Abstract
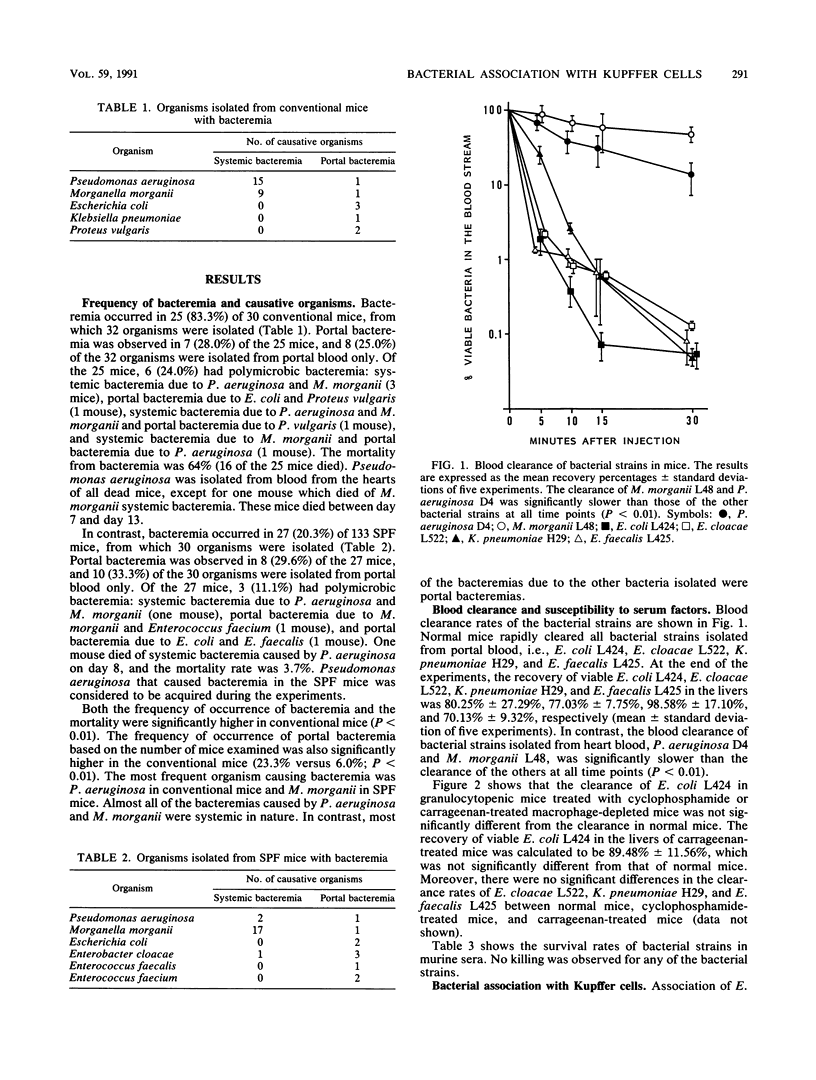
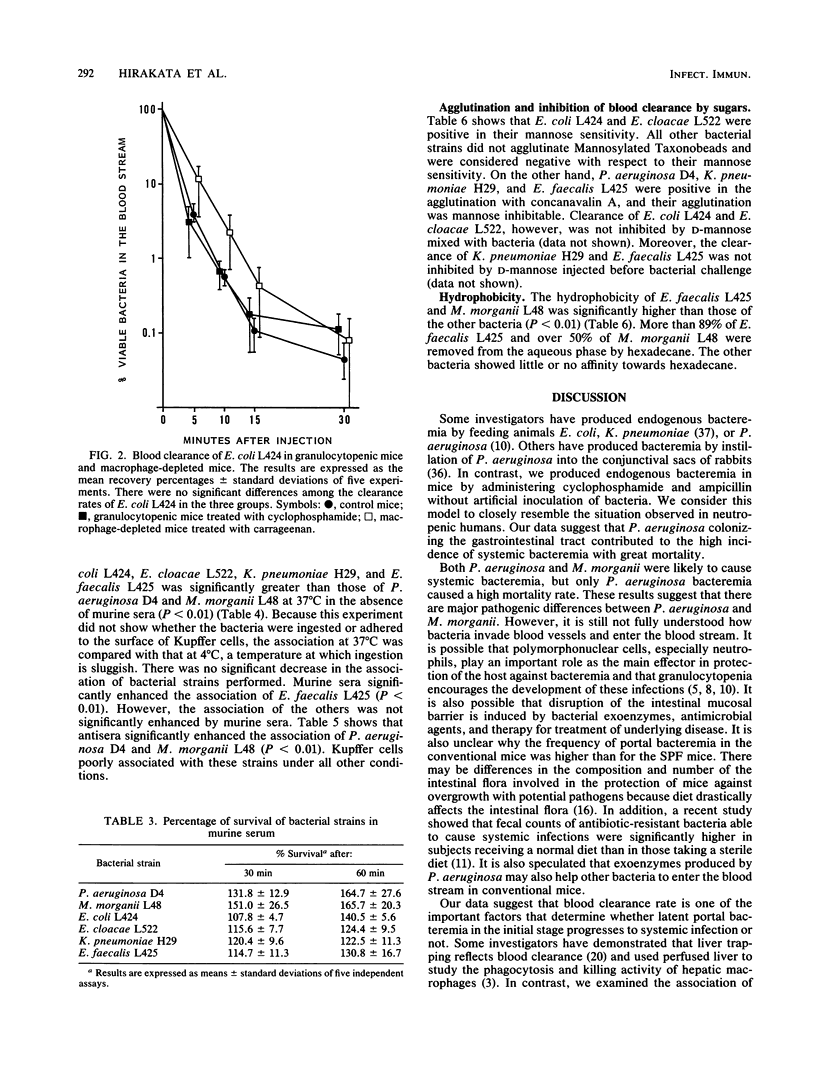
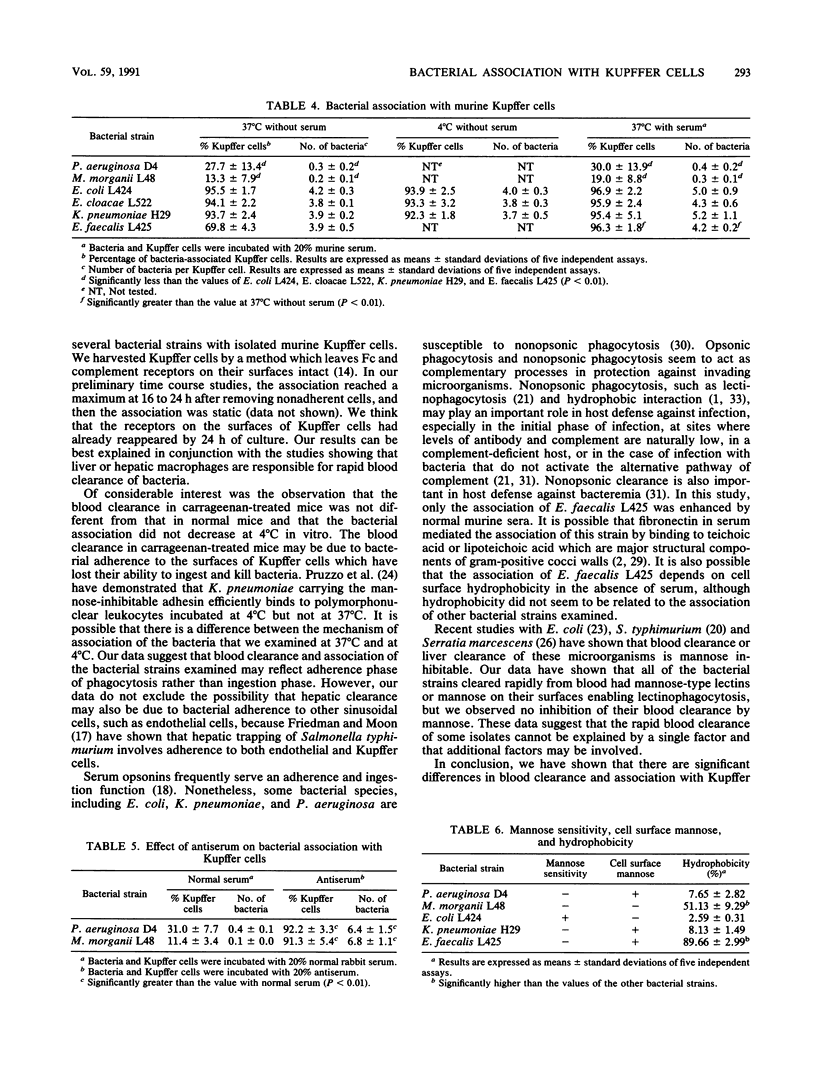
Bacteremia in immunocompromised hosts often arises from their endogenous intestinal flora. We produced experimental endogenous bacteremia by administering cyclophosphamide and ampicillin to conventional and specific-pathogen-free mice. The frequencies of bacteremia and mortality in the conventional mice were significantly higher than for the specific-pathogen-free mice. Pseudomonas aeruginosa was the major pathogen causing systemic bacteremia in conventional mice and was associated with a high mortality rate. Morganella morganii caused systemic bacteremia in both conventional and specific-pathogen-free mice. In contrast, Escherichia coli, enterococci, or other species most often caused portal bacteremia only. To determine the mechanism of occurrence of systemic bacteremia, we investigated bacterial blood clearance in mice and association with murine Kupffer cells, using several bacterial strains isolated from mice with bacteremia. Blood clearance rates and the abilities of isolated Kupffer cells to associate with bacteria were significantly greater for the organisms causing portal bacteremia than for those causing systemic bacteremia. There were no significant differences between the blood clearance rates in carrageenan-treated mice and that in normal mice. Moreover, association at 4 degrees C was not different from that at 37 degrees C. The results suggest that blood clearance of bacteria reflects bacterial adherence to Kupffer cells and that the resistance of bacteria to association with Kupffer cells plays an important role in the occurrence of overwhelming systemic bacteremia in this animal model.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Absolom D. R. The role of bacterial hydrophobicity in infection: bacterial adhesion and phagocytic ingestion. Can J Microbiol. 1988 Mar;34(3):287–298. doi: 10.1139/m88-054. [DOI] [PubMed] [Google Scholar]
- Andreana A., Perna P., Utili R., Dilillo M., Ruggiero G. Increased phagocytosis and killing of Escherichia coli treated with subinhibitory concentrations of cefamandole and gentamicin in isolated rat livers. Antimicrob Agents Chemother. 1984 Feb;25(2):182–186. doi: 10.1128/aac.25.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andremont A., Marang B., Tancrède C., Baume D., Hill C. Antibiotic treatment and intestinal colonization by Pseudomonas aeruginosa in cancer patients. Antimicrob Agents Chemother. 1989 Aug;33(8):1400–1402. doi: 10.1128/aac.33.8.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROOKE B. N., SLANEY G. Portal bacteraemia in ulcerative colitis. Lancet. 1958 Jun 7;1(7032):1206–1207. doi: 10.1016/s0140-6736(58)91910-x. [DOI] [PubMed] [Google Scholar]
- Baltch A. L., Griffin P. E. Pseudomonas aeruginosa bacteremia: a clinical study of 75 patients. Am J Med Sci. 1977 Sep-Oct;274(2):119–129. [PubMed] [Google Scholar]
- Bennion R. S., Wilson S. E., Williams R. A. Early portal anaerobic bacteremia in mesenteric ischemia. Arch Surg. 1984 Feb;119(2):151–155. doi: 10.1001/archsurg.1984.01390140017003. [DOI] [PubMed] [Google Scholar]
- Bodey G. P. Epidemiological studies of Pseudomonas species in patients with leukemia. Am J Med Sci. 1970 Aug;260(2):82–89. doi: 10.1097/00000441-197008000-00002. [DOI] [PubMed] [Google Scholar]
- Braude A. I., Douglas H., Jones J. Experimental production of lethal Escherichia coli bacteremia of pelvic origin. J Bacteriol. 1969 Jun;98(3):979–991. doi: 10.1128/jb.98.3.979-991.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins H. H., Cross A. S., Dobek A., Opal S. M., McClain J. B., Sadoff J. C. Oral ciprofloxacin and a monoclonal antibody to lipopolysaccharide protect leukopenic rats from lethal infection with Pseudomonas aeruginosa. J Infect Dis. 1989 Jun;159(6):1073–1082. doi: 10.1093/infdis/159.6.1073. [DOI] [PubMed] [Google Scholar]
- Corpet D. E. Antibiotic resistance from food. N Engl J Med. 1988 May 5;318(18):1206–1207. doi: 10.1056/nejm198805053181818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eade M. N., Brooke B. N. Portal bacteraemia in cases of ulcerative colitis submitted to colectomy. Lancet. 1969 May 17;1(7603):1008–1009. doi: 10.1016/s0140-6736(69)91802-9. [DOI] [PubMed] [Google Scholar]
- Filice G. A. Antimicrobial properties of Kupffer cells. Infect Immun. 1988 Jun;56(6):1430–1435. doi: 10.1128/iai.56.6.1430-1435.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flick M. R., Cluff L. E. Pseudomonas bacteremia. Review of 108 cases. Am J Med. 1976 Apr;60(4):501–508. doi: 10.1016/0002-9343(76)90716-6. [DOI] [PubMed] [Google Scholar]
- Freter R., Abrams G. D. Function of various intestinal bacteria in converting germfree mice to the normal state. Infect Immun. 1972 Aug;6(2):119–126. doi: 10.1128/iai.6.2.119-126.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. L., Moon R. J. Hepatic clearance of Salmonella typhimurium in silica-treated mice. Infect Immun. 1977 Jun;16(3):1005–1012. doi: 10.1128/iai.16.3.1005-1012.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. Phagocytosis of microorganisms. Rev Infect Dis. 1982 Jan-Feb;4(1):104–123. doi: 10.1093/clinids/4.1.104. [DOI] [PubMed] [Google Scholar]
- Leunk R. D., Moon R. J. Association of type 1 pili with the ability of livers to clear Salmonella typhimurium. Infect Immun. 1982 Jun;36(3):1168–1174. doi: 10.1128/iai.36.3.1168-1174.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. II. Prog Allergy. 1962;6:30–154. doi: 10.1159/000313795. [DOI] [PubMed] [Google Scholar]
- Ofek I., Sharon N. Lectinophagocytosis: a molecular mechanism of recognition between cell surface sugars and lectins in the phagocytosis of bacteria. Infect Immun. 1988 Mar;56(3):539–547. doi: 10.1128/iai.56.3.539-547.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry A., Ofek I. Inhibition of blood clearance and hepatic tissue binding of Escherichia coli by liver lectin-specific sugars and glycoproteins. Infect Immun. 1984 Jan;43(1):257–262. doi: 10.1128/iai.43.1.257-262.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce de Leon S., Wenzel R. P. Hospital-acquired bloodstream infections with Staphylococcus epidermidis. Review of 100 cases. Am J Med. 1984 Oct;77(4):639–644. doi: 10.1016/0002-9343(84)90354-1. [DOI] [PubMed] [Google Scholar]
- Pruzzo C., Guzmán C. A., Calegari L., Satta G. Impairment of phagocytosis by the Klebsiella pneumoniae mannose-inhibitable adhesin-T7 receptor. Infect Immun. 1989 Mar;57(3):975–982. doi: 10.1128/iai.57.3.975-982.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumelt S., Metzger Z., Kariv N., Rosenberg M. Clearance of Serratia marcescens from blood in mice: role of hydrophobic versus mannose-sensitive interactions. Infect Immun. 1988 May;56(5):1167–1170. doi: 10.1128/iai.56.5.1167-1170.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHATTEN W. E., DESPREZ J. D., HOLDEN W. D. A bacteriologic study of portal-vein blood in man. AMA Arch Surg. 1955 Sep;71(3):404–409. doi: 10.1001/archsurg.1955.01270150098011. [DOI] [PubMed] [Google Scholar]
- Schimpff S. C., Young V. M., Greene W. H., Vermeulen G. D., Moody M. R., Wiernik P. H. Origin of infection in acute nonlymphocytic leukemia. Significance of hospital acquisition of potential pathogens. Ann Intern Med. 1972 Nov;77(5):707–714. doi: 10.7326/0003-4819-77-5-707. [DOI] [PubMed] [Google Scholar]
- Speert D. P., Eftekhar F., Puterman M. L. Nonopsonic phagocytosis of strains of Pseudomonas aeruginosa from cystic fibrosis patients. Infect Immun. 1984 Mar;43(3):1006–1011. doi: 10.1128/iai.43.3.1006-1011.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speert D. P., Loh B. A., Cabral D. A., Salit I. E. Nonopsonic phagocytosis of nonmucoid Pseudomonas aeruginosa by human neutrophils and monocyte-derived macrophages is correlated with bacterial piliation and hydrophobicity. Infect Immun. 1986 Jul;53(1):207–212. doi: 10.1128/iai.53.1.207-212.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tancrède C. H., Andremont A. O. Bacterial translocation and gram-negative bacteremia in patients with hematological malignancies. J Infect Dis. 1985 Jul;152(1):99–103. doi: 10.1093/infdis/152.1.99. [DOI] [PubMed] [Google Scholar]
- Yang K. D., Bathras J. M., Shigeoka A. O., James J., Pincus S. H., Hill H. R. Mechanisms of bacterial opsonization by immune globulin intravenous: correlation of complement consumption with opsonic activity and protective efficacy. J Infect Dis. 1989 Apr;159(4):701–707. doi: 10.1093/infdis/159.4.701. [DOI] [PubMed] [Google Scholar]
- Young L. S., Stevens P., Kaijser B. Gram-negative pathogens in septicaemic infections. Scand J Infect Dis Suppl. 1982;31:78–94. [PubMed] [Google Scholar]
- Ziegler E. J., Douglas H. Pseudomonas aeruginosa vasculitis and bacteremia following conjunctivitis: a simple model of fatal pseudomonas infection in neutropenia. J Infect Dis. 1979 Mar;139(3):288–296. doi: 10.1093/infdis/139.3.288. [DOI] [PubMed] [Google Scholar]
- Ziegler E. J., Douglas H., Sherman J. E., Davis C. E., Braude A. I. Treatment of E. coli and klebsiella bacteremia in agranulocytic animals with antiserum to a UDP-gal epimerase-deficient mutant. J Immunol. 1973 Aug;111(2):433–438. [PubMed] [Google Scholar]
- van Oss C. J. Phagocytosis as a surface phenomenon. Annu Rev Microbiol. 1978;32:19–39. doi: 10.1146/annurev.mi.32.100178.000315. [DOI] [PubMed] [Google Scholar]


