Abstract
Hop (Humulus lupulus L. Cannabaceae) is an economically important crop for the brewing industry, where it is used to impart flavor and aroma to beer, and has also drawn attention in recent years due to its potential pharmaceutical applications. Essential oils (mono- and sesquiterpenes), bitter acids (prenylated polyketides), and prenylflavonoids are the primary phytochemical components that account for these traits, and all accumulate at high concentrations in glandular trichomes of hop cones. To understand the molecular basis for terpene accumulation in hop trichomes, a trichome cDNA library was constructed and 9,816 cleansed expressed sequence tag (EST) sequences were obtained from random sequencing of 16,152 cDNA clones. The ESTs were assembled into 3,619 unigenes (1,101 contigs and 2,518 singletons). Putative functions were assigned to the unigenes based on their homology to annotated sequences in the GenBank database. Two mono- and two sesquiterpene synthases identified from the EST collection were expressed in Escherichia coli. Hop MONOTERPENE SYNTHASE2 formed the linear monterpene myrcene from geranyl pyrophosphate, whereas hop SESQUITERPENE SYNTHASE1 (HlSTS1) formed both caryophyllene and humulene from farnesyl pyrophosphate. Together, these enzymes account for the production of the major terpene constituents of the hop trichomes. HlSTS2 formed the minor sesquiterpene constituent germacrene A, which was converted to β-elemene on chromatography at elevated temperature. We discuss potential functions for other genes expressed at high levels in developing hop trichomes.
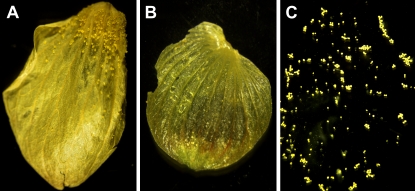
Hop (Humulus lupulus) is a perennial, dioecious plant that belongs to the Cannabaceae family. “Hops” is the common term for the female inflorescences of hop plants, well known for their use in beer flavoring. These inflorescences develop into cones upon maturation. The lower parts of the inner surface of the bracts of mature female hop cones are covered with glandular trichomes, termed lupulin glands (Fig. 1). Glandular trichomes, also referred to as secretory or peltate trichomes, are lipophilic glands comprising a group of secretory cells and a cuticle-enclosed cavity that fills with the secreted compounds (Oliveira and Pais, 1990; Saito et al., 1995). The plastids in glandular trichomes have less-defined membrane structures than chloroplasts and may be associated with synthesis and/or secretion of secondary metabolites, such as terpenoids and flavonoids (Oliveira and Pais, 1990).
Figure 1.
Isolation of glandular trichomes from female hop bracts. A, Glandular trichomes at the base of the female bracteole. B, Female bracteole after trichome isolation. C, Isolated glandular trichomes.
Three major classes of secondary metabolites are synthesized and accumulated in hop lupulin glands; essential oils, bitter acids, and prenylflavonoids. Commercial hop varieties often differ in the content of these components, which determines their use in bittering and finishing (adding flavor and aroma) of beer. Essential oils are the principal aroma components of hops. Essential oils make up 0.5% to 3% (v/w) of the whole hop cone, and terpenoids are abundant in this fraction (Eri et al., 2000), accounting for up to 90% of the oil. The composition of essential oils is characteristic of the hop genotype and, together with that of bitter acids and flavonoids, has been used for distinguishing different hop varieties. In addition to hydrocarbon compounds, which are predominantly terpenes, oxygenated compounds and small amounts of sulfur-containing compounds are also found. The major monoterpene and sesquiterpene components of hop essential oils are myrcene, α-humulene, and β-caryophyllene (Bernotiene et al., 2004). Most studies of hop terpenes have analyzed whole hop cones and there is little information on the content of these compounds in other tissues. It is also not clear whether the trichome is the exclusive organ for their biosynthesis and storage.
The bittering agents in beer are called bitter acids, which account for 10% to 20% of the hop cone by dry weight. The two representative bitter acids, α-acid and β-acid (humulone and lupulone), are prenylated polyketide derivatives. Prenylated flavonoids have also been identified from hops and beer and include the two prenylchalcones xanthohumol and desmethylxanthohumol and the three prenylflavanones isoxanthohumol, 8-prenylnaringenin, and 6-prenylnaringenin. Prenylchalcones and bitter acids accumulate at low levels at the onset of flowering and their concentrations gradually increase during the development of female hop cones (De Keukelere et al., 2003). The in vitro prenylation of the aromatic intermediates in the biosynthesis of bitter acids has been described using a crude hop extract (Zuurbier et al., 1998). In contrast to membrane-bound (iso)flavonoid prenyltransferases (Welle and Grisebach, 1991; LaFlamme et al., 1993; Sasaki et al., 2008), the hop bitter acid prenyltransferase activities were in the soluble fraction of the protein extract (Zuurbier et al., 1998). However, the bitter acid prenyltransferase remains to be characterized and it is not clear whether prenylflavonoids and bitter acids are synthesized by the same soluble prenyltransferase or whether polyketide prenylation is functionally related to other areas of terpene metabolism in hop trichomes.
In one of the first examples of applying genomics approaches to plant secondary metabolism, sequencing of a peppermint (Mentha piperita) oil gland cDNA library proved highly effective for studying essential oil biosynthesis in peppermint glandular trichomes (Lange et al., 2000). A similar approach has been reported recently with hop trichomes, leading to identification of an O-methyltransferase active in the biosynthesis of prenyl chalcone (Nagel et al., 2008). Essential oils, bitter acids, and prenylflavonoids are all derived from pathways of terpene metabolism, but the enzymes responsible for these processes have yet to be identified in hops. To facilitate gene discovery in hop natural product biosynthesis, a cDNA library was constructed from total RNA isolated from lupulin glands, and candidates for diverse terpene biosynthetic enzymes identified based on sequence similarities to previously known genes and direct functional identification through expression of recombinant terpene synthases in Escherichia coli. We here identify the mono- and sesquiterpene synthases involved in the formation of myrcene, humulene, and caryophyllene, and also describe the most highly expressed genes in the metabolically specialized hop trichomes.
RESULTS
Terpene Content and Composition of Hop Glandular Trichomes
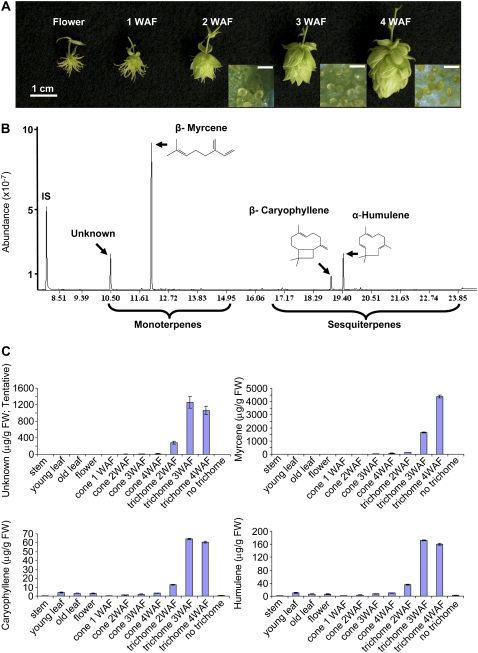
Hexane extracts of different tissues from hop cultivar Nugget were collected and analyzed by gas chromatography (GC)-mass spectrometry (MS). This revealed that the linear monoterpene myrcene and an unidentified compound of retention time 10.491 min were found exclusively in trichomes. MS analysis was most consistent with a structure of 2,7-dimethyl-1,6-octadiene (C10H18) for the unidentified compound, which accounted for approximately 15% (n = 3 biological replicates) of the total monoterpenes in the trichomes. The levels of both compounds increased during trichome development. In the trichomes of 4-week-old hop cones, myrcene comprised about 80% (n = 3) of the monoterpenes based on the area of peaks with monoterpene-specific fragments (10–15 min, m/z 136, 121, 93, 69) resolved by GC (Fig. 2). Other monoterpenes, such as linalool (retention time 14.007 min), were present in trace amounts and accumulated mainly in floral tissue (data not shown).
Figure 2.
Accumulation of mono- and sesquiterpenes in trichomes and other tissues of hop. A, Developmental sequence of developing hop flowers and trichomes. Bars on trichome photographs are 200 μm. WAF, Weeks after flowering. B, GC-MS analysis of terpenoids from mature trichomes from female bracts of hop cultivar Nugget. IS, Internal standard (toluene). C, Levels of unidentified compound (retention time 10.491 min; see B), myrcene, caryophyllene, and humulene in different tissue and in trichomes at different stages of development.
Humulene and caryophyllene were the two major sesquiterpenes in isolated hop trichomes. Together, they account for approximately 85% (n = 3) of the total sesquiterpenes in the trichomes (16.5–24 min, peaks with sesquiterpene-specific fragments of m/z 204, 161, 93, 69; Fig. 2). However, unlike myrcene and 2,7-dimethyl-1,6-octadiene (tentative), humulene and caryophyllene were not specific to trichomes and were also found in other tissues, such as leaves and flowers. Moreover, the ratios of humulene to caryophyllene in different tissues were almost identical, at about 3.0 based on peak areas (Fig. 2). This suggests the possibility that they may be formed by the same terpene cyclase enzyme (see below).
We also extracted the terpenoids from hop trichomes with ethyl acetate in place of hexane. This resulted in overall extraction of more terpenoids as determined by GC-MS and comparison to the internal standard (10% more myrcene and about 35% more humulene and caryophyllene), but the profiles included some nonterpenoid compounds eluting at higher retention times. Importantly, the patterns of terpenoid compounds were very similar using the two different extraction protocols and the ratio of humulene to caryophyllene was the same.
Construction, Annotation, and Functional Classification of a Hop Glandular Trichome EST Library Unigene Set
The Phoenix variety used for generation of the cDNA library has high essential oil and α-acid content and was developed in the UK as a dual-purpose hop for both bittering and finishing. It has 8% to 12% α-acids, 4.2% to 5.5% β-acids, 0.54% xanthohumol, and a total essential oil percentage of 1.2% to 2.5%.
Glandular trichomes were collected from female bracts (Fig. 1). The conventional method for cDNA library construction requires impractically large amounts of trichomes to extract sufficient quantities of mRNA. We therefore employed a PCR-based cDNA library construction method that uses small amounts of total RNA as starting material; 12,665 single-pass EST sequences were generated by random sequencing of 16,152 cDNA clones. Vector, low-quality, and short sequences (less than 100 bp) were excluded by using the cross-match program (http://www.phrap.org/phredphrapconsed.html) along with manual curation. A high percentage of ribosomal RNA contamination was observed and the corresponding sequences were removed during postsequencing cleansing of the ESTs, resulting in 9,816 cleansed high-quality EST sequences.
An EST database was generated containing the 9,816 sequences assembled into 1,101 contigs and 2,518 singletons. The contigs and singletons are collectively referred to as unigenes. The unigenes were searched against the National Center for Biotechnology Information (NCBI) nonredundant protein database (released on 9/30/07) using the BLASTX algorithm, and annotated according to their homologous sequences in the GenBank database. The most abundant unigenes are shown in Table I, and unigenes encoding enzymes of terpene and prenylflavonoid biosynthesis are listed in Table II and their frequencies depicted diagrammatically on metabolic pathways in Figure 3.
Table I.
The top 15 most abundant unigenes in the hop glandular trichome cDNA library
| Unigene | No. ESTs | BLAST Hit | GenBank ID | E Value |
|---|---|---|---|---|
| TCHL10783 | 194 | Cytochrome P450-like protein (Nicotiana tabacum) | BAA10929 | 4e-86 |
| TCHL10811 | 184 | VPS (Humulus lupulus) | O80400 | 0 |
| TCHL10806 | 167 | Major allergen Pru p1 (Prunus persica) | ABB78006 | 9e-56 |
| TCHL10947 | 137 | Cystatin-like protein (Citrus × paradisi) | AAG38521 | 6e-28 |
| TCHL11024 | 123 | Putative CHI (Lycopersicon esculentum) | AAQ55182 | 7e-64 |
| TCHL10880 | 120 | Metallothionein 1a (Populus balsamifera subsp. trichocarpa × Populus deltoides) | AAT02522 | 0.008 |
| TCHL10775 | 114 | Gly-rich protein (Citrus unshiu) | BAA92155 | 1e-06 |
| TCHL10835 | 114 | Short-chain dehydrogenase/reductase (SDR) family protein (Arabidopsis thaliana) | NP_567300 | 2e-59 |
| TCHL10005 | 85 | Selenium-binding protein (Medicago sativa) | CAC67501 | 5e-43 |
| TCHL10130 | 58 | CHS (CHS2) (Humulus lupulus) | BAB47194 | 0 |
| TCHL10382 | 56 | Nonspecific lipid transfer-like protein (Prosopis juliflora) | ABF06565 | 1e-32 |
| TCHL10509 | 51 | Isopentenyl pyrophosphate isomerase (Pueraria montana var. lobata) | AAQ84167 | 2e-122 |
| TCHL10255 | 32 | AMP-dependent synthetase and ligase family protein (Arabidopsis thaliana) | NP_179356 | 1e-75 |
| TCHL10134 | 32 | Senescence-associated protein (Nicotiana tabacum) | AAZ23261 | 2e-44 |
| TCHL10384 | 32 | Type 2 peroxiredoxin (Brassica rapa subsp. pekinensis) | AAD33602 | 4e-63 |
Table II.
Hop unigenes encoding enzymes of terpene and prenylflavonoid/bitter acid biosynthesis
| Unigene | No. ESTs | BLAST Hit | GenBank ID | E Value |
|---|---|---|---|---|
| TCHL10811 | 184 | VPS (Humulus lupulus) | O80400 | 0 |
| TCHL10130 | 58 | CHS (CHS2) (Humulus lupulus) | BAB47194 | 0 |
| TCHL10129 | 23 | CHS-like protein (CHS4) (Humulus lupulus) | CAD23044 | 0 |
| TCHL10662 | 10 | CHS (chs_H1) (Humulus lupulus) | CAK19318 | 1e-149 |
| TCHL10849 | 5 | Putative orcinol O-methyltransferase (Rosa odorata) | CAJ65661 | 1e-80 |
| TCHL10548 | 6 | 1-Deoxy-d-xylulose-5-P synthase (Pueraria montana) | AAQ84169 | 4e-53 |
| TCHL10613 | 2 | 1-Deoxy-d-xylulose-5-P reductoisomerase (Mentha × piperita) | AAD24768 | 1e-28 |
| TCHL10661 | 9 | 2-C-methyl-d-erythritol-2,4-cyclodiphosphate synthase | – | – |
| TCHL10150/11107 | 14 | Hydroxymethylbutenyl-4-diphosphate synthase | – | – |
| TCHL10138/10273 | 22 | 4-Hydroxy-3-methylbut-2-enyl diphosphate reductase (Arabidopsis thaliana ) | NP_567965 | 1e-47 |
| 19 | – | – | – | |
| TCHL10436 | 4 | PAL1 (Prunus avium) | AAC78457 | 2e-155 |
| TCHL10998 | 2 | PAL2 (Rubus idaeus) | AAF40224 | 3e-51 |
| ES654594 | 1 | PAL (Populus kitakamiensis) | BAA21643 | 3e-35 |
| EX516754 | 1 | PAL3 (Manihot esculenta) | AAK60273 | 1e-40 |
| TCHL10666 | 9 | C4H (Citrus × paradisi) | AAK57011 | 3e-96 |
| TCHL10606 | 2 | C4H (CYP73) (Catharanthus roseus) | CAA83552 | 1e-96 |
| ES652343 | 1 | C4H (Gossypium arboretum) | AAG10197 | 5e-63 |
| EX519255 | 1 | 4CL (Arabidopsis thaliana) | AAP03021 | 1e-27 |
| EX520059 | 1 | 4CL-like protein (Arabidopsis thaliana) | AAP03022 | 4e-97 |
| TCHL10609 | 2 | Pinene synthase (Quercus ilex) | CAK55186 | 2e-53 |
| TCHL10730 | 2 | (+)-δ-Cadinene synthase (Gossypium arboretum) | CAA77191 | 9e-14 |
| TCHL10281 | 6 | Sesquiterpene cyclase (Artemisia annua) | AAG24640 | 4e-03 |
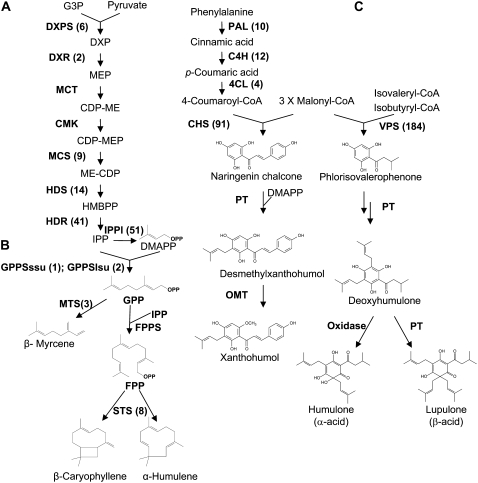
Figure 3.
Biosynthetic pathways for terpene-derived natural products found in hop trichomes, showing EST abundance in the hop glandular trichome cDNA library. A, The MEP pathway leading to DMAPP biosynthesis. B, General reactions of mono- and sesquiterpene biosynthesis. C, Prenylflavonoid and bitter acid biosynthesis. Chemical structures of selected compounds are shown. The abundance of ESTs corresponding to each biosynthetic gene in the hop glandular trichome cDNA library is indicated in parentheses. DXPS, 1-Deoxy-d-xylulose-5-P synthase; DXR, 1-deoxy-d-xylulose-5-P reductoisomerase; MCT, 2-C-methyl-d-erythritol-4-P cytidylyltransferase; CMK, 4-diphosphocytidyl-2-C-methyl-d-erythritol kinase; MCS, 2-C-methyl-d-erythritol-2,4-cyclodiphosphate synthase; HDS, 1-hydroxy-2-methyl-2-(E)-butenyl-4-diphosphate synthase; HDR, 1-hydroxy-2-methyl-2-(E)-butenyl-4-diphosphate reductase; IPPI, isopentenyl diphosphate/dimethylallyl diphosphate isomerase; OMT, O-methyltransferase; PT, prenyltransferase. Numbers in parentheses represent the number of occurrences of an EST corresponding to the particular enzyme in the 9,816 cleansed hop ESTs.
In addition to annotation based on sequence similarities to NCBI database entries, the unigenes were also classified into three major functional categories—cellular component, molecular function, and biological process—according to the standard Gene Ontology (GO; www.geneontology.org) terms, by searching the GO standard protein database released in August 2007 (BLASTX, e-value <1 e-04; Supplemental Fig. S1). Of the cellular component GO terms, 44% and 34% of hop unigenes were related to cell part and organelle, respectively. In the category of molecular function, 47% of hop unigenes were involved in catalytic activity and 26% in binding activity. Under the category of biological process, 31% and 30% were involved in cellular process and metabolic process, respectively, indicating that the hop glandular trichomes are highly metabolically active. The hop glandular trichome unigene sequences and GO classifications were deposited in the TrichOME database along with trichome EST sequences from other species and are publicly available for viewing and searching (http://trichome.noble.org/trichomedb).
The Most Abundant Hop Trichome Unigenes
The 15 most highly represented unigenes in the hop glandular trichome cDNA library are listed in Table I. The abundance of their transcripts suggests critical roles in secondary metabolite synthesis and transport and in glandular trichome development. Indeed, four of these highly represented unigenes encoded enzymes involved in secondary metabolite synthesis, namely, valerophenone synthase (VPS), chalcone synthase (CHS), chalcone isomerase (CHI)-like protein, and isopentenyl diphosphate (IPP) isomerase. Unigene TCHL10255 encoded an AMP-dependent synthetase and ligase family protein (also known as acyl-activating enzyme; Shockey et al., 2003). Acyl-activating enzymes convert carboxylic acids to acyl-AMP intermediates and then to acyl-CoAs, and could potentially be involved in the formation of branched-chain acyl-CoAs as substrates for VPS.
ESTs homologous to food allergens and nonspecific lipid transfer proteins (LTPs) were also highly expressed in hop glandular trichomes. Trichomes are also known for their roles in storage and secretion of heavy metals and in defense (Küpper et al., 2000; Choi et al., 2001), and unigene TCHL10880 (containing 120 ESTs) was similar to metallothioneins and TCHL10947 (composed of 137 ESTs) was homologous to cystatin. Metallothioneins are heavy-metal binding proteins that play dual roles in heavy-metal detoxification and metal ion uptake/transport, and cystatin is a Cys protease inhibitor that is induced by biotic and abiotic stresses. TCHL10134 showed strong sequence similarity to a tobacco (Nicotiana tabacum) senescence-related protein, the expression of which was transiently increased upon bacterial (Rhodococcus fascians) infection (Simon-Mateo et al., 2006), suggesting a potential role in pathogen defense.
Biosynthesis of Early Terpene Pathway Precursors
Hop bitter acids and prenylflavonoids are formed by the transfer of one or more single prenyl (dimethylallyl diphosphate; DMAPP) groups to a polyketide acceptor molecule, and mono- and sesquiterpenes are derived from longer chain allylic pyrophosphates formed from DMAPP/IPP. Both the cytosolic mevalonate (MVA) pathway and the plastidial 2-C-methyl-d-erythritol-4-P (MEP) pathway can produce IPP, which is then converted to its allylic isomer, DMAPP, through the action of IPP isomerase (Lichtenthaler, 1999). Based on the stable isotope-labeling pattern of dimethylallyl groups in humulone, it was concluded that hop bitter acids are derived from the MEP pathway (Goese et al., 1999). Consistent with these labeling results, unigenes encoding the MEP pathway enzymes 1-deoxy-d-xylulose-5-P synthase, 1-deoxy-d-xylulose-5-P reductoisomerase, 2-C-methyl-d-erythritol-2,4-cyclodiphosphate synthase, 1-hydroxy-2-methyl-2-(E)-butenyl-4-diphosphate synthase, and 1-hydroxy-2-methyl-2-(E)-butenyl-4-diphosphate reductase, were present in the hop glandular trichome cDNA library (Fig. 3; Table II). ESTs encoding 2-C-methyl-d-erythritol-4-P cytidylyltransferase and 4-diphosphocytidyl-2-C-methyl-d-erythritol kinase were not identified, indicating that, although abundant in biosynthetic genes, our hop unigene set did not contain all of the transcripts for secondary metabolite synthesis in glandular trichomes. In a recent report on a hop trichome EST collection from cultivars Taurus and Nugget, both of these enzymes were identified, although the libraries in this case were a mixture of normalized and non-normalized (Nagel et al., 2008). It should, however, be noted that no ESTs corresponding to any of the enzymes of the MVA pathway were represented in the present collection.
Hop essential oils are synthesized from geranyl diphosphate (GPP) and farnesyl diphosphate (FPP). A cDNA encoding FPP synthase (FPPS) was cloned and characterized from hop that showed both GPP synthase and FPPS activities, but not prenyltransferase activity toward phlorisovalerophenone (PIVP) in bitter acid synthesis (Okada et al., 2001). We could not identify this FPPS gene or its homolog in our glandular trichome cDNA library. Unigenes homologous to GPP synthase large and small subunits were, however, present in the EST database (data not shown), and the biochemistry of the heterodimeric hop GPP synthase will be described elsewhere.
Functional Identification of Hop Monoterpene Synthases
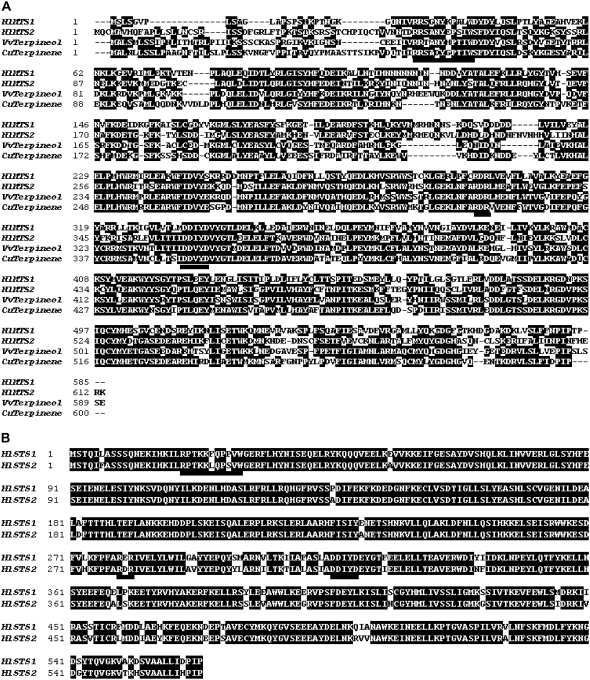
A search of the hop trichome EST database for potential terpene synthases revealed three different unigenes with high protein sequence identity to functionally identified monoterpene synthases (designated as HlMTS1, HlMTS2, and HlMTS3). The EST clones of HlMTS1 and HlMTS2 were found to be truncated at their N termini. 5′-RACE was therefore used to clone the corresponding full-length cDNAs for these two putative monoterpene synthases. The full-length cDNA of HlMTS1 (2,095 bp) encodes a peptide sequence of 585 amino acids with a calculated molecular mass of 67,510 D and a pI of 4.9. The full-length cDNA of HlMTS2 (1,953 bp) contains an open reading frame of 1,842 nucleotides that encode a predicted protein of 613 amino acids with a pI of 5.68. HlMTS1 and HlMTS2 share 46.7% amino acid identity to each other. Both have a plastid-targeting peptide at the N terminus (the first 31 and 46 amino acids for MTS1 and MTS2, respectively; predicted with TargetP 1.1 software). The deduced proteins of HlMTS1 and HlMTS2 show 48.9 and 52% amino acid identity with Vitis vinifera (−)-α-terpineol synthase (Martin and Bohlmann, 2004), respectively (Fig. 4A). According to BLAST results, HlMTS3 is an ortholog of linalool synthase, and was highly expressed in flower tissues where linalool accumulation was highest. We did not attempt to further characterize HlMTS3.
Figure 4.
Alignments of hop MTS (A) and STS (B) sequences. Other MTS sequences are from Citrus unshiu (accession no. BAD27259) and V. vinifera (AAS79352). Identical amino acids are shown as white letters on a black background. The RR(P)X8W, RXR, and DDXXD motifs are underlined.
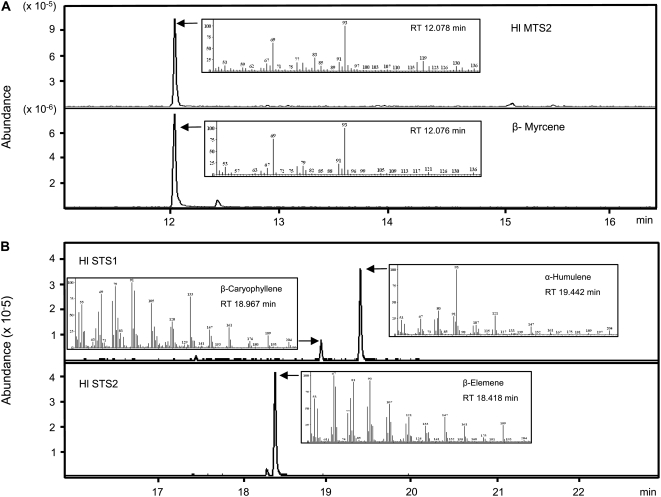
Truncated forms of HlMTS1 and HlMTS2 with the predicted plastid-targeting peptides removed were expressed in E. coli. After induction of protein expression with isopropylthio-β-d-galactoside at 16°C for 16 h, crude bacterial extracts were used for terpene synthase assays using GPP (for monoterpene synthase), FPP (for sesquiterpene synthase), and geranylgeranyl diphosphate (GGPP; for diterpene synthase) as substrates. GC-MS analysis showed that myrcene was the only product when the extract containing HlMTS2 was incubated with GPP (Fig. 5A). No product could be detected with FPP or GGPP as substrates. These results therefore indicate that HlMTS2 activity leads to the formation of myrcene in hop trichomes. We were unable to detect monoterpene, sesquiterpene, or diterpene synthase activity in extracts containing HlMTS1.
Figure 5.
GC-MS analysis of enzymatic products from recombinant hop mono- and sesquiterpene synthases. A, Portion of chromatogram showing the product of the reaction of GPP with HlMTS1 and its corresponding mass spectrum (inset). Bottom, Same for an authentic standard of myrcene. B, Portion of chromatogram showing the two products of the reaction of FPP with HlSTS1 and their corresponding mass spectra (inset). Bottom, Same for HlSTS2.
Kinetic analysis of purified His-tagged recombinant HlMTS2 revealed a Km value for geranyl pyrophosphate of 7.65 ± 2.40 μm (n = 3).
Functional Identification of Hop Sesquiterpene Synthases
ESTs corresponding to two different sesquiterpene synthase-like genes (designated as HlSTS1 and HlSTS2) were also were identified from the hop trichome library. The full-length cDNAs of HlSTS1 and HlSTS2 were obtained using 5′-RACE. The full-length cDNA of HlSTS1 (1,842 bp) encodes a peptide sequence of 563 amino acids with a calculated molecular mass of 66,218 D and a pI of 5.1. The full-length cDNA of HlSTS2 (1,863 bp) contains an open reading frame of 1,692 nucleotides that encode a predicted protein of 563 amino acids with a pI of 5.44. HlSTS1 and HlSTS2 share 92.4% identity to each other at the protein level (Fig. 4B) and 50% amino acid identity with a functionally characterized V. vinifera (−)-germacrene D synthase (Lücker et al., 2004).
HlMTS1, HlMTS2, HlSTS1, and HlSTS2 all contain the RR(P)X8W, RXR, and DDXXD (X is any amino acid) motifs, which are key features of most angiosperm terpene synthases (Keeling and Bohlmann, 2006; Fig. 4).
HlSTS1 and H1STS2 were expressed in E. coli using the same strategy as employed for the monoterpene synthases. Both enzymes were active with FPP as substrate, but not with GPP or GGPP. Terpenoid products were identified by GC-MS. The major sesquiterpene products of HlSTS1 were identified as humulene (70% of total products) and caryophyllene (25%; Fig. 5B). This ratio of humulene to caryophyllene was similar to the value in the essential oil of the hop trichomes.
In spite of the very close sequence identity between HlSTS1 and HlSTS2, the products of HlSTS2 are not humulene and caryophyllene (Fig. 5B). The major product of HlSTS2 was shown by GC-MS analysis to be β-elemene, a relatively minor component of hop essential oil. However, β-elemene can be formed in vitro by the rearrangement of germacrene A at high temperatures, such as used in the present GC-MS analysis. We therefore analyzed the product of the STS2 enzymatic reaction by GC-MS using lower injection temperatures (150°C and 180°C instead of the usual 280°C). β-Elemene was no longer observed and was replaced by a broad peak corresponding to germacrene A).
Kinetic analysis of purified His-tagged recombinant HlSTS1 and H1STS2 revealed Km values for farnesyl pyrophosphate of 0.70 ± 0.07 (n = 3) and 0.49 ± 0.04 (n = 3) μm, respectively.
Tissue-Specific and Developmental Expression of Hop Terpene Synthases
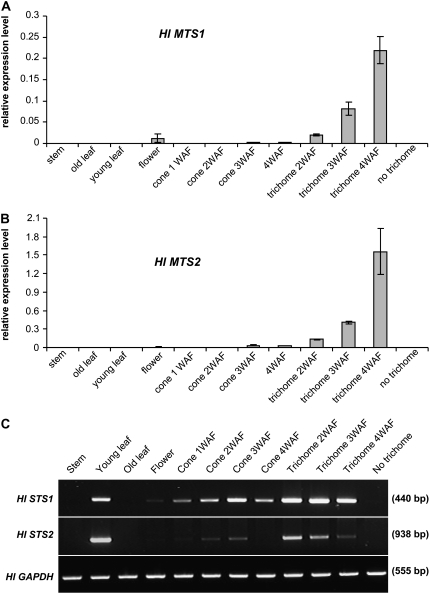
To test whether the patterns of terpene metabolite production in hops can be explained by the expression of the above-characterized terpene synthases, real-time PCR was first performed to examine the expression of HlMTS1 and HlMTS2 in different tissues and in trichomes at different developmental stages. HlMTS1 and HlMTS2 transcript levels followed the same developmental pattern as the monoterpene metabolites (Fig. 6, A and B). Both HlMTS1 and HlMTS2 transcript levels were highest in trichomes from cones at 4 weeks after flowering (Fig. 6, A and B).
Figure 6.
Tissue-specific expression of hop terpene synthases. A, Quantitative real-time PCR analysis of HlMTS1 transcript levels in different hop tissues and different developmental stages of cones and trichomes. No trichome = mature cones with trichomes removed. B, As above, for HlMTS2 transcripts. C, Semiquantitative RT-PCR analysis of HlSTS1 and H1STS2 transcripts in different hop tissues and different developmental stages of cones and trichomes.
Because of the high sequence identity between HlSTS1 and HlSTS2, we could not find good primers for real-time reverse transcription (RT)-PCR to distinguish between the two genes. Semiquantitative RT-PCR was therefore used to analyze the expression patterns of HlSTS1 and HlSTS2. HlSTS1 transcripts were abundant in those tissues with high levels of humulene and caryophyllene, and also paralleled the levels of these compounds in hop trichomes during development. HlSTS2 transcripts were abundant in young leaf tissue, although they were also detected in other tissues (Fig. 6C).
Polyketide Biosynthesis and Prenylation
Hop prenylflavonoids are formed by transfer of a 5-carbon prenyl group to a chalcone precursor, itself formed by the condensation of malonyl-CoA and 4-coumaroyl-CoA by CHS. 4-Coumaroyl-CoA is derived from l-Phe by the sequential actions of l-Phe ammonia-lyase (PAL), cinnamate 4-hydroxylase (C4H), and 4-coumarate:CoA ligase (4CL). Four PAL gene homologs were present in the hop glandular trichome cDNA library (Table II), and three unigenes were similar to C4H from different plant species; TCHL10606 matched to the N terminus of the enzyme, whereas the closely related TCHL10666 and the singleton ES652343 matched to the C terminus. Arabidopsis (Arabidopsis thaliana) 4CL-like protein homologs were also found in the cDNA library.
Bitter acid and prenylflavonoid biosynthesis share several features, particularly the involvement of a polyketide synthase and subsequent prenyltransferases. The polyketide synthase for bitter acid biosynthesis, VPS, is related to CHS, and produces PIVP from malonyl-CoA and isovaleryl-CoA. VPS and CHS from hop have been cloned and functionally characterized (Paniego et al., 1999; Okada and Ito, 2001; Okada et al., 2004). In contrast to a single VPS gene in hops, CHS constitutes a family of four genes, CHS2, CHS3, CHS4, and CHS_H1. VPS and CHS2 were among the most abundant unigenes in the present hop glandular trichome cDNA library, represented by 184 and 58 ESTs, respectively (Table I). CHS4 and CHS_H1 were also present, whereas CHS3 was not identified (Table II).
Plant CHIs are classified into four subfamilies according to their phylogenetic relationship (Ralston et al., 2005). Proteins in the CHI3 and CHI4 subfamilies show sequence similarity to previously characterized CHIs, but have not been biochemically characterized to date and are therefore described as CHI-like proteins. HlCHI-like 1, which was among the most abundant unigenes in the glandular trichome cDNA library (Table I), was homologous to a tomato (Solanum lycopersicum) CHI-like protein (LeCHI-like) that belongs to the CHI3 subfamily, and HlCHI-like 2 was similar to an Arabidopsis CHI-like protein (At5g05270) that belongs to the CHI4 subfamily. Neither HlCHI-like 1 nor HlCHI-like 2 exhibited CHI activity when expressed as recombinant proteins in E. coli (data not shown).
Transfer of DMAPP to polyketide acceptor molecules is likely catalyzed by aromatic prenyltransferases in hops (Stevens and Page, 2004). Aromatic prenyltransferases have been isolated and characterized from bacteria and plants and can be grouped into two classes: the soluble bacterial prenyltransferases and the membrane-bound bacterial and plant UbiA family prenyltransferases (Sasaki et al., 2008; Tian et al., 2008). The UbiA family prenyltransferases share the common motif (N/D)DXXD for prenyl diphosphate binding (Bräuer et al., 2004). By searching hop unigenes with the prenyl diphosphate-binding motif, a single unigene was identified that showed strong sequence similarity to a membrane-bound prenyltransferase involved in ubiquinone synthesis (data not shown).
DISCUSSION
The generation and sequencing of an EST library from hop lupulin glands has enabled us to identify and characterize the enzymes involved in the biosynthesis of the major mono- and sesquiterpene aroma compounds produced in these trichomes. As in a previous study (Nagel et al., 2008), ESTs corresponding to MEP pathway enzymes were highly abundant as compared with MVA pathway transcripts. Although this suggests that the DMAPP/IPP for all classes of terpene synthesis in the hop trichomes originates primarily from the MEP pathway, it is perhaps dangerous to equate EST counts to expressed enzymatic activities. Nevertheless, the MEP pathway has previously been shown to provide precursors for both mono- and sesquiterpene biosynthesis in snapdragon (Antirrhinum majus) flowers (Dudareva et al., 2005).
On comparing the overall transcript abundances in our EST library with those in the two non-normalized libraries previously generated from trichomes of hop cultivars Taurus and Nuggett (Nagel et al., 2008), 47.5% of the ESTs (1,087 of 2,290) in the Taurus/Nuggett libraries could hit targets in the cultivar Phoenix library described in this article, and 33.4% of the Phoenix ESTs could hit targets in the Taurus/Nuggett libraries. The lack of closer coincidence could arise from differences in both hop cultivar and developmental stage. However, all of the terpene synthases identified in this article could be found in the Taurus/Nuggett libraries, and two of the three O-methyltransferases previously identified (Nagel et al., 2008) were also represented in the Phoenix library.
Myrcene, caryophyllene, and humulene represent the bulk of the terepene component of hop essential oil. The relative proportions of caryophyllene and humulene were very similar in all tissues in which these compounds were produced and the same as produced by recombinant HlSTS1 in vitro. Furthermore, we did not observe one of the compounds without the other in any of the hop tissues analyzed.
H1STS1 and HlSTS2 are 92.4% identical at the amino acid level, but make different products. β-Elemene, the initially measured product of HlSTS2, has been previously described as a minor component of hop essential oils (Katsiotis et al., 1989), although, as shown here, it may be derived nonenzymatically from germacrene A. It is likely that the active-site cavities of HlSTS1 and HlSTS2 differ in size, shape, or polarity as a result of the few amino acids that are different between the two enzymes, leading to different modes of cyclization.
The previously described myrcene synthase from grand fir (Abies grandis) is more closely related to sesquiterpene and diterpene synthases from conifers than it is to monoterpene synthases from angiosperms (Bohlmann et al., 1997). The hop myrcene synthase exhibited 52% amino acid identity with V. vinifera (−)-α-terpineol synthase (Martin and Bohlmann, 2004), but only 29% identity to grand fir myrcene synthase. However, the hop myrcene synthase is also only 29.8% identical to myrcene synthase from snapdragon (Dudareva et al., 2003); it is more closely related (40.8%) to Arabidopsis myrcene synthase (At2g24210), although this enzyme, unlike that from hop, can also produce ocimene from GPP (Bohlmann et al., 2000).
It is well known that sesquiterpene synthases with the ability to form humulene can also form caryophyllene. Thus, rice (Oryza sativa) caryophyllene synthase produces caryophyllene as the major product, although several other products are made with FPP as substrate, including humulene and β-elemene (Cheng et al., 2007). It is interesting that this latter product is produced by a separate enzyme (HlSTS2) in hop. Similar product complexity to that observed with rice sesquiterpene synthases is seen in Arabidopsis, where genetic evidence has shown that only two enzymes account for most of the complex mixture of over 20 sesquiterpenes in the floral scent (Tholl et al., 2005). HlSTS1 appears to be a less promiscuous sesquiterpene synthase than those of rice and Arabidopsis, and its dual product specificity in vitro correlates with the humulene to caryophyllene ratio in the different hop tissues in which this enzyme is expressed, suggesting that it is the major determinant of the levels of these two compounds.
Although the two monoterpene synthases described in this article have clear N-terminal plastid-targeting sequences, we could not detect similar sequences in the two sesquiterpene synthases using TargetP 1.1 software. If these latter enzymes are truly cytoplasmic and if, as we suggest, the plastidial MEP pathway is responsible for most, if not all, of the formation of the building blocks for GPP and FPP, there would need to be transport of IPP/DMAPP between plastid and cytosol in hop trichomes, as occurs in snapdragon flowers (Dudareva et al., 2005).
Although the genomics approach applied in this article successfully identified the genes involved in mono- and sesquiterpene biosynthesis in hop trichomes, the prenylation step in the formation of bitter acids and prenylflavonoids still requires elucidation. Recently, the first plant flavonoid prenyltransferase was described, an enzyme from Sophora flavescens that prenylates the flavanone naringenin (Sasaki et al., 2008). This enzyme is a member of the membrane-associated UbiA family of plant prenyltransferases. However, preliminary biochemical evidence suggests that the hop bitter acid prenyltransferase is a soluble enzyme (Zuurbier et al., 1998). It is not clear whether the same or different enzymes catalyze the prenylation of the different polyketide (naringenin chalcone and PIVP) intermediates in prenylflavonoid and bitter acid biosynthesis. We could only identify a single EST representing a plant UbiA family prenyltransferase in our EST collection. In contrast, 23 hop ESTs annotated as potential aromatic prenyltransferases were reported from an EST collection derived from normalized and non-normalized libraries, although none was functionally identified (Nagel et al., 2008). This suggests that the aromatic prenyltransferases of bitter acid and prenyl flavonoid biosynthesis in hop are, if similar to currently known prenyltransferases, expressed at relatively low levels in comparison to the corresponding polyketide synthases.
Bacterial prenyltransferases involved in antibiotic synthesis were identified recently and are soluble proteins that contain a conserved protein fold but share low similarity at the primary sequence level (Kuzuyama et al., 2005). The bacterial prenyltransferase sequences were also used to search against our hop unigenes, but no homologous sequences were identified.
Several previous studies have revealed a high proportion of LTP transcripts in plant trichomes (Lange et al., 2000; Aziz et al., 2005). LTPs are small basic polypeptides that bind to fatty acid derivatives and are secreted to the cell walls in plants (Kader, 1996). Some of the food allergens belong to the LTP family (Pastorello et al., 1999). The function of LTPs in trichomes is unknown, although it has been suggested that LTPs may play a role in plant defense against pathogens (García-Olmedo et al., 1998). It has also been suggested that the LTPs in peppermint glandular trichomes are involved in intracellular trafficking and secretion of essential oils (Lange et al., 2000).
In conclusion, we have described the construction and analysis of a hop glandular trichome EST database. Mining the sequences in the database resulted in the identification of many of the genes involved in terpene natural product biosynthesis, and mono- and sesquiterpene synthases responsible for formation of the major hop essential oil components were functionally characterized. The database, which is publicly available as a part of the Noble Foundation's TrichOME database (http://trichome.noble.org/trichomedb), provides a resource for further characterization of the molecular basis of hop trichome development and metabolism, as well as being a potential source of molecular markers to facilitate hop breeding.
MATERIALS AND METHODS
Plant Material
For cDNA library construction, mid-developmental stage female cones were collected from hop (Humulus lupulus ‘Phoenix’) plants, grown at the Hop Research Institute, Wye, Kent, UK. The large cones (unlikely to develop much further) and the small cones (containing few trichomes) were discarded and only the medium-sized cones were used.
Rhizomes of hops of cultivar Nugget were purchased from Northern Brewer Company and grown in the greenhouse (after flowering, the daylength was reduced from 16 to 14.5 h to initiate production of cones). Young leaves (1–2 cm in diameter), old leaves (8–10 cm in diameter), cones, and glandular trichomes (see below) were collected and stored at −80°C until used for chemical extraction or analysis of transcript levels.
Preparation of Trichomes
A total of 10 female cones were used for each batch of RNA isolated. The cones were frozen in liquid nitrogen and kept on ice while each bract was removed from the cone using a fine tip forceps. The bracts were transferred to a chilled 50-mL falcon tube and cold diethyl pyrocarbonate-treated water was added to submerge all the plant material. Approximately 2 g of glass beads (Sigma glass beads; 600 μm; acid washed) were added and the tube firmly capped. Glandular trichomes were separated by vortexing the tube for 1 to 2 min while keeping the tube in a horizontal position. Trichomes were sifted through a 500-μm metal mesh followed by low-speed centrifugation to collect trichomes in the bottom of the tube. The trichomes were used immediately for total RNA extraction.
RNA Isolation, cDNA Library Construction, and EST Sequencing
Total RNA was isolated from trichomes using the cold-phenol method as described (Carpenter and Simon, 1998). RNA concentration and quality were determined with a Nanodrop spectrophotometer (Thermo Fisher Scientific) and by formamide gel electrophoresis. First-strand cDNA was synthesized from 1 μg total RNA using a Creator Smart cDNA library construction kit following the manufacturer's protocol (CLONTECH); 16,152 colonies were randomly selected and used for inoculating liquid cultures. Plasmids were extracted from bacterial clones using Biomek 2000 robots and were submitted to single-pass 5′ sequencing.
EST Sequence Analysis and Annotation
Vector, low-quality, and short sequences (<100 bp) were subtracted from the EST database. The remaining sequences were inspected manually to further improve the quality of EST trimming. Cleansed EST sequences were used for assembling into unigenes (contigs and singletons). The parameters used for sequence assembly were minimum sequence overlap of 40 bp and minimum percentage of sequence identity of 94%.
Hop unigenes were annotated based on their best BLASTX hits in the NCBI nonredundant protein database. Unigenes with E < 10−4 were classified as no hit. Sequences showing high similarity to ribosomal RNA and genomic DNA were excluded from the EST database. For functional classification of the hop glandular trichome unigenes, the sequences were searched against the protein database with standard GO classifications (www.geneontology.org). The relative frequency of unigene counts assigned to each functional category was displayed in pie charts using Microsoft Excel.
Terpene Analysis in Hop Tissues
Fresh plant material was ground to a fine powder in liquid N2 using a mortar and pestle. The powder was soaked in hexane (10:1 [v/w], hexane to tissue) containing 0.03% toluene as internal standard and extracted for 2 h at room temperature in 4-mL glass vials with tightly sealed rubber septa caps. After centrifugation at 3,000 rpm for 30 min, the clear hexane layer was transferred into another vial for GC-MS analysis. For analysis of terpenes in trichomes, the hop cones were broken in liquid nitrogen and the trichomes isolated by filtration and centrifugation as described above, followed by extraction with hexane. Samples were injected at a 1:1 split ratio, and the inlet and transfer line were held at 280°C. Separation was achieved with a temperature program of 40°C for 2 min, then ramped at 10°C/min to 300°C and held for 20 min, on a 60-m DB-5MS column (J&W Scientific; 0.25 mm i.d., 0.25-μm film thickness) with constant flow of 1.0 mL/min. Three independent biological replicates were analyzed for each data point. The myrcene, humulene, and caryophyllene contents were calculated from standard curves constructed with authentic samples. The content of the unidentified compound at retention time 10.491 min was calculated based on the standard curve of myrcene.
Generation, Expression, and Assay of Recombinant Terpene Synthases
To obtain the N-terminal sequences of MTS1 and MTS2, 5′-RACE was performed using the MTS1-specific reverse primer 5′-TCACCCTTTTGGTACAGTAGCATTGCCCCCC-3′ and the MTS2-specific reverse primer 5′-CCGGTCCTCCTATTGAAAGCCAAGCCATCTC-3′, respectively. The open reading frames of MTS1 and MTS2 were obtained by RT-PCR using the primers 5′-ATGTCACTTTCAGGAGTGCCATTATCGGCTGG-3′ and 5′-CTAAGGAGTGGGAATTGGATTGAAAAACAAGGAG-3′ for MTS1, and 5′-ATGCAGTGCATGGCTGTTCACCAATTTGC-3′ and 5′-TCACTTTCTCTCATGAAAATTAATAGGATTAATAATCAGTGC-3′ for MTS2. The resulting PCR products were subcloned into pEXP5-CT/TOPO vector (Invitrogen) and the DNA sequences of MTS1 and MTS2 confirmed by sequencing at least five independent clones.
To obtain soluble proteins for expression in Escherichia coli, the terpene synthase inserts were truncated at the 5′-end to remove the putative N-terminal signal peptides. For MTS1 and MTS2 expression constructs, artificial open reading frames were obtained by PCR using the forward primer 5′-ATGAGGCGATCCGGCAATTACAAACC-3′ and the reverse primer 5′-CTAAGGAGTGGGAATTGGATTGAAAAACAAGGAG-3′ (for MTS1), and the forward primer 5′-ATGCGAAGATCAGCCAACTATGAACCCTC-3′ and the reverse primer 5′-TCACTTTCTCTCATGAAAATTAATAGGATTAATAATCAGTGC-3′ (for MTS2). The resulting PCR fragments were cloned into pEXP5-CT/TOPO vector following the manufacturer's instructions. Recombinant enzymes were induced by treating the cells with 0.5 mm isopropylthio-β-galactoside overnight at 16°C.
Full-length cDNA cloning and protein expression in E. coli for STS1 and STS2 was performed as above, but using the following primers for STS1: 5′-GCCTCTCAAGGCTCTTTCTCAATGGCCTCTC-3′ for 5′-RACE and 5′-ATGTCCACTCAAATCTTAGCATCATC-3′ and 5′-TCATGGAATTGGATCAATAAGCAACGCAGCAACACTATCTTTG-3′ for production of N-terminal truncated open reading frames for protein expression in E. coli. Corresponding primers for STS2 were 5′-GGTGAGAAATAAACAACTTTATTTATTAATTTTAACAAGC-3′ for 5′-RACE and 5′-ATGTCCACTCAAATCTTTGCATCATC-3′ and 5′-TCATGGAATTGGATGAATAAGCAACGC-3′ for generation of protein expression constructs.
Monoterpene and sesquiterpene synthase assays were preformed as described previously (Dudareva et al., 2003; Tholl et al., 2005).
Quantitative RT-PCR Analysis of MTS1 and MTS2 Transcript Levels
Total RNA for real-time RT-PCR analysis of terpene synthase transcripts in different tissues was isolated using the cold-phenol method after a DNA digestion step (Carpenter and Simon, 1998). Equal amounts of total RNA after treatment with the RNA Cleanup kit (Qiagen) were used for cDNA generation using Superscript III (Invitrogen) according to the manufacturer's instructions. Primer design and real-time PCR were performed by following the manufacturer's instructions. The relative amounts of transcripts for different genes were normalized to glyceraldehyde-3-P dehydrogenase (GAPDH) transcript levels using LinRegPCR software. Every PCR reaction was repeated with three independent biological replicates, each of which was represented by three technical replicates. Gene-specific primers were as follows: MTS1 forward, 5′-CTTCTCCATCCAACAAACCAACT-3′, MTS1 reverse, 5′-TGCCGGATCGCCTAACAA-3′; MTS2 forward, 5′-GGCGACGTTCCTAAATCAATTC-3′, MTS2 reverse, 5′-TCACGAGCTTCGTCTTCTGAAG-3′; and GAPDH forward, 5′-TCTCCCAGCTCTCAACGGTAA-3′, GAPDH reverse, 5′-TGAGACATCGACGGTAGGAACA-3′.
Gene-specific primers for semiquantitative RT-PCR of HlSTS1 and HlSTS2 were STS1 forward, 5′-TATGGACCGCAAGATTATTAGGGCATCTTC-3′, STS1 reverse, 5′-TTCTGTTATTTACACACTTATTATATAGAAGAGATATCC-3′; and STS2 forward, 5′-GGATATTAGCAGTCTATTATGAACCCCAATACTATT-3′, STS2 reverse, 5′-GGTGAGAAATAAACAACTTTATTTATTAATTTTAACAAGC-3′.
Sequence data from this article can be found in the GenBank dbEST database under accession numbers ES652314-ES658722 and EX515309-EX521564 (12,665 single-pass EST sequences), and in the GenBank gene database as accession numbers EU760348-EU760351 (HlMTS1, HlMTS2, HlSTS1, and HlSTS2, respectively).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Functional classification of the sequences in the hop glandular trichome cDNA library.
Supplementary Material
Acknowledgments
We thank David Huhman and Dr. Wensheng Li for assistance with GC-MS analysis, Dr. Peter Darby for provision of Phoenix hop cones, and Dr. Marina Naoumkina and Dr. Hui Shen for critical reading of the manuscript.
This work was supported by the National Science Foundation Plant Genome Program (grant no. DBI–0605033 to R.A.D.) and by the Samuel Roberts Noble Foundation.
Any opinions, findings, and conclusions or recommendations expressed in this article are those of the authors and do not necessarily reflect the views of the National Science Foundation.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Richard A. Dixon (radixon@noble.org).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Aziz N, Paiva NL, May GD, Dixon RA (2005) Profiling the transcriptome of alfalfa glandular trichomes. Planta 221 28–38 [DOI] [PubMed] [Google Scholar]
- Bernotiene G, Nivinshiene O, Butkiene R, Mochkute D (2004) Chemical composition of essential oils of hops (Humulus lupulus L.) growing wild in Aukstaitija. Chemija 15 31–36 [Google Scholar]
- Bohlmann J, Martin D, Oldham NJ, Gershenzon J (2000) Terpenoid secondary metabolism in Arabidopsis thaliana: cDNA cloning, characterization, and functional expression of a myrcene/(E)-beta-ocimene synthase. Arch Biochem Biophys 375 261–269 [DOI] [PubMed] [Google Scholar]
- Bohlmann J, Steele CL, Croteau R (1997) Monoterpene synthases from grand fir (Abies grandis) cDNA isolation, characterization and functional expression of myrcene synthase, (2)-(4S)-limonene synthase, and (2)-(1S,5S)-pinene synthase. J Biol Chem 272 21784–21792 [DOI] [PubMed] [Google Scholar]
- Bräuer L, Brandt W, Wessjohann LA (2004) Modeling the E. coli 4-hydroxybenzoic acid oligoprenyltransferase (ubiA transferase) and characterization of potential active sites. J Mol Model 10 317–327 [DOI] [PubMed] [Google Scholar]
- Carpenter CD, Simon AE (1998) Preparation of RNA. Methods Mol Biol 82 85–89 [DOI] [PubMed] [Google Scholar]
- Cheng AX, Xiang CY, Li JX, Yang CQ, Hu WL, Wang LJ, Lou YG, Chen XY (2007) The rice (E)-β-caryophyllene synthase (OsTPS3) accounts for the major inducible volatile sesquiterpenes. Phytochemistry 68 1632–1641 [DOI] [PubMed] [Google Scholar]
- Choi YE, Harada E, Wada M, Tsuboi H, Morita Y, Kusano T, Sano H (2001) Detoxification of cadmium in tobacco plants: formation and active excretion of crystals containing cadmium and calcium through trichomes. Planta 213 45–50 [DOI] [PubMed] [Google Scholar]
- De Keukelere J, Ooms G, Heyerock A, Roldan-Ruiz I, Van Bockstaele E, De Keukelere D (2003) Formation and accumulation of α-acids, β-acids, desmethylxanthohumol, and xanthohumol during flowering of hops (Humulus lupulus L.). J Agric Food Chem 51 4436–4441 [DOI] [PubMed] [Google Scholar]
- Dudareva N, Andersson S, Orlova I, Gatto N, Reichelt N, Rhodes D, Boland W, Gershenzon J (2005) The nonmevalonate pathway supports both monoterpene and sesquiterpene formation in snapdragon flowers. Proc Natl Acad Sci USA 192 933–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva N, Martin D, Kish CM, Kolosova N, Gorenstein N, Fäldt J, Miller B, Bohlmann J (2003) (E)-beta-ocimene and myrcene synthase genes of floral scent biosynthesis in snapdragon: function and expression of three terpene synthase genes of a new terpene synthase subfamily. Plant Cell 15: 1227–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eri S, Khoo BK, Lech J, Hartman TG (2000) Direct thermal desorption-gas chromatography and gas chromatography-mass spectrometry profiling of hop (Humulus lupulus L.) essential oils in support of varietal characterization. J Agric Food Chem 48 1140–1149 [DOI] [PubMed] [Google Scholar]
- García-Olmedo F, Molina A, Alamillo JM, Rodríguez-Palenzuéla P (1998) Plant defense peptides. Biopolymers 47 479–491 [DOI] [PubMed] [Google Scholar]
- Goese M, Kammhuber K, Bacher A, Zenk MH, Eisenreich W (1999) Biosynthesis of bitter acids in hops. A (13)C-NMR and (2)H-NMR study on the building blocks of humulone. Eur J Biochem 263 447–454 [DOI] [PubMed] [Google Scholar]
- Kader JC (1996) Lipid-transfer proteins in plants. Annu Rev Plant Biol 47 627–654 [DOI] [PubMed] [Google Scholar]
- Katsiotis ST, Langezaal CR, Scheffer JJC, Verpoorte R (1989) Comparative study of the essential oils from hops of various Humulus lupulus L. cultivars. Flavour Fragrance J 4 187–191 [Google Scholar]
- Keeling CI, Bohlmann J (2006) Genes, enzymes and chemicals of terpenoid diversity in the constitutive and induced defence of conifers against insects and pathogens. New Phytol 170 657–675 [DOI] [PubMed] [Google Scholar]
- Küpper H, Lombi E, Zhao FJ, McGrath SP (2000) Cellular compartmentation of cadmium and zinc in relation to other elements in the hyperaccumulator Arabidopsis halleri. Planta 212 75–84 [DOI] [PubMed] [Google Scholar]
- Kuzuyama T, Noel JP, Richard SB (2005) Structural basis for the promiscuous biosynthetic prenylation of aromatic natural products. Nature 435 983–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFlamme P, Khouri H, Gulick P, Ibrahim R (1993) Enzymatic prenylation of isoflavones in white lupin. Phytochemistry 34 147–151 [Google Scholar]
- Lange BM, Wildung MR, Stauber EJ, Sanchez C, Pouchnik D, Croteau R (2000) Probing essential oil biosynthesis and secretion by functional evaluation of expressed sequence tags from mint glandular trichomes. Proc Natl Acad Sci USA 97 2934–2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK (1999) The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 50 47–65 [DOI] [PubMed] [Google Scholar]
- Lücker J, Bowen P, Bohlmann J (2004) Vitis vinifera terpenoid cyclases: functional identification of two sesquiterpene synthase cDNAs encoding (+)-valencene synthase and (-)-germacrene D synthase and expression of mono- and sesquiterpene synthases in grapevine flowers and berries. Phytochemistry 65 2649–2659 [DOI] [PubMed] [Google Scholar]
- Martin DM, Bohlmann J (2004) Identification of Vitis vinifera (-)-alpha-terpineol synthase by in silico screening of full-length cDNA ESTs and functional characterization of recombinant terpene synthase. Phytochemistry 65 1223–1229 [DOI] [PubMed] [Google Scholar]
- Nagel J, Culley LK, Lu Y, Liu E, Matthews PD, Stevens JF, Page JE (2008) EST analysis of hop glandular trichomes identifies an O-methyltransferase that catalyzes the biosynthesis of xanthohumol. Plant Cell 20 186–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Ito K (2001) Cloning and analysis of valerophenone synthase gene expressed specifically in lupulin gland of hop (Humulus lupulus L.). Biosci Biotechnol Biochem 65 150–155 [DOI] [PubMed] [Google Scholar]
- Okada Y, Sano Y, Kaneko T, Abe I, Noguchi H, Ito K (2004) Enzymatic reactions by five chalcone synthase homologs from hop (Humulus lupulus L.). Biosci Biotechnol Biochem 68 1142–1145 [DOI] [PubMed] [Google Scholar]
- Okada Y, Sugimoto M, Ito K (2001) Molecular cloning and expression of farnesyl pyrophosphate synthase gene responsible for essential oil biosynthesis in hop (Humulus lupulus). J Plant Physiol 158 1183–1188 [Google Scholar]
- Oliveira MM, Pais MS (1990) Glandular trichomes of Humulus lupulus var. Brewer's gold (hops): ultrastructural aspects of peltate trichomes. J Submicrosc Cytol Pathol 22 241–248 [Google Scholar]
- Paniego NB, Zuurbier KWM, Fung SY, van der Heijden R, Scheffer JJC, Verpoorte R (1999) Phlorisovalerophenone synthase, a novel polyketide synthase from hop (Humulus lupulus L.) cones. Eur J Biochem 262 612–616 [DOI] [PubMed] [Google Scholar]
- Pastorello EA, Farioli L, Pravettoni V, Ortolani C, Ispano M, Monza M, Baroglio C, Scibola E, Ansaloni R, Incorvaia C, et al (1999) The major allergen of peach (Prunus persica) is a lipid transfer protein. J Allergy Clin Immunol 103 520–526 [DOI] [PubMed] [Google Scholar]
- Ralston L, Subramanian S, Matsuno M, Yu O (2005) Partial reconstruction of flavonoid and isoflavonoid biosynthesis in yeast using soybean type I and type II chalcone isomerases. Plant Physiol 137 1375–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Hirosawa T, Horiuchi S, Murakami A, Matsushima H (1995) A study of SEM examination on fresh hop (Humulus lupulus L.) peltate glandular trichomes. J Electron Microsc (Tokyo) 44 39–44 [Google Scholar]
- Sasaki K, Mito K, Ohara K, Yamamoto H, Yazaki K (2008) Cloning and characterization of naringenin 8-prenyltransferase, a flavonoid-specific prenyltransferase of Sophora flavescens. Plant Physiol 146 1075–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockey JM, Fulda MS, Browse J (2003) Arabidopsis contains a large superfamily of acyl-activating enzymes. Phylogenetic and biochemical analysis reveals a new class of acyl-coenzyme A synthetases. Plant Physiol 132 1065–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon-Mateo C, Depuydt S, de Oliveira Manes CL, Cnudde F, Holsters M, Goethals K, Vereecke D (2006) The phytopathogen Rhodococcus fascians breaks apical dominance and activates axillary meristems by inducing plant genes involved in hormone metabolism. Mol Plant Pathol 7 103–112 [DOI] [PubMed] [Google Scholar]
- Stevens JF, Page JE (2004) Xanthohumol and related prenylflavonoids from hops and beer: to your good health! Phytochemistry 65 1317–1330 [DOI] [PubMed] [Google Scholar]
- Tholl D, Chen F, Petri J, Gershenzon J, Pichersky E (2005) Two sesquiterpene synthases are responsible for the complex mixture of sesquiterpenes emitted from Arabidopsis flowers. Plant J 42 757–771 [DOI] [PubMed] [Google Scholar]
- Tian L, Pang YZ, Dixon RA (2008) Biosynthesis and genetic engineering of proanthocyanidins and (iso)flavonoids. Phytochem Rev 7 445–465 [Google Scholar]
- Welle R, Grisebach H (1991) Properties and solubilization of the prenyltransferase of isoflavonoid phytoalexin biosynthesis in soybean. Phytochemistry 30 479–484 [Google Scholar]
- Zuurbier KWM, Fung SY, Scheffer JJC, Verpoorte R (1998) In-vitro prenylation of aromatic intermediates in the biosynthesis of bitter acids in Humulus lupulus. Phytochemistry 49 2315–2322 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.