Abstract
Purpose
Results in previous reports have demonstrated that immunization of the EAU-prone B6 mouse activates both CD4 and CD8 IRBP-specific T cells. The purpose of this study was to investigate structural and functional differences between CD4 and CD8 autoreactive T cells activated by the uveitogenic peptide.
Methods
Purified CD4 and CD8 isolated from B6 mice immunized with an uveitogenic peptide, interphotoreceptor retin-oid-binding protein (IRBP)1-20, were stimulated in vitro with various doses of immunizing peptide. The activated T cells were determined for cytokine production, expression of Foxp3, and suppressor activity.
Results
CD4 autoreactive T cells underwent full activation when stimulated with high or medium concentrations of immunizing peptide, whereas a high dose of antigenic peptide resulted in only modest activation of CD8 autoreactive T cells. When stimulated by a low dose (<0.1 μg/mL) of antigen or by of a high dose of antigen and a small amount of TGF-β1, the minimally activated CD8 T cells expressed a high level of Foxp3 and gained suppressor function.
Conclusions
Minimally activated CD8 autoreactive T cells can be functionally suppressive and may neutralize the tissue-damaging effect of the CD4 autoreactive T cells.
The immune system has evolved complex mechanisms to avoid an autoimmune response while providing protective immunity, and transgenic animal studies have shown that animals can keep autoaggression in check despite the presence of a large number of self-reactive cells.1,2 Further studies are needed to determine whether the effects of T cells on the autoimmune response and protective immunity are controlled by distinct regulatory T cell (Treg) subsets or by shared Treg subsets activated to different levels. Recent studies have demonstrated that, among the CD4 T cells, a subset expressing CD25 has strong suppressor activity.3,4 There is also evidence that some subsets of CD8 T cells are functionally suppressive.5–9 In the present study, we showed that, among the CD8 T cells in the B6 mouse that are reactive to the uveitogenic peptide interphotoreceptor retinoid-binding protein (IRBP)1-20, a subset of cells was preferentially activated to exert suppressor activity if these T cells were minimally activated.
In previous reports, we demonstrated that adoptive transfer of IRBP-specific T cells induces more severe uveitis than the same disease induced by antigen immunization.10 The increased disease-inducing ability of IRBP-specific T cells is associated with the appearance of a high number of activated, CD8 IRBP-specific T cells, which is promoted by in vitro stimulation of the in vivo primed IRBP-specific T cells.11–13 Moreover, in vitro activated IRBP-specific T cells induce much milder disease on transfer to β2m−/− mice, which do not express major histocompatibility complex (MHC) class I antigens,11 indicating a synergistic effect of CD4 and CD8 autoreactive T cells in the disease pathogenesis. Since the pathogenic activity of CD4 autoreactive T cells is closely associated with their degree of activation,13–15 we wanted to determine whether this association also applies to the pathogenic activity of CD8 autoreactive T cells.
In the present study, we showed that, depending on the degree of activation acquired, in vivo primed CD8 IRBP-spe-cific T cells varied greatly in their ability to produce proinflam-matory cytokines and in Foxp3 expression. Minimally activated CD8 cells produced little cytokine, but expressed greatly increased amounts of Foxp3 and were functionally suppressive when exposed to a low dose of immunizing antigen or to a high dose of immunizing antigen in the presence of TGF-β1. Our results show that CD8 activation may generate completely opposite functions, depending on the degree of activation, with weakly activated CD8 cells having increased suppressor activity. Thus, activated CD8 autoreactive T cells may act as a two-edged sword, and whether these cells are pathogenic or protective depends on their degree of activation.
MATERIALS AND METHODS
Animals and Reagents
Pathogen-free female C57BL/6 mice (10–14 weeks old) were purchased from the Jackson Laboratory (Bar Harbor, ME) and were housed and maintained in the animal facilities of the University of Louisville. Institutional approval was obtained, and all procedures adhered to institutional guidelines regarding animal experimentation. The recombinant porcine TGF-β1 was purchased from R&D Systems (Minneapo-lis, MN). All animal studies conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Preparation of IRBP1-20-Specific T Cells
Donor mice were immunized with subcutaneous injection of 200 μL of an emulsion containing 200 μg IRBP1-20 (amino acids 1-20 of human IRBP; Sigma-Aldrich, St. Louis, MO) and 500 μg of Mycobacterium tuberculosis H37Ra (Difco, Detroit, MI) in incomplete Freund’s adjuvant (Sigma-Aldrich), distributed over six spots at the tail base and on the flank. At 13 days after immunization, T cells were isolated from spleen cells by passage through a nylon wool column, and then 1 × 107 cells in 2 mL of RPMI medium in a six-well plate (Costar; Corning, Corning, NY) were stimulated with 10 μg/mL IRBP1-20 in the presence of 1 × 107 irradiated syngeneic spleen cells as antigen-presenting cells (APCs). After 2 days, the activated lymphoblasts were isolated by gradient centrifugation (Lymphoprep; Robbins Scientific, Mountain View, CA) and cultured in RPMI 1640 medium supplemented with IL-2-containing medium (10 ng/mL).
Proliferation Assay
T cells from IRBP1-20-immunized wild-type B6 mice were prepared and seeded at 4 × 105 cells/well in 96-well plates and cultured at 37°C for 48 hours in a total volume of 200 μL of medium, with or without IRBP1-20, in the presence of irradiated syngeneic spleen APCs (1 × 105), and [3H] thymidine incorporation during the last 8 hours was assessed with a microplate scintillation counter (Packard; PerkinElmer, Meriden, CT). The proliferative response is expressed as the mean counts per minute ± SD of triplicate determinations.
Purification of CD4 and CD8 T Cells
Purified CD4 and CD8 T cells were prepared from the spleens using CD4 and CD8 isolation kits (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). The spleen cells were first incubated for 10 minutes at 4°C with a cocktail of biotin-conjugated antibodies against mouse CD8 (CD8a, Ly-2) or CD4 (CD4, L3T4) T cells (H57 to 597), B cells (CD45R, B220), NK cells (CD49b,DX5), hematopoietic cells (CD11b, Mac-1), and erythroid cells (Ter119) and then for 15 minutes at 4°C with anti-biotin microbeads. The cells were then separated into bound and nonbound cells on a magnetic separator column (auto-MACS; Miltenyi Biotec GmbH) and washed with 15 mL of medium, according to the manufacturer’s protocol. The flow-through fraction containing CD4- or CD8-enriched cells was collected, and the purity of the isolated cell fraction was determined by flow cytometric analysis with FITC-conjugated anti-TCR antibody and PE-conjugated antibody against mouse CD8 or CD4 (BD Biosciences, San Jose, CA). Data collection and analysis were performed on a flow cytometer (FACSCalibur using CellQuest software; BD Biosciences).
Real-Time PCR
RT-PCR was performed as described previously.16 Total RNA was extracted from the freshly purified CD8 and CD4 T-cell subsets by using RNA isolation kits (Invitrogen, Carlsbad, CA). Total RNA was reverse transcribed into cDNA, amplified, and quantified by real-time PCR (TaqMan assays on an Mx3000P system; Stratagene, La Jolla, CA). Primer and probe sequences were as follows: for Foxp3, forward primer 5′-CTTCAGAAACCACCCCGCCA-3′, reverse primer 5′-CTCGCTCTCCACTCGCACAA-3′, and probe 5′-TGCCATCCGCCA-CAACCTGAGCCT-3′; and for ′-actin, forward primer 5′-ATCTAC-GAGGGCTATGCTCTCC-3′, reverse primer 5′-ACGCTCGGTCAG-GATCTTCAT-3′, and probe 5′-CCTGCGTCTGGACCTGGCTGGC-3. Multiplex reactions were run in duplicate and samples normalized to the internal control, β2-microglobulin. The increase (x-fold) was compared by the Foxp3 expression of T cells under investigation relative to known low-expressing T cells.17
Treg Cell Preparation
To isolate CD4+CD25+ and CD8+CD122+ Treg cells, purified CD4 and CD8 T cells were incubated with PE-conjugated anti-CD25 antibody (PC61, 1 μg/106 cells) or anti-CD122 antibody (TM-β1; BD Biosciences; 1 μg/106 cells, antibody depleted of sodium azide by overnight dialysis against phosphate-buffered saline [PBS]) in PBS containing 0.5% fetal bovine serum for 30 minutes at 4°C, washed, and incubated for 15 minutes at 4°C with anti-PE-conjugated microbeads (20 μL/107 cells; Miltenyi Biotec GmbH). CD4+CD25− and CD8+CD122− Treg cells were then isolated on a magnetic separator column (auto-MACS; Mil-tenyi Biotec GmbH). The purity of the isolated cell fraction was determined by flow cytometric analysis using FITC-conjugated anti-TCR antibody and PE-conjugated antibody directed against mouse CD4, CD25, CD8, or CD122 (BD Biosciences).
Flow Cytometry Analysis
Aliquots of 2 × 105 cells were double stained with combinations of FITC- or PE-conjugated monoclonal antibodies against mouse αβTCR (H57-597), CD4, or CD8. Data collection and analysis were performed on a flow cytometer (FACSCalibur using CellQuest software; BD Biosciences).
Enzyme-Linked Immunosorbent Assay
IL-2 and IFN-γ were measured by using commercially available ELISA kits (R&D Systems).
Statistics
Statistical analyses of all data were performed by using unpaired Student’s t-tests. The data are expressed as the mean ± SD. Each experiment was repeated at least three times.
RESULTS
Effect of In Vitro Low-Dose Antigen Stimulation on In Vivo Primed CD8 IRBP-Specific T Cells
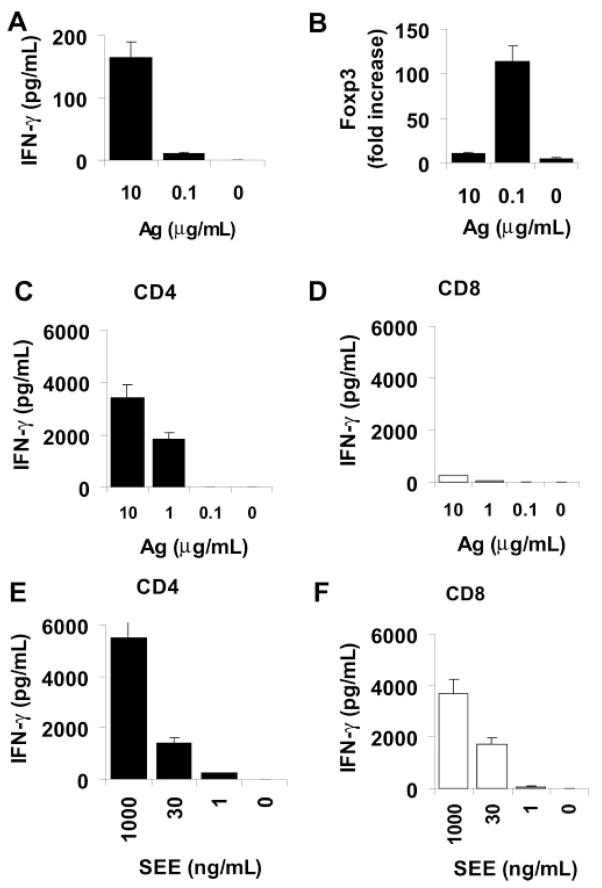
In a previous report, we showed that, on exposure in vitro to the immunizing peptide, in vivo primed CD8 IRBP-specific T cells proliferate marginally and produce limited amounts of proinflammatory cytokines, such as IL-2 and IFN-γ.11 In the present study, we exposed purified CD4 and CD8 T cells from IRBP1-20-immunized mice to graded doses of immunizing antigen ranging from 0.1 to 10 μg/mL in the presence of synge-neic APCs (irradiated spleen cells); then, 48 hours later, the culture supernatants were assessed for cytokines, including IL-2 and IFN-γ, and the cells were cultured with exogenous IL-2 for a further 3 days and analyzed by PCR for Foxp3. As shown in Figure 1, CD8 cells exposed to a high dose of antigen (10 μg/mL) produced a modest level of cytokines (Fig. 1A) and expressed limited levels of Foxp3 (Fig. 1B). In contrast, after exposure to a low dose of immunizing antigen (0.1 μg/mL), these cells expressed much more Foxp3 (Fig. 1B) without producing a significant amount of cytokines (Fig. 1A), whereas the same T cells cultured with APCs alone in the absence of antigen did not express IFN-γ and expressed a very low level of Foxp3 (Figs. 1A, 1B). Thus, the expression of Foxp3 by CD8 IRBP-specific T cells correlated inversely with the degree of activation. To exclude the possibility that such cells were defective in cytokine-producing ability, we exposed the separated CD4 or CD8 T cells to 0.1 to 10 μg/mL of immunizing antigen or 1 to 1000 ng/mL of SEE in the presence of syngeneic APCs (Figs. 1C, 1F). The results showed that the CD4 T cells responded to both stimuli, whereas the CD8 cells, although not responding to antigen, produced large amounts of cytokines when exposed to SEE, showing that they were not defective in inflammatory cytokine production and that, unlike CD4 IRBP-specific T cells, immunizing antigen alone was not sufficient to cause full activation of CD8 cells.
Figure 1.
IFN-γ production and Foxp3 expression by CD8 IRBP-specific T cells correlated inversely with their degree of activation. (A, B) MACS column–purified CD8 T cells (4 × 106/well) were stimulated for 48 hours in 12-well plates with IRBP1-20 (10, 0.1, or 0 μg/mL) and tested for IFN-γ production (A) or Foxp3 expression (B; P < 0.01). (C–F) MACS column-purified CD4 (C, E) or CD8 (D, F) T cells (4 × 106/well) were stimulated for 48 hours in 12-well plates with IRBP1-20 (10, 1, 0.1, or 0 μg/mL) (C, D) or SEE (E, F; 1000, 30, 1, or 0 ng/mL; P < 0.01). The data are the mean ± SD of results in three separate experiments. Note that the CD8 IRBP-specific T cells were only partially activated by immunizing autoantigen (D), but were fully capable of producing proinflammatory cytokines if properly activated (F).
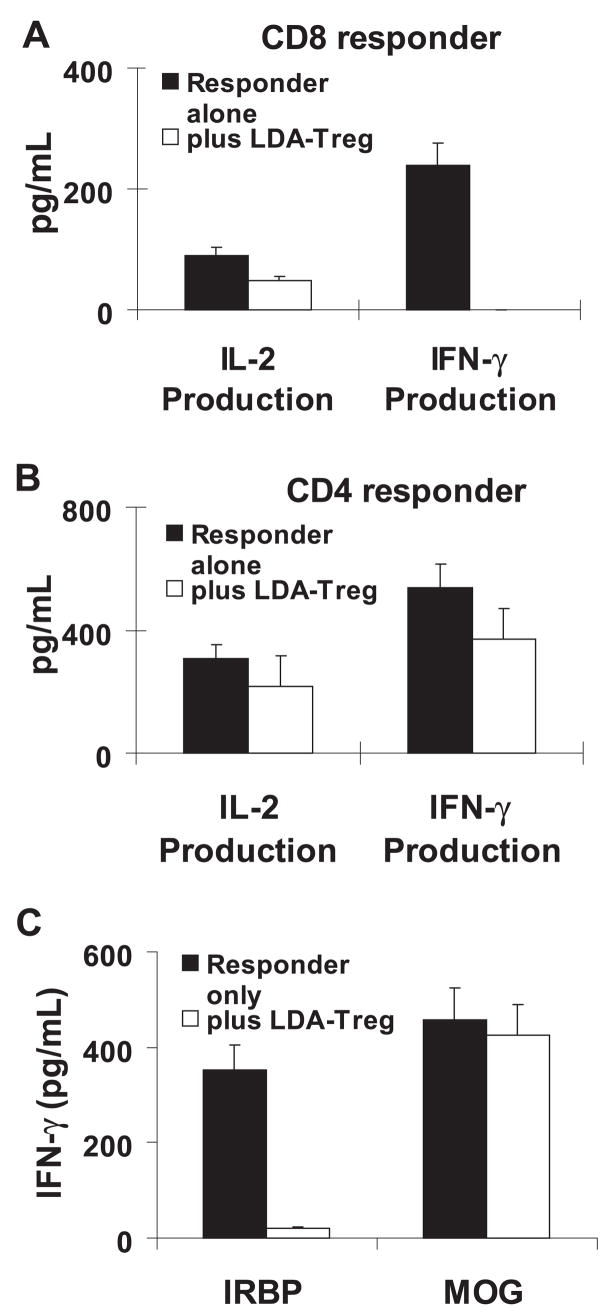
To determine further whether these low-dose antigen-in-duced regulatory (LDA-Treg) T cells showed increased suppressor activity, we prepared CD4 and CD8 responder T cells from IRBP-immunized mice and stimulated them in a 96-well plate of IRBP peptide in the absence or presence of LDA-Treg cells. The cells significantly inhibited cytokine production by LDA-Treg the responder CD8 (Fig. 2A) and CD4 (Fig. 2B) T cells, whereas CD8 T cells exposed to a high dose of antigen (10 μg/mL) did not show significant inhibition of cytokine production, and the LDA-Treg cells alone did not produce detectable cytokines (not shown). It is interesting to note that they were more suppressive of CD8 responder T cells (Fig. 2A) than of CD4 responder T cells (Fig. 2B). To determine whether the effect of such regulatory T cells is antigen-specific, we also performed a functional test to assess the suppressive effect of the regulatory T cells isolated from IRBP-induced EAU on uveitogenic and encephalitogenic T cells. Our results showed that the regulatory T cells isolated from IRBP-induced mice were more suppressive of the IRBP-specific uveitogenic T cells than of the myelin oligodendrocyte/glycoprotein (MOG)-specific enceph-alitogenic T cells (Fig. 2C).
Figure 2.
In vitro suppressive effect of Foxp3high CD8 T cells. (A, B) Foxp3high CD8 T cells have a strong inhibitory effect on IFN-γ production by responder T cells and are more inhibitory for CD8 than CD4 responder T cells. Responder CD8 (A) or CD4 (B) T cells were separated from IRBP1-20-immunized B6 mice using flow cytometry, then incubated for 48 hours in 12-well plates (4 × 106 cells/well) with immunizing peptide (10 μg/mL) and APCs in the absence or presence of Foxp3high CD8 T cells at a regulatory T to effector T cell ratio of 1:4, and then IL-2 and IFN-γ levels in the culture supernatants were measured by ELISA. The result shown is representative of those obtained in more than five experiments (P < 0.01). (C) Regulatory T cells isolated from IRBP-induced mice were more suppressive of the uveitogenic T cells than the encephalitogenic T cells. Foxp3high CD8 T cells were isolated from IRBP-immunized B6 mice. Nylon wool column–enriched responder T cells (4 × 106) isolated either from IRBP-or from MOG-immunized B6 mice were incubated for 48 hours in 12-well plates with immunizing peptide (10 μg/mL) and APCs in the absence or presence of Foxp3high CD8 T cells at a regulatory T to effector T cell ratio of 1:4, and IFN-γ levels in the culture supernatants were measured by ELISA.
Effect of TGF-β1 on the Balance between the Generation of Suppressor and Nonsuppressor CD8 Autoreactive T Cells
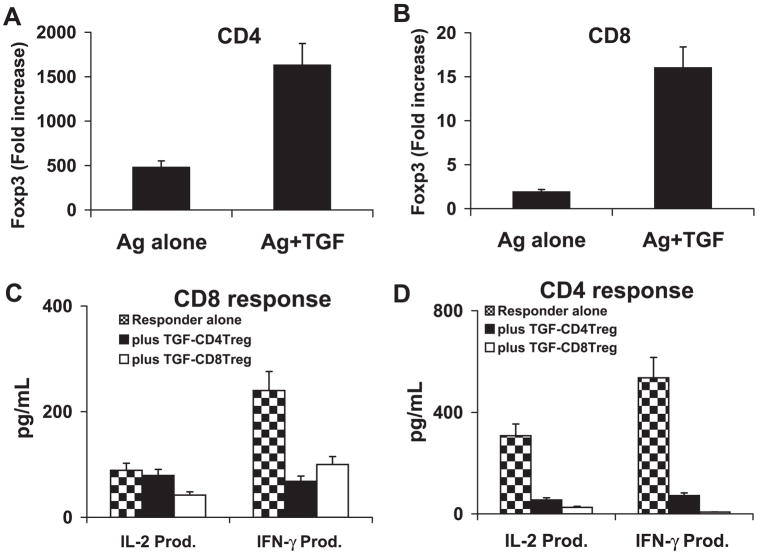
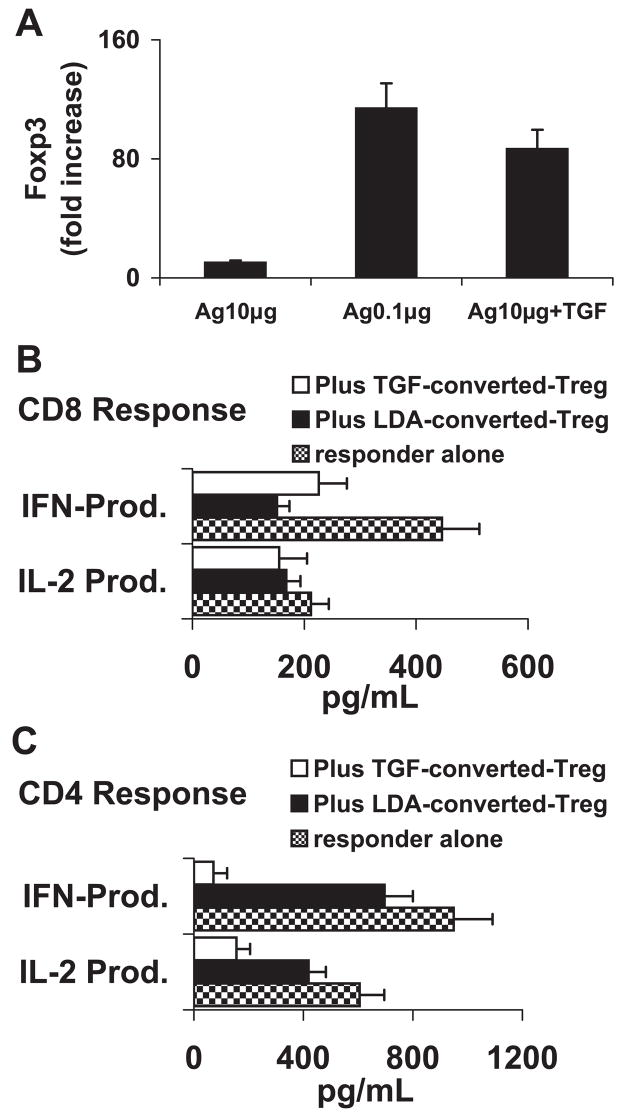
Previous studies have demonstrated that TGF-β1 suppresses immune responses by maintaining or promoting the development of suppressor T cells.18,19 To determine the effect of TGF-β1 on the generation of regulatory T cells from CD4 and CD8 IRBP-specific autoreactive T cells, in vivo primed CD4 and CD8 IRBP-specific T cells were cultured with a high dose of immunizing antigen (10 μg/mL) in the presence or absence of TGF-β1 (1 ng/mL; Figs. 3A, 3B), then, 3 days later, the T cells were separated, analyzed for Foxp3 expression and tested for suppressor activity. As shown in Figures 3A and 3B, addition of TGF-β1 greatly promoted the expansion of Foxp3-expressing cells from both CD4 (Fig. 3A) and CD8 (Fig. 3B) IRBP-specific T-cells. We repeatedly observed that the TGF-β1-induced CD8 Treg cells were more functionally suppressive of CD8 responder T cells, whereas the similarly induced CD4 Treg were more functionally suppressive of CD4 responder T cells (Figs. 3C, 3D).
Figure 3.
TGF-β1 activated CD8 and CD4 suppressor T cells. (A, B) Responder CD4 (A) or CD8 (B) T cells were separated from IRBP1-20-immunized B6 mice by flow cy-tometry and were stimulated in vitro with antigen (10 μg/mL) and APCs in the absence or presence of 1 ng/mL of TGF-β1 for 3 days. The T cells were then separated by single-density gradient centrifugation, and cultured in IL-2 (10 ng/mL) medium for another 24 hours before the real-time PCR assessment of Foxp3 expression. The x-fold increase was then calculated (P < 0.01). (C, D) After the 3-day in vitro stimulation with antigen and TGF-β1, the CD8 (3C) or CD4 (D) T cells were assessed for suppressor activity of CD8 (C) and CD4 (D) T-cell responses. The responder T cells were freshly prepared CD8+ CD122− or CD4+ CD25− T cells from immunized B6 mice. After coculture of responder (4 × 106 cells/well) and suppressor T cells (ratio 4:1) for 48 hours in 12-well plates with immunizing peptide (10 μg/mL) and APCs, the culture supernatants were sampled for measurement of IL-2 and IFN-γ by ELISA. The result shown is representative of those obtained in more than five experiments. TGF-CD4 Treg: TGFβ1-induced regulatory CD4 cells; TGF-CD8 Treg: TGFβ1-induced regulatory CD8 cells (P < 0.01).
Immunosuppressive Effect of the CD8+CD122+ T-Cell Subset
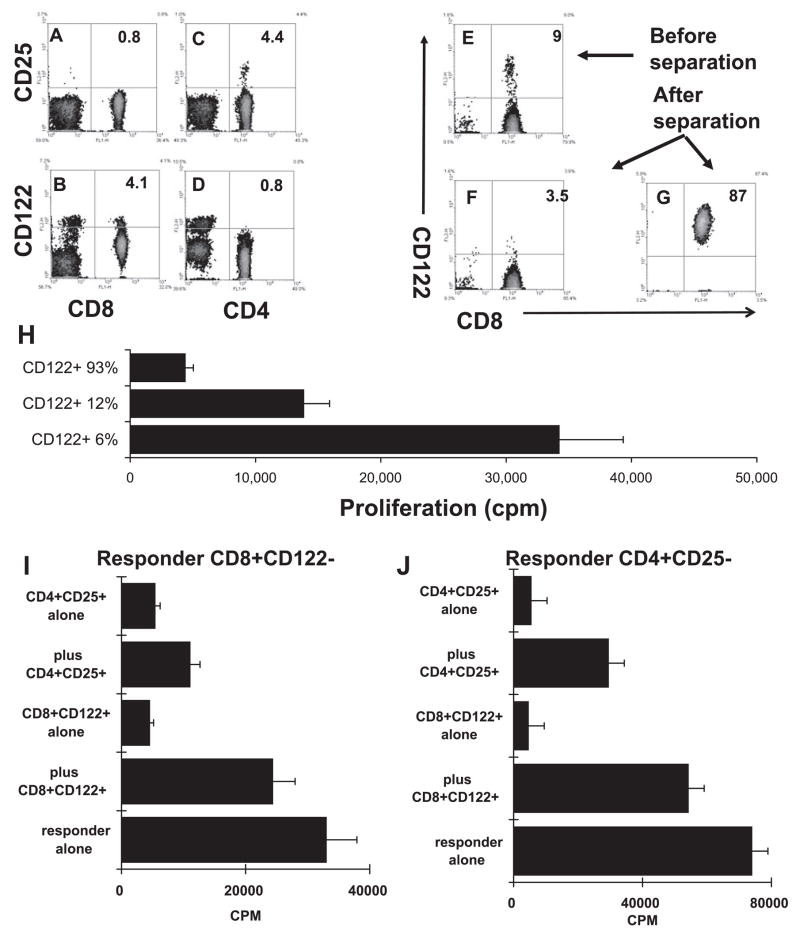
To distinguish between the possibilities that (1) low-dose antigen or TGF-β1 preferentially drives CD8 T subsets that are functionally suppressive and (2) the suppressor activity of CD8 T cells is not an inherent property of specific T-cell subsets, but the degree of T-cell activation determines whether an activated CD8 T cell is functionally suppressive, we first examined whether suppressor CD8 autoreactive T-cell subsets express specific surface markers. Figure 4 shows that 4% to 5% of total splenic T cells expressed CD25 and CD4 (Fig. 4C), but CD25+ was undetectable on the CD8 T cells (Fig. 4A). In contrast, 4.1% of the total T cells (12% of the CD8 T cells) expressed CD122, and the frequency of CD122+ cells was much lower among the CD4 cells (Figs. 4B, 4D). To determine whether the CD122+ CD8 T cells, like the CD25+ CD4 cells, were functionally suppressive, we partially depleted CD122+ cells from the MACS-purified CD8 T cells (Fig. 4F) or enriched the CD122+ cells (Fig. 4G) before these T cells were stimulated by an immunogenic dose of immunizing IRBP peptide. As shown in Figure 4H, responder T cells containing a high percentage of CD122−CD8+ T cells responded more vigorously, whereas those containing a low percentage of CD122+CD8+ T cells responded poorly. The results shown in Figures 4I and 4J demonstrate that highly enriched CD122+ CD8+ T cells were functionally suppressive of both the CD4 and CD8 T-cell responses.
Figure 4.
Suppressor effect of the CD8+CD122+ T cell subset. (A–D) Unfractionated T cells from IRBP1-20-immuned mice were double-stained with PE-conjugated anti-CD25 or anti-CD122 antibody and FITC-conjugated anti-CD4 or anti-CD8 antibody. Some of the CD8 T cells express CD122, but not CD25, whereas some of the CD4 T cells express CD25, but not CD122. (E–G) Separation of CD8+CD122+ T cells from CD8+ CD122− T cells on a magnetic column (auto-MACS; Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). (H) Unfractionated CD8 T cells (12% CD122+), CD122+-enriched CD8 T cells (93%), and CD8 T cells partially depleted of CD122+ cells (6% CD122+) were stimulated with 10 μg/mL of IRBP 1-20 in the presence of APCs and assessed for thymidine incorporation. CD8 IRBP-specific T cells showed increased proliferation when the CD122+ subset was partially depleted (P < 0.01). (I–J) Isolated CD8+CD122+ and CD4+ CD25+ T cells were suppressive of the CD4 (I) and CD8 (J) response. Magnetic bead-separated CD8+CD122− or CD4+ CD25− responder T cells were incubated for 48 hours in 96-well plates (4 × 105 cells/well) with immunizing peptide (10 μg/mL) and APCs in the absence or presence of the indicated freshly prepared CD8+CD122+ or CD4+CD25+ Treg cells (1 × 105 cells/well), and [3H] thymidine incorporation during the last 8 hours was assessed (P < 0.01).
Conversion (Acquisition) of the Regulatory Activity of CD8+CD122− Cells
To determine whether the CD8+ CD122− T cells could gain suppressive activity if treated with a low dose of immunizing antigen or with TGF-β1, we separated the CD122+ CD8 T cells from the CD122− CD8 T cells and cultured the CD122− CD8 cells for 3 days with a high or low dose of antigen or a high dose of antigen in the presence of TGF-β1. As shown in Figure 5A, increased expression of Foxp3+ was seen in CD8+ CD122− T cells treated with a low dose of IRBP or with a high dose of IRBP plus TGF-β1, and the functional assay demonstrated that these cells were functionally suppressive of both CD4 and CD8 responder T cells (Fig. 5B). Together, these results show that regulatory T cells are found in both CD122+ and CD122− CD8 T cells.
Figure 5.
CD8+CD122− T cells gained suppressor activity after stimulation with low-dose antigen or high-dose antigen in the presence of TGF-β1. (A) CD8+CD122− T cells were isolated from IRBP1-20-immunized B6 mice, and stimulated for 48 hours in 12-well plates with 10 or 0.1 μg/mL IRBP1-20 10 μg/mL of IRBP1-20 plus 1 ng/mL TGF-β1. After an in vitro stimulation by low-dose antigen or high-dose antigen plus TGF-β1, the CD8+CD122− T cells expressed increased levels of Foxp3 (P < 0.01). (B, C) Freshly prepared CD8+CD122− (B) or CD4+CD25− (C) responder cells were incubated for 48 hours in 12-well plates (4 × 106 cells/well) with immunizing peptide (10 μg/mL) and APCs, in the absence or presence of the indicated Foxp3high CD8+CD122− Treg cells at a regulatory T cell to responder T cell ratio of 1:4, then IL-2 and IFN-γ in the culture supernatants were measured by ELISA. The results are representative of more than five experiments. LDA-converted-Treg: CD8CD122− T cells converted by low-dose antigen; TGF-β-converted Treg: CD8CD122− T cells converted by TGFβ1 (P < 0.01).
DISCUSSION
Although the immunoregulatory ability of Treg cells is widely accepted,20 questions such as how these T cells become functionally active and the factors that regulate their function remain largely unknown. The initial goal of this study was to compare the activation requirements of CD4 and CD8 autore-active T cells in an IRBP-induced uveitis model in the B6 mouse, based on our previous reports that, in both rat and mouse uveitis models, CD8 autoreactive T cells can be readily demonstrated.11–13 We wanted to determine how these two T cell populations are generated and interact in the pathogenesis of this autoimmune disease and determine the immunologic conditions that contribute to the activation of the either or both (CD4 and CD8) autoreactive T cells, given that the pathogenic activity of the T cells is closely related to their degree of activation, rather than their number.21,22
In this study, we found that IRBP-specific CD8 T cells differed greatly from their CD4 counterparts in gaining activation-dependent functional properties. The uveitogenic peptide, IRBP1-20, strongly stimulated full in vitro activation of CD4 cells from the immunized mouse, but elicited only a modest proliferative response in the CD8 cells, which produced limited amounts of proinflammatory cytokines. Parallel studies showed that the CD8 cells mounted a strong proliferative response and produced high levels of cytokines when stimulated with the superantigen SEE, supporting our assumption that, unlike antigen-primed CD4 T cells, a high degree of activation of CD8 autoreactive T cells is not achieved by au-toantigen alone but requires additional activation signals.23,24 This may explain our previous findings that induction of EAU by active immunization of disease-prone rodents with pathogenic peptides induces acute, monophasic disease, whereas adoptive transfer of in vitro activated IRBP-specific T cells induces chronic recurrent disease.10,13 Conceivably, antigen immunization causes moderate CD4 activation in vivo in the EAU-prone rodent with a low degree of activation of CD8 autoreactive T cells, whereas, in the adoptive transfer model, the in vitro stimulation of the IRBP-specific T cells promotes a high degree of activation of CD8 autoreactive T cells.11,13 As a result, the synergistic pathogenic effect of both the activated CD4 and CD8 autoreactive T cells leads to severe and recurrent disease. The results of this study also demonstrated that exposure of CD8 autoreactive T cells to a low dose of immunizing antigen stimulated them to express high levels of Foxp3, a cytoplasmic molecule that is commonly expressed in suppressor T cells.25–27 This implies that confrontation with a low-dose autoantigen causes CD8 autoreactive T cells to gain suppressor activity, which then neutralizes the pathogenic activity of both CD8 and CD4 autoreactive T cells.
Using a similar working model, we were able to show that in vitro stimulation of CD8 T cells with a high dose of immunizing antigen in the presence of TGF-β1 also induced the cells to express Foxp3. This observation agrees with previous reports that TGF-β1 supports the activation and expansion of Treg cells.28–32 Both CD4 and CD8 cells showed increased expression of Foxp3 after TGF-β treatment. Although CD4 cells expressed higher levels of Foxp3, functional tests showed that TGF-induced CD8 cells were functionally more active than TGF-treated CD4 cells, especially in the suppression of the CD8 response (Fig. 3). The fact that CD8 autoreactive T cells show increased activation when cytokines produced by CD4 T cells are provided11 suggests that the activation of CD4 T cells, either specific or nonspecific, provides additional activation requirements for CD8 T cells, leading to augmented disease severity.
Several recent studies have demonstrated the regulatory function of the CD4+CD25+ T cell subset4,33–35; however, CD8 T-cell subsets have also repeatedly been shown to have suppressor activity.5,7,36–38 CD8+ T cells are reported to be essential for the protective effect of T-cell vaccination, and they also participate in oral tolerance.39,40 In contrast, CD8+ T cells act as pathogenic cells of autoimmune disease.11,13,41–43 Thus, CD8+ T cells or subsets may act as effectors or regulators of immune responses. Our studies further support the previous observation that subsets of CD8 cells can express high levels of Foxp3 and inhibit T-cell activation.44 The fact that the regulatory T cells isolated from IRBP-induced EAU were more inhibitory of the uveitogenic T cells than of the MOG-induced encephalitogenic T cells and they were more suppressive of CD8 responder T cells than of CD4 responder T cells (Fig. 2) indicates that this regulatory cell subset may be antigen-spe-cific regulatory cells. It remains to be determined whether the Foxp3+ cells in our system are a specific CD8 T cell subset that constantly expresses Foxp3 or whether CD8 IRBP-specific T-cell subsets express various levels of Foxp3, depending on their differentiation or activation status. Our current data cannot distinguish between these possibilities. However, our results indicate that activation of CD8 autoreactive T cells during autoimmune disease is a two-edged sword, as a high degree of activation may promote the pathogenic process and the exacerbation of the disease.11 A low degree of activation of CD8 autoreactive T cells may favor the suppression of disease by a mechanism by which suppressor CD8 cells are preferentially activated.
In summary, our study demonstrated that, unlike CD4 au-toreactive T cells, the activation and expansion of which is solely dependent on the availability of antigen and APCs, full activation of CD8 autoreactive T cells is only achieved when various growth factors become available. Minimally activated CD8 autoreactive T cells express high levels of Foxp3 and gain suppressive function. As a result, exposure of autoreactive T cells to a low dose of antigen induces suppressive T-cell activity, which favors protection against autoimmune disease.
Acknowledgments
The authors thank Tom Barkas for editorial assistance.
Supported in part by National Eye Institute Grants EY12974, EY14599 (HS) and EY014366, EY017373 (DS); Vision Research Infrastructure Development Grant R24 EY015636; National Multiple Sclerosis Society Grant RG3413A4, and the Commonwealth of Kentucky Research Challenge Trust Fund.
Footnotes
Disclosure: Y. Peng, None; H. Shao, None; Y. Ke, None; P. Zhang, None; G. Han, None; H.J. Kaplan, None; D. Sun, None
References
- 1.Katz JD, Wang B, Haskins K, et al. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74:1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 2.Goverman J, Woods A, Larson L, et al. Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell. 1993;72:551–560. doi: 10.1016/0092-8674(93)90074-z. [DOI] [PubMed] [Google Scholar]
- 3.Shevach EM. Regulatory T cells in autoimmunity. Annu Rev Im-munol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 4.Suripayer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreac-tive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212–1218. [PubMed] [Google Scholar]
- 5.Sun D, Qin Y, Chluba J, et al. Suppression of experimentally-induced autoimmune encephalomyelitis by cytolytic T-T cell interactions. Nature. 1988;332:843–846. doi: 10.1038/332843a0. [DOI] [PubMed] [Google Scholar]
- 6.Sun D, Whitaker JN, Wilson DB. Regulatory T cells in experimental allergic encephalomyelitis. I. Frequency and specificity analysis in normal and immune rats of a T cell subset that inhibits disease. Int Immunol. 1999;11:307–315. doi: 10.1093/intimm/11.3.307. [DOI] [PubMed] [Google Scholar]
- 7.Jiang H, Zhang S-I, Pernis B. Role of CD8+ T cells in murine experimental allergic encephalomyelitis. Science. 1992;256:1213–1215. doi: 10.1126/science.256.5060.1213. [DOI] [PubMed] [Google Scholar]
- 8.Endharti AT, Rifa’IM, Shi Z, et al. Cutting edge: CD8+CD122+ regulatory T cells produce IL-10 to suppress IFN-γ production and proliferation of CD8+ T Cells. J Immunol. 2005;175:7093–7097. doi: 10.4049/jimmunol.175.11.7093. [DOI] [PubMed] [Google Scholar]
- 9.Najafian N, Chitnis T, Salama AD, et al. Regulatory functions of CD8+CD28- T cells in an autoimmune disease model. J Clin Invest. 2003;112:1037–1048. doi: 10.1172/JCI17935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shao H, Liao T, Ke Y, et al. Severe chronic experimental autoim-mune uveitis (EAU) of the C57BL/6 mouse induced by adoptive transfer of IRBP1–20-specific T cells. Exp Eye Res. 2006;82:323–331. doi: 10.1016/j.exer.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Peng Y, Shao H, Ke Y, et al. In vitro activation of CD8 interpho-toreceptor retinoid-binding protein-specific T cells requires not only antigenic stimulation but also exogenous growth factors. J Immunol. 2006;176:5006–5014. doi: 10.4049/jimmunol.176.8.5006. [DOI] [PubMed] [Google Scholar]
- 12.Shao H, Peng Y, Liao T, et al. A shared epitope of the interphoto-receptor retinoid-binding protein (IRBP) recognized by the CD4+ and CD8+ autoreactive T cells. J Immunol. 2005;175:1851–1857. doi: 10.4049/jimmunol.175.3.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shao H, Sun SL, Kaplan HJ, Sun D. Characterization of rat CD8+ uveitogenic T cells specific for interphotoreceptor retinal-binding protein 1177-1191. J Immunol. 2004;173:2849–2854. doi: 10.4049/jimmunol.173.4.2849. [DOI] [PubMed] [Google Scholar]
- 14.Sun D. Staphylococcal enterotoxin enhances the activation of rat encephalitogenic T cells by myelin basic protein. J Neuroimmu-nol. 1993;46:5–10. doi: 10.1016/0165-5728(93)90227-p. [DOI] [PubMed] [Google Scholar]
- 15.Sun D, Le J, Yang S, et al. Major role of antigen-presenting cells in the response of rat encephalitogenic T cells to myelin basic proteins. J Immunol. 1993;151:111–118. [PubMed] [Google Scholar]
- 16.Xystrakis E, Dejean AS, Bernard I, et al. Identification of a novel natural regulatory CD8 T-cell subset and analysis of its mechanism of regulation. Blood. 2004;104:3294–3301. doi: 10.1182/blood-2004-03-1214. [DOI] [PubMed] [Google Scholar]
- 17.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 18.Quere P, Thorbecke GJ. Multiple suppressive effects of transforming growth factor β1 on the immune response in chickens. Cell Immunol. 1990;129:468–477. doi: 10.1016/0008-8749(90)90221-c. [DOI] [PubMed] [Google Scholar]
- 19.Takeuchi M, Alard P. TGF-β promotes immune deviation by altering accessory signals of antigen-presenting cells. J Immunol. 1998;160:1589–1597. [PubMed] [Google Scholar]
- 20.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 21.Shao H, Lei S, Sun S, et al. CpG-ODN1826 converts the weak uveitogenic rat IRBP1181–91 peptide into a strong uveitogen. J Immunol. 2003;171:4780–4785. doi: 10.4049/jimmunol.171.9.4780. [DOI] [PubMed] [Google Scholar]
- 22.Shao H, Song L, Sun SL, et al. Conversion of monophasic to recurrent autoimmune disease by autoreactive T cell subsets. J Im-munol. 2003;171:5624–5630. doi: 10.4049/jimmunol.171.10.5624. [DOI] [PubMed] [Google Scholar]
- 23.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med. 2003;197:1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt CS, Mescher MF. Peptide antigen priming of naive, but not memory, CD8 T cells requires a third signal that can be provided by IL-12. J Immunol. 2002;168:5521–5529. doi: 10.4049/jimmunol.168.11.5521. [DOI] [PubMed] [Google Scholar]
- 25.Schubert LA, Jeffery E, Zhang Y, et al. Scurfin (FOXP3) acts as a repressor of transcription and regulates T cell activation. J Biol Chem. 2001;276:37672–37679. doi: 10.1074/jbc.M104521200. [DOI] [PubMed] [Google Scholar]
- 26.Ramsdell F. Foxp3 and natural regulatory T cells: key to a cell lineage? Immunity. 2003;19:165–168. doi: 10.1016/s1074-7613(03)00207-3. [DOI] [PubMed] [Google Scholar]
- 27.Cobbold SP, Castejon R, Adams E, et al. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J Immunol. 2004;172:6003–6010. doi: 10.4049/jimmunol.172.10.6003. [DOI] [PubMed] [Google Scholar]
- 28.Belghith M, Bluestone JA, Barriot S, et al. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003;9:1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 29.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25+ naive T cells to CD4+CD25− regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y, Kuchroo VK, Inobe J, et al. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encepha-lomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 31.Seddon B, Mason D. Regulatory T cells in the control of autoimmunity: the essential role of transforming growth factor β and interleukin 4 in the prevention of autoimmune thyroiditis in rats by peripheral CD4+CD45RC− cells and CD4+CD8− thymo-cytes. J Exp Med. 1999;189:279–288. doi: 10.1084/jem.189.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu S, Zhang N, Yopp AC, et al. TGF-β induces Foxp3+ T-regulatory cells from CD4+ CD25− precursors. Am J Transplant. 2004;4:1614–1627. doi: 10.1111/j.1600-6143.2004.00566.x. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immu-nol. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 35.Yamazaki S, Iyoda T, Tarbell K, et al. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing den-dritic cells. J Exp Med. 2003;198:235–247. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nanda NK, Thomson E, Mason I. Murine suppressor T cell clones specific for minor histocompatibility antigens express CD4, CD8 and αβ T cell receptor molecules. Int Immunol. 1990;2:1063–1071. doi: 10.1093/intimm/2.11.1063. [DOI] [PubMed] [Google Scholar]
- 37.Bloom BR, Modlin RL, Salgame P. Stigma variations: observations on suppressor T cells and leprosy. Ann Rev Immunol. 1992;10:453–488. doi: 10.1146/annurev.iy.10.040192.002321. [DOI] [PubMed] [Google Scholar]
- 38.Sun D, Whitaker JN, Wilson DB. Regulatory T cells in experimental allergic encephalomyelitis. II. CD8+ T cells functionally antagonistic to CD4+ encephalitogenic MBP-specific T cells show persistent expression of FasL. J Neurosci Res. 1999;58:357–366. [PubMed] [Google Scholar]
- 39.Miller A, Lider O, Roberts AB, et al. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor β after antigen-specific triggering. Proc Natl Acad Sci USA. 1992;89:421–425. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang H, Braunstein NS, Yu B, et al. CD8+ T cells control the TH phenotype of MBP-reactive CD4+ T cells in EAE mice. Proc Natl Acad Sci USA. 2001;98:6301–6306. doi: 10.1073/pnas.101123098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graser RT, DiLorenzo TP, Wang FM, et al. Identification of a CD8 T cell that can independently mediate autoimmune diabetes development in the complete absence of CD4 T cell helper functions. J Immunol. 2000;164:3913–3918. doi: 10.4049/jimmunol.164.7.3913. [DOI] [PubMed] [Google Scholar]
- 42.Tsuchida T, Parker KC, Turner RV, et al. Autoreactive CD8+ T-cell responses to human myelin protein-derived peptides. Proc Natl Acad Sci USA. 1994;91:10859–10863. doi: 10.1073/pnas.91.23.10859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vizler C, Bercovici N, Cornet A, et al. Role of autoreactive CD8+ T cells in organ-specific autoimmune diseases: insight from trans-genic mouse models. Immunol Rev. 1999;169:81–92. doi: 10.1111/j.1600-065x.1999.tb01308.x. [DOI] [PubMed] [Google Scholar]
- 44.Cosmi L, Liotta F, Lazzeri E, et al. Human CD8+CD25+ thymo-cytes share phenotypic and functional features with CD4+CD25+regulatory thymocytes. Blood. 2003;102:4107–4114. doi: 10.1182/blood-2003-04-1320. [DOI] [PubMed] [Google Scholar]