experiments; bars indicate ± ses.
DISCUSSION
Characterization of a Novel ROMT from Grapevine
Although pinosylvin closely resembles resveratrol, PMT is only able to methylate pinosylvin on one hydroxyl group, to yield pinosylvin 3-O-methyl ether (Chiron et al., 2000a). Therefore, we hypothesized that the putative ROMT enzyme was more likely to resemble rose OOMTs, which catalyze the biosynthesis of the dimethylated molecule 3,5-dimethoxytoluene (Fig. 2; Lavid et al., 2002; Scalliet et al., 2002). Identification of OOMT homologs among grapevine EST collections allowed the cloning of a candidate ROMT cDNA from Cabernet Sauvignon. The predicted amino acid sequence of this putative ROMT shared 69.5% and 30% identity with rose OOMT1 and P. sylvestris PMT, respectively (Fig. 3A). Phylogenetic analysis of selected members of the plant OMT gene family showed that PMT and ROMT belong to different subfamilies. Indeed, PMT is related to the caffeic acid OMT (COMT) gene family, whereas ROMT belongs to a distinct clade, which includes rose OOMT, chavicol, and eugenol OMT from basil (Ocimum basilicum; Gang et al., 2002; Fig. 3B). Stilbenes occur naturally in a small number of unrelated plant species and phylogenetic analysis of the STS and chalcone synthase (CHS) gene family showed that STS genes have evolved from CHS genes several times independently in the course of evolution (Tropf et al., 1994). Consistent with this repeated evolution of STS genes, the stilbene methylation enzymes PMT and ROMT also evolved independently in Scots pine and grapevine, respectively, from different OMT ancestors. Unlike PMT, which was shown to be active with a broad range of substrates (Chiron et al., 2000a), ROMT exhibited remarkable specificity for resveratrol and RME (Table I). Determination of ROMT kinetic parameters showed that Km values for resveratrol and RME were similar (12 and 14 μm, respectively); however, ROMT exhibited a significantly higher specific activity with RME as a substrate than with resveratrol (Table I). This may explain why, when ROMT was incubated with resveratrol in the presence of SAM, the RME intermediate accumulated only if the incubation time was short (Fig. 4A). When longer incubation times were used, the only product observed was pterostilbene and no accumulation of the monomethyl intermediate was detected (Fig. 4B). Pterostilbene accumulation following coexpression of ROMT and STS in tobacco (Fig. 5C) confirmed that ROMT alone was sufficient to catalyze both resveratrol methylation steps to yield pterostilbene. In contrast, the biosynthesis of DMT from orcinol in roses (Fig. 2) involves two different OOMT1 and OOMT2 enzymes, which operate sequentially to catalyze the first and the second methylation step of orcinol, respectively (Lavid et al., 2002). The evolution of OOMT1 from the duplicated OOMT2 gene most probably corresponds to an optimization of DMT biosynthesis in Chinese roses (Scalliet et al., 2008). A BLAST search of the genome of the highly homozygous PN40024 grapevine cultivar (Jaillon et al., 2007) identified a small family of four putative ROMT genes located on chromosome 12 (accession nos. CAO69896, CAO69893, CAO69817, and CAO69813), all of which were very similar to ROMT from Cabernet Sauvignon. However, none of these genes was identical to the ROMT gene characterized in this article, which probably represents an allele of the CAO69896 gene of PN40024. Indeed, the protein deduced from the CAO69896 gene differed in only three amino acid positions from ROMT from Cabernet Sauvignon. Additional work will be needed to characterize the function of the other members of this gene family.
ROMT Gene Expression Is Induced by Biotic and Abiotic Stresses
UV light, AlCl3 treatments and fungal infection have been shown to induce the accumulation of stilbenes in grapevine leaves (Fritzmeier and Kindl, 1981; Adrian et al., 1996; Douillet-Breuil et al., 1999). However, in grapevine tissues, pterostilbene usually accumulates in low amounts compared to other resveratrol derivatives (Langcake et al., 1979; Pezet and Pont, 1988). Inoculation of Cabernet Sauvignon leaves with P. viticola resulted in an accumulation of pterostilbene corresponding to about 5% total stilbene content (Fig. 1). Stilbene accumulation following UV treatment was much higher, reaching 10-fold the abundance detected after fungal infection. However, pterostilbene accumulation in UV-treated leaves was relatively lower, corresponding to only 1.5% of total stilbenes. This could be explained by a limiting ROMT activity in Cabernet Sauvignon leaves. Although ROMT gene expression was induced following both P. viticola infection and UV exposure, ROMT enzyme activity may not have been sufficient to produce high amounts of pterostilbene. trans- and cis-piceid accounted for 30% to 50% of total stilbenes in P. viticola-infected or UV-treated leaves (Fig. 1), indicating that glucosyltransferases may have competed with ROMT for the resveratrol substrate (Hall and De Luca, 2007). Furthermore, stilbene quantification following elicitation of grape cell suspensions showed that most of the resveratrol was secreted into the culture medium (Aziz et al., 2003). Therefore, resveratrol methylation may also be impaired by secretion mechanisms that lower its availability to ROMT.
In Scots pine, PMT gene transcription was shown to be induced by various stresses, including ozone treatment, fungal infection, and wounding (Chiron et al., 2000b). Semiquantitative RT-PCR analysis of ROMT expression showed that, like PAL and STS genes, ROMT expression was induced in P. viticola-infected and in UV- and AlCl3-treated leaf discs. ROMT transcript accumulated transiently, reaching a maximum 24 h posttreatment, and decreased markedly afterward. These results were confirmed using real-time quantitative RT-PCR (Fig. 7). Quantification of ROMT transcripts in response to P. viticola infection showed that, although they accumulated following infection, their absolute number remained low in comparison to PAL or STS transcripts. This relatively low level of ROMT transcript accumulation, together with the weak activity of the corresponding enzymes in grapevine extracts, may explain why earlier attempts to characterize ROMT were unsuccessful (Jeandet et al., 2002).
Pterostilbene Accumulates as the Major Stilbene following in Planta Coexpression of STS and ROMT Genes
Although STS enzymes occur naturally in only a few plant species, the substrates necessary to synthesize stilbenes are present in all higher plants. In consequence, the ability to synthesize stilbenes can be conferred to many plant species by expressing an STS gene (Hain et al., 1990; Hipskind and Paiva, 2000; Yu et al., 2006). In this work, we used tobacco leaves capable of stilbene biosynthesis due to the transient expression of the grapevine STS VST1 cDNA to characterize the activity of ROMT in planta. Unlike grapevine, where pterostilbene accumulates to relatively low amounts, pterostilbene was the major stilbene in tobacco leaves coexpressing STS and ROMT (Fig. 5). Given the antibiotic properties of stilbenes, STS genes have been used as targets for transgenic crop enhancement. STS genes from peanut (Arachis hypogaea) and grape have been expressed in tobacco and alfalfa (Medicago sativa), respectively, and in both cases, enhanced resistance to pathogenic fungi was conferred to the transgenic host plants (Hain et al., 1990; Hipskind and Paiva, 2000). Due to the higher fungitoxicity of pterostilbene compared to resveratrol (Pezet and Pont, 1990; Pezet et al., 2004), the combination of STS and ROMT genes in transgenic plants may lead to improved resistance characteristics. In particular, such transgenic plants will allow detailed investigation of the fungitoxic properties of pterostilbene in vivo to complete the data obtained from in vitro studies (Langcake et al., 1979; Pezet and Pont, 1990). Recent transcript-profiling experiments have been aimed at identifying the molecular pathways affected by pterostilbene exposure in yeast (Saccharomyces cerevisiae; Pan et al., 2008). These included lipid and Met metabolism and further characterization of cellular targets of pterostilbene may provide new insights into the molecular basis of its fungitoxicity.
In conclusion, we characterized a grapevine ROMT catalyzing the efficient biosynthesis of pterostilbene from resveratrol, both in vitro and in planta. The anticancer, hypolipidemic, and antidiabetic properties of pterostilbene have recently attracted much interest (Rimando et al., 2005). In particular, the high hypolipidemic activity of pterostilbene may provide alternative treatments of dyslipidemia in the future. Thus, the identification of ROMT constitutes a major step in understanding the biosynthesis of a plant stilbene with promising pharmacological properties.
MATERIALS AND METHODS
Chemicals and Radiochemicals
trans-Resveratrol and trans-pterostilbene were from Sigma-Aldrich. trans-Piceid, trans-δ-viniferin, and trans-ɛ-viniferin standards were kindly provided by R. Pezet (Changins, Switzerland). cis forms of stilbenes were obtained by photoisomerization under UV light of trans-stilbene standard solutions at 5 μg mL−1. [14C]SAM (55 mCi/mmol) was from GE Healthcare-Amersham Biosciences. RME (3-methoxy-5,4′-dihydroxy-trans-stilbene) was obtained from a partial chemical methylation of resveratrol. One hundred milligrams of resveratrol were incubated with equimolar amounts of iodomethane and K2CO3 in acetone (8 mL final volume) for 3 h at 50°C. RME was purified using preparative TLC and HPLC, and characterized by GC-MS). RME characteristics corresponded to published data (λmax 307 nm, 320 nm in 50% acetonitrile, ion at m/z 242; Katsuyama et al., 2007; Mikstacka et al., 2007). HPLC-grade solvents (acetonitrile, methanol) were from Merck and used in combination with sterilized water from Aguettant. All other chemicals and reagents were from Sigma.
Plant Material and Leaf Sample Preparation
Grapevine (Vitis vinifera ‘Cabernet Sauvignon’) plantlets were obtained from cuttings and grown on potting soil in a greenhouse at a temperature of 22°C and 19°C (day and night, respectively), with a photoperiod of 16 h of light (supplemental light provided by sodium lamp illumination), until they developed 12 to 14 fully expanded leaves. The sixth leaf, counted from the apex, was detached, rinsed with sterile demineralized water, and leaf discs (14-mm diameter) were punched. For each sample, leaves from four different plants were used and four leaf discs were pooled, each coming from a different plant, to represent the same individuals in every time points.
For AlCl3 treatment, leaf discs were placed (abaxial face up) in petri dishes on filter paper soaked with 5 mL of a freshly prepared 1% AlCl3 solution. For UV treatment, leaf discs were placed abaxial face up in petri dishes on wet filter paper and exposed for 6 min to UV light (90 μW cm−2) from a UVC tube (Osram; 30 W, 254 nm).
Plasmopara viticola isolate was harvested on grapevine cv Chardonnay-infected leaves in the experimental vineyard plot at INRA-Colmar in 2006 and maintained on grapevine (cv Muscat ottonel) seedlings. Leaf discs were infected by immersion in a suspension of P. viticola sporangia in sterile water (2 × 104 mL−1) and then transferred on humid filter paper in sealed petri dishes. Mock-inoculated leaf discs were immersed in sterile water. All leaf discs were kept at 21°C under 16/8-h photoperiod (200 μmol m−2 s−1). Leaf discs were collected at 0, 6, 24, and 48 h after treatment, immediately frozen in liquid nitrogen, and conserved at −80°C until RNA extraction.
Stilbene Analyses
Stilbene extractions and HPLC-DAD analyses were performed as described previously (Poutaraud et al. 2007). GC-MS analyses were performed as described previously (Mikstacka et al., 2007).
Cloning of ROMT cDNA
ROMT coding region was amplified by RT-PCR using the upstream primer 5′-ATGGATTTGGCAAACGCTGTGATATCAGCTGA-3′ and the downstream primer 5′-TCAAGGATAAACCTCAATGAGGGACCTCAAACC-3′. Primers were designed based on ESTs CF202077 and CF209780. RT was performed as indicated below. PCR amplification was carried out for 30 cycles of denaturation at 94°C for 30 s, annealing at 52°C for 30 s, and extension at 72°C for 1 min with a final extension of 5 min, in a GeneAmp PCR system 9700 cycler (Perkin-Elmer), using Ex Taq DNA polymerase (TaKaRa). Amplified DNA fragments were cloned into pGEM-T Easy (Promega) and the inserts sequenced.
Phylogenetic Analysis
OMT sequences were obtained from GenBank. Nucleic acid and protein sequences were aligned using ClustalW (Thompson et al., 1994). The Phylo_win program (Galtier et al., 1996) was used to construct phylogenetic trees, using the neighbor-joining method, with 500 bootstrap replicates.
Characterization of Recombinant ROMT
ROMT cDNA was amplified by PCR using the upstream primer 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTTGGTTCCGCGTGGATCCATGGATTTGGCAAACGCTGTG-3′ and the downstream primer 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTTCATCAAGGATAAACCTCAATGAG-3′, and cloned into pDONR207 Gateway-compatible vector (Invitrogen). ROMT cDNA was sequenced to verify that no mutation had been introduced, and subsequently transferred into the pHNGWA destination vector (accession no. EU680842; Busso et al., 2005). ROMT enzyme was expressed as His-tagged NusA-fusion protein. Recombinant ROMT was purified using TALON metal affinity resin (CLONTECH) and characterized after cleavage of the NusA moiety using thrombin (GE Healthcare-Amersham). Purified recombinant ROMT was incubated in a final volume of 25 μL with 35 μm [14C]SAM and 200 μm to 1 mm of stilbene substrates in 0.1 m Tris, pH 7.5, containing 20% glycerol (v/v), 5 mm MgCl2, and 14 mm 2-mercaptoethanol. Ranges of stilbene substrate concentrations of between 5 μm and 1 mm were used for Km determination and the incorporated radioactivity was measured by liquid scintillation. Km and Vmax values were calculated from Lineweaver-Burk plots. Reaction products were analyzed by TLC on silica gel (Merck) with dichloromethane/acetone (40/3 [v/v]) as the solvent, using a Molecular Imager FX phosphorimager (Bio-Rad). Enzyme reaction products were identified by comigrating with standards.
Transient Expression in Tobacco
For Agrobacterium-mediated transient expression, ROMT cDNA was transferred into the GATEWAY-compatible binary vector pB2GW7 (Karimi et al., 2002). Grapevine STS was expressed in tobacco (Nicotiana benthamiana) using a 35S:VST1 construct described previously (Santos-Rosa et al., 2008) and m-GFP5-HDEL was used as a control (Haseloff et al., 1997). All constructs were introduced into Agrobacterium tumefaciens strain C58 (pMP90) by electroporation. Tobacco leaves were infiltrated with A. tumefaciens cultures (OD600 0.1–0.3) according to Batoko et al. (2000). Disks were punched from tobacco leaves 48 h after Agrobacterium infiltration and analyzed for stilbene content.
RNA Isolation and Semiquantitative RT-PCR
Total RNAs were isolated using RNeasy plant mini kit (Qiagen) according to the manufacturer's instructions and quantified using a Nanodrop ND-1000 spectrophotometer (Thermo Scientific). Residual genomic DNA was removed by performing on-column DNase I digestion with the RNase-Free DNase set (Qiagen). One microgram of total RNAs was used as template for RT, using the RevertAid moloney murine leukemia virus reverse transcriptase (Fermentas), with 0.5 μg of oligo(dT)18, for 1 h at 42°C. PCR amplifications were performed on 5 μL of the 10× diluted cDNA solution using Taq DNA Polymerase from Promega, with 28 cycles of 94°C for 15 s, 55°C for 30 s, and 72°C for 30 s. Primers are described below. All PCR products were separated onto a 2% agarose gel stained with EtBr and image processing was carried out with a Bio-Rad GelDoc apparatus (Bio-Rad), bands analyzed with Quantity One software, version 4.4.1 (Bio-Rad).
Real-Time Quantitative RT-PCR
Transcript levels were determined by real-time one-step RT-PCR using iCycler iQ thermal cycler (Bio-Rad), and QuantiTect SYBR Green RT-PCR kit (Qiagen). Total RNAs were adjusted to 2.5 ng/μL before use. RT-PCR reactions were carried out in triplicate in 96-well plates (25 μL/well) in a buffer containing 1× QuantiTect SYBR Green RT-PCR master mix (including HotStarTaq DNA Polymerase, dNTPs, SYBR Green dye), 0.25 μL of QuantiTect RT mix (containing Omniscript and Sensiscript reverse transcriptases), 10 nm fluorescein (used as dynamic well factor), 500 nm forward and reverse primers, and 12.5 ng of total RNAs. One-step RT-PCR reactions were performed under the following conditions: 50°C for 30 min (RT), 95°C for 15 min (RT degradation and Taq DNA polymerase activation), followed by 40 cycles of denaturation at 94°C for 15 s, annealing at 60°C for 30 s, extension at 72°C for 30 s, and data acquisition at 77°C for 15 s. Identical conditions were used for all transcripts, except for grapevine PAL (annealing at 58°C, acquisition at 76°C) and P. viticola actin (acquisition at 81°C). Transcript levels were calculated using the standard-curve method and normalized using grapevine actin gene as internal control. Results presented are means of duplicate data of one representative experiment. The following primers were used for RT-PCR amplifications: 5′-GTGCCAATTTATGAAGGTTATGC-3′ and 5′-CCCTCTCAGTTAGAATCTTCATCAG-3′ for grapevine actin (accession no. AF369525), 5′-GGGATTTCACTGTTGCATATACTC-3′ and 5′-CATAGAATAGAAAGCGCGAGG-3′ for PAL (CF511227), 5′-AAAGAAACTCGAAGCAACGAGG-3′ and 5′-TGGCCCTCTCCCCCTTAA-3′ for STS (X76892), 5′-TGCCTCTAGGCTCCTTCTAA-3′ and 5′-TTTGAAACCAAGCACTCAGA-3′ for ROMT (FM178870). Actin from P. viticola was amplified using the primers 5′-GTTCGAGACGTTCAACGTGC and 5′-CATGATGGTCTGGAACGTGC. To design the primers, actin sequence from the Oomycetes Phytophthora infestans, Pythium splendens, and Achlya bisexualis were aligned together with actin from grapevine. Primer targeted sequences conserved in all three oomycetes, but divergent in grapevine. Absence of amplification in healthy grapevine samples confirmed the specificity of the primers.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession number FM178870.
Acknowledgments
We would like to thank Jean-Pierre Dutasta and Jean-Christophe Mulatier (Laboratoire de Chimie, Ecole Normale Supérieure de Lyon, France) for help with resveratrol methyl ether synthesis. We are indebted to Prof. Peter Beyer for the use of his laboratory facilities at the Center for Applied Biosciences (Freiburg, Germany). We thank Pascale Coste, Bernard Delnatte, Denise Hartmann, Charlotte Knichel, Jacky Misbach, and Christian Vivant (INRA, Colmar, France) for assistance with plant material. We are grateful to J. Mark Cock (Algal Genetics Group, UMR7139, Roscoff, France) for critically reading the manuscript.
This work was supported by INRA.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Philippe Hugueney (philippe.hugueney@ens-lyon.fr).
References
- Adrian M, Jeandet P, Bessis R, Joubert JM (1996) Induction of phytoalexin (resveratrol) synthesis in grapevine leaves treated with aluminum chloride (AlCl3). J Agric Food Chem 44 1979–1981 [Google Scholar]
- Aziz A, Poinssot B, Daire X, Adrian M, Bézier A, Lambert B, Joubert JM, Pugin A (2003) Laminarin elicits defense responses in grapevine and induces protection against Botrytis cinerea and Plasmopara viticola. Mol Plant Microbe Interact 16 1118–1128 [DOI] [PubMed] [Google Scholar]
- Batoko H, Zheng HQ, Hawes C, Moore I (2000) A Rab1 GTPase is required for transport between the endoplasmic reticulum and Golgi apparatus and for normal Golgi movement in plants. Plant Cell 12 2201–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA (2006) Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5 493–506 [DOI] [PubMed] [Google Scholar]
- Busso D, Delagoutte-Busso B, Moras D (2005) Construction of a set Gateway-based destination vectors for high-throughput cloning and expression screening in Escherichia coli. Anal Biochem 343 313–321 [DOI] [PubMed] [Google Scholar]
- Chiron H, Drouet A, Claudot A-C, Eckerskorn C, Trost M, Heller W, Ernst D, Sandermann H (2000. a) Molecular cloning and functional expression of a stress-induced multifunctional O-methyltransferase with pinosylvin methyltransferase activity from Scots pine (Pinus sylvestris L.). Plant Mol Biol 44 733–745 [DOI] [PubMed] [Google Scholar]
- Chiron H, Drouet A, Lieutier F, Payer HD, Ernst D, Sandermann H (2000. b) Gene induction of stilbene biosynthesis in Scots pine in response to ozone treatment, wounding, and fungal infection. Plant Physiol 124 865–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commun K, Mauro MC, Chupeau Y, Boulay M, Burrus M, Jeandet P (2003) Phytoalexin production in grapevine protoplasts during isolation and culture. Plant Physiol Biochem 41 317–323 [Google Scholar]
- Coutos-Thévenot P, Poinssot B, Bonomelli A, Yean H, Breda C, Buffard D, Esnault R, Hain R, Boulay M (2001) In vitro tolerance to Botrytis cinerea of grapevine 41B rootstock in transgenic plants expressing the stilbene synthase Vst1 gene under the control of a pathogen-inducible PR 10 promoter. J Exp Bot 52 901–910 [DOI] [PubMed] [Google Scholar]
- Douillet-Breuil AC, Jeandet P, Adrian M, Bessis R (1999) Changes in the phytoalexin content of various Vitis spp. in response to ultraviolet C elicitation. J Agric Food Chem 47 4456–4461 [DOI] [PubMed] [Google Scholar]
- Dudareva N, Murfitt LM, Mann CJ, Gorenstein N, Kolosova N, Kish CM, Bonham C, Wood K (2000) Developmental regulation of methyl benzoate biosynthesis and emission in snapdragon flowers. Plant Cell 12 949–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzmeier KH, Kindl H (1981) Coordinate induction by UV light of stilbene synthase, phenylalanine ammonia-lyase and cinnamate 4-hydroxylase in leaves of Vitaceae. Planta 151 48–52 [DOI] [PubMed] [Google Scholar]
- Galtier N, Gouy M, Gautier C (1996) SEAVIEW and PHYLO_WIN: two graphic tools for sequence alignment and molecular phylogeny. Comput Appl Biosci 12 543–548 [DOI] [PubMed] [Google Scholar]
- Gang DR, Lavid N, Zubieta C, Chen F, Beuerle T, Lewinsohn E, Noel JP, Pichersky E (2002) Characterization of phenylpropene O-methyltransferases from sweet basil: Facile change of substrate specificity and convergent evolution within a plant O-methyltransferase family. Plant Cell 14 505–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hain R, Bieseler B, Kindl H, Schröder G, Stöcker R (1990) Expression of a stilbene synthase gene in Nicotiana tabacum results in synthesis of the phytoalexin resveratrol. Plant Mol Biol 15 325–335 [DOI] [PubMed] [Google Scholar]
- Hall D, De Luca V (2007) Mesocarp localization of a bi-functional resveratrol/hydroxycinnamic acid glucosyltransferase of Concord grape (Vitis labrusca). Plant J 49 579–591 [DOI] [PubMed] [Google Scholar]
- Haseloff J, Siemering KR, Prasher DC, Hodge S (1997) Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis brightly. Proc Natl Acad Sci USA 94 2122–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XZ, Dixon RA (1996) Affinity chromatography, substrate/product specificity, and amino acid sequence analysis of an isoflavone O-methyltransferase from alfalfa (Medicago sativa L.). Arch Biochem Biophys 336 121–129 [DOI] [PubMed] [Google Scholar]
- Hipskind JD, Paiva NL (2000) Constitutive accumulation of a resveratrol-glucoside in transgenic alfalfa increases resistance to Phoma medicaginis. Mol Plant Microbe Interact 13 551–562 [DOI] [PubMed] [Google Scholar]
- Jaillon O, Aury JM, Noel B, Policriti A, Clepet C, Casagrande A, Choisne N, Aubourg S, Vitulo N, Jubin C, et al (2007) The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 449 463–467 [DOI] [PubMed] [Google Scholar]
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CV, Beecher CWWB, Fong HHS, Farnsworth NR, Kinghorn AD, Mehta RG, et al (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275 218–220 [DOI] [PubMed] [Google Scholar]
- Jeandet P, Douillet-Breuil AC, Bessis R, Debord S, Sbaghi M, Adrian M (2002) Phytoalexins from the Vitaceae: biosynthesis, phytoalexin gene expression in transgenic plants, antifungal activity, and metabolism. J Agric Food Chem 50 2731–2741 [DOI] [PubMed] [Google Scholar]
- Karimi M, Inzé D, Depicker A (2002) GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7 193–195 [DOI] [PubMed] [Google Scholar]
- Katsuyama Y, Funa N, Horinouchi S (2007) Precursor-directed biosynthesis of stilbene methyl ethers in Escherichia coli. Biotechnol J 2 1286–1293 [DOI] [PubMed] [Google Scholar]
- Kersten S (2008) Peroxisome proliferator activated receptors and lipoprotein metabolism. PPAR Res 2008 132960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortekamp A (2006) Expression analysis of defence-related genes in grapevine leaves after inoculation with a host and a non-host pathogen. Plant Physiol Biochem 44 58–67 [DOI] [PubMed] [Google Scholar]
- Langcake P, Cornford CA, Pryce RJ (1979) Identification of pterostilbene as a phytoalexin from Vitis Vinifera leaves. Phytochemistry 18 1025–1027 [Google Scholar]
- Lavid N, Wang J, Shalit M, Guterman I, Bar E, Beuerle T, Menda N, Shafir S, Zamir D, Adam Z, et al (2002) O-methyltransferases involved in the biosynthesis of volatile phenolic derivatives in rose petals. Plant Physiol 129 1899–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton MA, Lamb CJ (1987) Transcriptional activation of plant defense genes by fungal elicitor, wounding, and infection. Mol Cell Biol 7 335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SM, Sykes SR, Clingeleffer PR (2003) A method using leafed single-node cuttings to evaluate downy mildew resistance in grapevine. Vitis 42 173–180 [Google Scholar]
- Manickam M, Ramanathan M, Jahromi MAF, Chansouria JPN, Ray AB (1997) Antihyperglycemic activity of phenolics from Pterocarpus marsupium. J Nat Prod 60 609–610 [DOI] [PubMed] [Google Scholar]
- Melchior F, Kindl H (1991) Coordinate- and elicitor-dependent expression of stilbene synthase and phenylalanine ammonia-lyase genes in Vitis cv. Optima. Arch Biochem Biophys 288 552–557 [DOI] [PubMed] [Google Scholar]
- Mikstacka R, Przybylska D, Rimando AM, Baer-Dubowska W (2007) Inhibition of human recombinant cytochromes P450 CYP1A1 and CYP1B1 by trans-resveratrol methyl ethers. Mol Nutr Food Res 51 517–524 [DOI] [PubMed] [Google Scholar]
- Noel JP, Dixon RA, Pichersky E, Zubieta C, Ferrer JL (2003) Structural, functional, and evolutionary basis for methylation of plant small molecules. In JT Romeo, ed, Recent Advances in Phytochemistry, Vol 37. Elsevier Science, Oxford, pp 37–58
- Pan Z, Agarwal AK, Xu T, Feng Q, Baerson SR, Duke SO, Rimando AM (2008) Identification of molecular pathways affected by pterostilbene, a natural dimethylether analog of resveratrol. BMC Med Genomics 1 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezet R, Gindro K, Viret O, Richter H (2004) Effects of resveratrol, viniferins and pterostilbene on Plasmopara viticola zoospore mobility and disease development. Vitis 43 145–148 [Google Scholar]
- Pezet R, Pont P (1988) Mise en evidence de pterostilbene dans les grappes de Vitis vinifera. Plant Physiol Biochem 26 603–607 [Google Scholar]
- Pezet R, Pont V (1990) Ultrastructural observations of pterostilbene fungitoxicity in dormant conidia of Botrytis cinerea Pers. J Phytopathol 129 29–30 [Google Scholar]
- Poutaraud A, Latouche G, Martins S, Meyer S, Merdinoglu D, Cerovic ZG (2007) Fast and local assessment of stilbene content in grapevine leaf by in vivo fluorometry. J Agric Food Chem 55 4913–4920 [DOI] [PubMed] [Google Scholar]
- Rimando AM, Cuendet M, Desmarchelier C, Mehta RG, Pezzuto JM, Duke SO (2002) Cancer chemopreventive and antioxidant activities of pterostilbene, a naturally occurring analogue of resveratrol. J Agric Food Chem 50 3453–3457 [DOI] [PubMed] [Google Scholar]
- Rimando AM, Kalt W, Magee JB, Dewey J, Ballington JR (2004) Resveratrol, pterostilbene, and piceatannol in vaccinium berries. J Agric Food Chem 52 4713–4719 [DOI] [PubMed] [Google Scholar]
- Rimando AM, Nagmani R, Feller DR, Yokoyama W (2005) Pterostilbene, a new agonist for the peroxisome proliferator-activated receptor alpha-isoform, lowers plasma lipoproteins and cholesterol in hypercholesterolemic hamsters. J Agric Food Chem 53 3403–3407 [DOI] [PubMed] [Google Scholar]
- Santos-Rosa M, Poutaraud A, Merdinoglu D, Mestre P (2008) Development of a transient expression system in grapevine via agro-infiltration. Plant Cell Rep 27 1053–1063 [DOI] [PubMed] [Google Scholar]
- Scalliet G, Journot N, Jullien F, Baudino S, Magnard JL, Channelière S, Vergne P, Dumas C, Bendahmane M, Cock JM, et al (2002) Biosynthesis of the major scent components 3,5-dimethoxytoluene and 1,3,5-trimethoxybenzene by novel rose O-methyltransferases. FEBS Lett 523 113–118 [DOI] [PubMed] [Google Scholar]
- Scalliet G, Lionnet L, Le Bechec M, Dutron D, Magnard JL, Baudino S, Bergougnoux V, Jullien F, Chambrier P, Vergne P, et al (2006) Role of petal-specific orcinol O-methyltransferases in the evolution of rose scent. Plant Physiol 14 18–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalliet G, Piola F, Douady CJ, Réty S, Raymond O, Baudino S, Bordji K, Bendahmane M, Dumas C, Cock JM, et al (2008) Scent evolution in Chinese roses. Proc Natl Acad Sci USA 105 5927–5932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder G, Wehinger E, Schröder J (2002) Predicting the substrates of cloned plant O-methyltransferases. Phytochemistry 59 1–8 [DOI] [PubMed] [Google Scholar]
- Seshadri TR (1972) Polyphenols of Pterocarpus and Dalbergia woods. Phytochemistry 11 881–898 [Google Scholar]
- Suh N, Paul S, Hao X, Simi B, Xiao H, Rimando AM, Reddy BS (2007) Pterostilbene, an active constituent of blueberries, suppresses aberrant crypt foci formation in the azoxymethane-induced colon carcinogenesis model in rats. Clin Cancer Res 13 350–355 [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res 22 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomzik J, Stenzel K, Stöcker R, Schreier PH, Hain R, Stahl D (1997) Synthesis of a grapevine phytoalexin in transgenic tomatoes (Lycopersicon esculentum Mill.) conditions resistance against Phytophthora infestans. Physiol Mol Plant Pathol 51 265–278 [Google Scholar]
- Tropf S, Lanz T, Rensing SA, Schröder J, Schröder G (1994) Evidence that stilbene synthases have developed from chalcone synthases several times in the course of evolution. J Mol Evol 38 610–618 [DOI] [PubMed] [Google Scholar]
- Wang J, Dudareva N, Bhakta S, Raguso RA, Pichersky E (1997) Floral scent production in Clarkia breweri (Onagraceae). II. Localization and developmental modulation of the enzyme SAM:(iso)eugenol O-methyltransferase and phenylpropanoid emission. Plant Physiol 114 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Watanabe N, Mita S, Dohra H, Ueda Y, Shibuya M, Ebizuka Y (2004) The key role of phloroglucinol O-methyltransferase in the biosynthesis of Rosa chinensis volatile 1,3,5-trimethoxybenzene. Plant Physiol 135 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CK, Lam CN, Springob K, Schmidt J, Chu IK, Lo C (2006) Constitutive accumulation of cis-piceid in transgenic Arabidopsis overexpressing a sorghum stilbene synthase gene. Plant Cell Physiol 47 1017–1021 [DOI] [PubMed] [Google Scholar]