Abstract
A central issue in the control of apoptosis is whether its essential mediators Bax and Bak must be restrained by Bcl-2-like prosurvival relatives to prevent their damaging mitochondria and unleashing apoptosis. The issue is particularly vexed for Bax, which is largely a cytosolic monomer in unstressed cells. To determine whether Bax regulation requires its binding by prosurvival relatives, we replaced a conserved aspartate in its BH3 interaction domain with arginine. Bax D68R functioned and behaved like wild-type Bax in localization and activation but had greatly impaired binding to the prosurvival family members. Nevertheless, Bcl-xL remained able to block apoptosis induced by Bax D68R. Whereas cells with sufficient Bcl-xL tolerated expression of Bax D68R, it provoked apoptosis when Bcl-xL was absent, downregulated, or inactivated. Moreover, Bax D68R rendered membrane bound by a C-terminal anchor mutation overwhelmed endogenous Bcl-xL and killed cells. These unexpected results suggest that engagement of Bax by its prosurvival relatives is a major barrier to its full activation. We propose that the Bcl-2-like proteins must capture the small proportion of Bax molecules with an exposed BH3 domain, probably on the mitochondrial membrane, to prevent Bax-imposed cell death, but that Bcl-xL also controls Bax by other mechanisms.
Keywords: BH3 domain, control of apoptosis, mitochondria, protein association, membrane association
Programmed cell death (apoptosis) is essential for development and tissue homeostasis, and its defective control underlies many disorders, including cancer. Interactions between 3 subgroups of the Bcl-2 protein family govern apoptosis (1, 2). Bcl-2 and close relatives Bcl-xL, Bcl-w, Mcl-1, and A1 promote cell survival, whereas the essential cell death mediators Bax and Bak provoke the mitochondrial damage that leads to cell death. Their shared structural similarities include 3 Bcl-2 homology (BH) regions. Members of the proapoptotic “BH3-only” subgroup, such as Bid, Bim, or Bad, share only the BH3 domain, an amphipathic α-helix essential for their interaction with prosurvival relatives and initiation of apoptosis.
Understanding Bax and Bak regulation has been deemed “the ‘holy grail' of apoptosis research” (2). The original rheostat model (3), in which Bcl-2 directly sequesters Bax, their balance determining cell fate, fell from favor when their association proved to be provoked largely by the detergents used to liberate the proteins from membranes (4, 5). Also, whereas Bcl-2 is an integral membrane protein, Bax in healthy cells is predominantly a cytosolic monomer (4, 5). In cytosolic Bax, the hydrophobic C-terminal helix is tucked within a surface groove, and the BH3 domain, which is required for its binding to prosurvival relatives (6–8), is not exposed (9).
Thus, whether the prosurvival family members must engage Bax and Bak to maintain cell survival remains uncertain (1, 10, 11). It has been suggested that they block apoptosis exclusively by sequestering the BH3-only proteins (12). If certain BH3-only proteins can activate Bax directly, as some findings (12–14) but not others (15–17) suggest, the need for prosurvival inhibition of Bax might be obviated. The issue remains challenging because triggering apoptosis requires only a small proportion of the Bax molecules (18, 19).
If Bax is kept inert by direct interaction with its prosurvival relatives, then a mutation that freed it from their control might unleash Bax's prodeath activity. We found that a mutation engineered into the Bax BH3 domain greatly impaired its binding to prosurvival family members, but the mutant Bax remained subject to control by Bcl-xL. Importantly, the mutant, but not WT Bax, killed cells when Bcl-xL availability was reduced or when more of the mutant was targeted to membranes. These findings implicate direct interaction of prosurvival Bcl-2 homologues with Bax, via its BH3 domain, as central to Bax regulation. Surprisingly, however, they also suggest that Bcl-xL may restrain Bax independently of its ability to bind the Bax BH3 domain. Nevertheless, when all prosurvival constraint is absent or overwhelmed, the results suggest that no additional stimulus need be imposed to unshackle the proapoptotic activity of Bax.
Results
A Bax Mutant Impaired for Binding Prosurvival Bcl-2 Proteins.
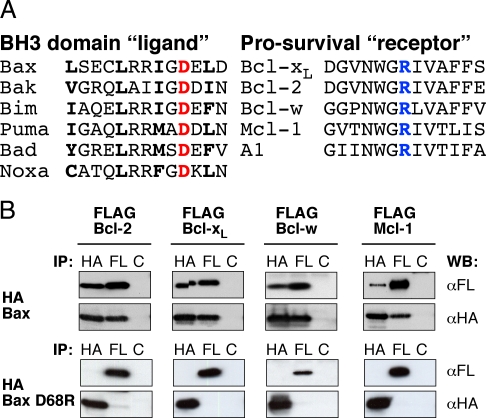
The BH3 domain of Bax is essential for its interaction with prosurvival relatives (6, 8) and binds into their surface groove (7). Mutagenesis and structural studies of prosurvival proteins complexed with BH3 peptides (e.g., refs. 7, 20) indicate that the critical BH3 residues (Fig. 1A) include not only 4 conserved hydrophobic residues (boldface) but also an invariant aspartate (red), which consistently binds an invariant arginine in the receptor groove (blue), as depicted for the Bcl-xL:BimBH3 complex [supporting information (SI) Fig. S1A] (20). We focused on this electrostatic interaction because replacing the solvent-exposed Bax D68 (Fig. S1B) (9) is unlikely to perturb its overall conformation. To convert the electrostatic attraction in the heterodimer to charge repulsion, we replaced the acidic aspartate with the basic arginine in human Bax, creating Bax D68R.
Fig. 1.
Designing a deregulated Bax mutant. (A) Sequence alignments illustrate key conserved residues. The BH3 domains from selected proapoptotic Bcl-2 family members (Left) show the invariant aspartate (red D) and the conserved hydrophobic residues in bold, whereas the sequences from the hydrophobic receptor grooves of the mammalian prosurvival relatives (Right) show the invariant arginine (R) in blue. (B) The D68R mutation compromises Bax binding to prosurvival proteins. Lysates prepared from 293T cells overexpressing HA-tagged human Bax or the D68R mutant, and FLAG-tagged prosurvival Bcl-2 proteins were immunoprecipitated with mouse monoclonal antibodies recognizing the HA, FLAG (FL), or an irrelevant control (C) tag. The immunoprecipitates were subjected to SDS-PAGE, transferred onto membranes, and the blots probed with rat anti-HA or -FLAG antibodies.
To evaluate whether association of Bax D68R with its prosurvival relatives was impaired, we tested whether the overexpressed tagged proteins coimmunoprecipitated from lysates prepared using the nonionic detergent Triton X-100, which promotes heterodimerization. As expected, WT Bax interacted with all 4 prosurvival proteins tested (Fig. 1B), whereas any association of Bax D68R was below the level of detection (more sensitive association studies are discussed below). The mouse prosurvival proteins also failed to bind Bax D68R detectably (Fig. S1C).
Bax D68R Is Localized Normally and Activated in Response to Cytotoxic Drugs.
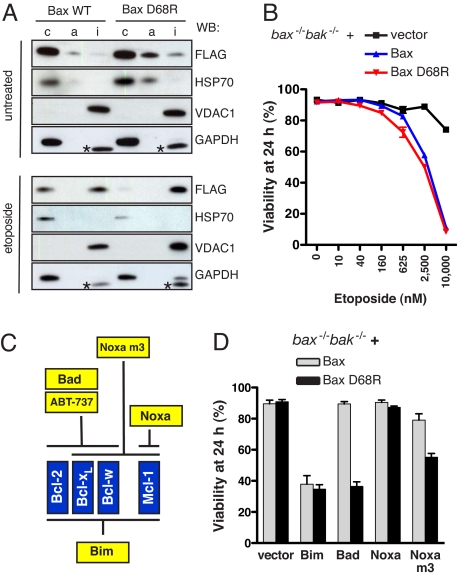
We next compared the localization and function of Bax D68R with WT Bax. To preclude any complications due to endogenous Bax or Bak, we analyzed bax−/−bak−/− mouse embryo fibroblasts (MEF) reconstituted with FLAG-tagged Bax or Bax D68R. These proteins were stably expressed at comparable levels (Figs. 2A and S2A), only approximately 3-fold higher than endogenous Bax in MEF (data not shown). Like WT Bax, Bax D68R localized predominantly in the cytosol of unstressed cells (Figs. 2A and S2B) but shifted into the membrane-integrated (carbonate-resistant) fraction when the cells were stressed (Fig. 2A). Importantly, Bax D68R retained full proapoptotic activity. The cells bearing Bax D68R and WT Bax were comparably sensitive to etoposide (Fig. 2B), and monitoring Bax activation with a conformation-specific antibody showed that both proteins were activated at a similar rate (Fig. S2C).
Fig. 2.
Bax D68R is functional but deregulated. (A) Like Bax, the D68R mutant is predominantly cytosolic but becomes membrane integrated after an apoptotic stimulus. Untreated or etoposide-treated (10 μM for 24 h) bax−/−bak−/− MEF stably expressing FLAG-tagged Bax or Bax D68R were separated into cytosolic (c), membrane-associated (a), and the carbonate-resistant membrane-integrated (i) fractions (see Materials and Methods). HSP70, cytosolic marker; VDAC1, integral mitochondrial outer membrane protein; GAPDH, loading control. *VDAC1 bands carried over from a previous blot. (B) Bax D68R retains full apoptotic function. The viability of reconstituted bax−/−bak−/− MEF (described in A) after etoposide treatment (0–10 μM) for 24 h was assessed by propidium iodide exclusion using flow cytometry. (C) Selectivity of BH3-only ligands for their prosurvival targets (see text). (D) Neutralization of Bcl-xL, Bcl-w, or both activates Bax D68R but not WT Bax. Viability of reconstituted bax−/−bak−/− MEF was determined 24 h after infection with retroviruses expressing the indicated BH3-only proteins. Data in B and D represent means ± 1 SEM of 3 or more independent experiments.
Prosurvival Control of Bax D68R Is Perturbed.
Because Bax D68R was not constitutively active (Figs. 2B and S2C), we wondered whether Bcl-2, Bcl-xL, Bcl-w, or Mcl-1, each of which directly restrains WT Bax (15), might still regulate Bax D68R. To explore this, we used a panel of BH3-only ligands that bind to overlapping subsets of the prosurvival proteins (14, 16). As summarized in Fig. 2C, Bim targets all 4 of the prosurvival protein expressed in MEF; A1 is not expressed (17). The more selective Bad, or its mimic ABT-737 (21, 22), targets Bcl-2, Bcl-xL, and Bcl-w but not Mcl-1; conversely, Noxa neutralizes only Mcl-1, whereas the Noxa BH3 mutant m3 also targets Bcl-xL and Bcl-w but not Bcl-2 (16).
We assessed the viability of bax−/−bak−/− MEF expressing Bax or Bax D68R after infection with retroviruses encoding these BH3-only ligands (Fig. 2D), or treatment with ABT-737 (Fig. S3A). As expected, the cells expressing Bax were killed by Bim but not by the more selective Bad, Noxa, or Noxa m3 (15, 16), nor by ABT-737. Surprisingly, however, cells expressing Bax D68R were also sensitive to Bad, ABT-737, and Noxa m3 (Figs. 2D and S3A). Given that neither Bad nor ABT-737 targets Mcl-1, this result implies that endogenous Mcl-1 is not sufficient to restrain Bax D68R. Similarly, the sensitivity to Noxa m3 implies that Bcl-2 cannot prevent Bax D68R activation. We therefore inferred that either Bcl-xL or Bcl-w (or both) might constrain Bax D68R.
Bcl-xL Deficiency Renders Bax D68R Fully Active.
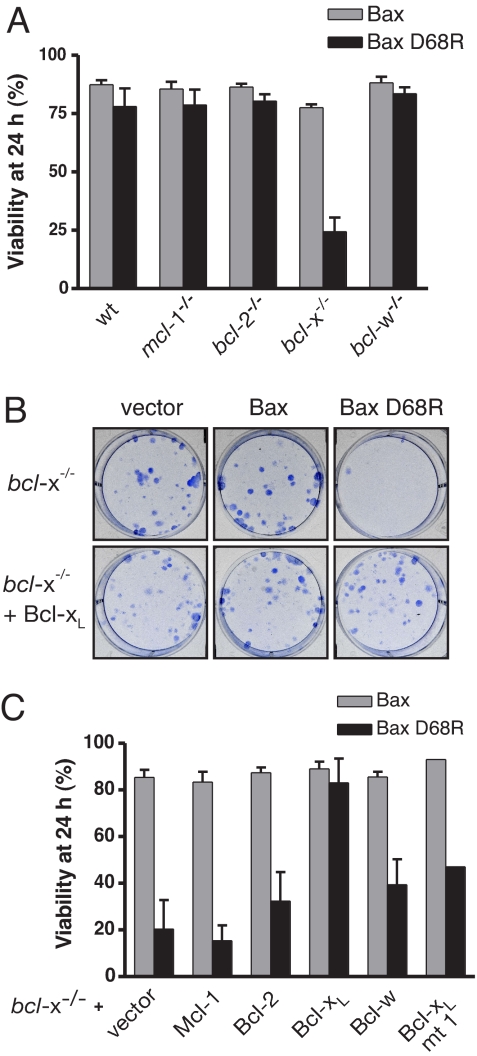
If only Bcl-xL or Bcl-w restrained Bax D68R, we reasoned that cells lacking either might become vulnerable to Bax D68R. To test this hypothesis, we infected MEF lacking specific prosurvival proteins with retroviruses expressing Bax or Bax D68R. Whereas each knockout line tolerated expression of WT Bax, Bax D68R induced apoptosis in the bcl-x−/− MEF but not the others (Fig. 3A). Furthermore, expression of Bax D68R abolished colony formation of bcl-x−/− MEF (Fig. 3B, Top) but coexpressed Bcl-xL rescued the colony formation (Fig. 3B, Lower). These results implicated Bcl-xL as the sole functional block to Bax D68R activation.
Fig. 3.
Bax D68R is constitutively active in the absence of Bcl-xL. (A) Bax D68R kills MEF lacking Bcl-xL. The viability of MEF lacking the indicated prosurvival proteins was determined by propidium iodide exclusion 24 h after infection with retroviruses expressing either WT or D68R Bax. (B) Bax D68R prevents colony formation by Bcl-xL-deficient MEF. The bcl-x−/− MEF or a subclone stably expressing Bcl-xL were infected with retroviruses expressing Bax, Bax D68R, or an empty control vector and colony formation assessed 6 d later. (C) Only Bcl-xL counters apoptosis induced by Bax D68R in MEF lacking Bcl-xL. The viability of bcl-x−/− MEF stably expressing the indicated prosurvival proteins was determined 24 h after reinfection with a Bax or Bax D68R retrovirus. Data in A and C (except for Bcl-xL mt1) represent means ± 1 SEM of 3 or more independent experiments.
To address the possibility that endogenous Bcl-xL prevented Bax D68R activation simply because its level was higher than that of other family members, we overexpressed each prosurvival protein in bcl-x−/− MEF before introducing Bax or Bax D68R. Bcl-2 and Bcl-xL were highly expressed and Bcl-w and Mcl-1 modestly (Fig. S3B). Even though Bcl-2, Bcl-xL, and Bcl-w protected against killing by etoposide (Fig. S3C), only Bcl-xL rescued the cells from killing by Bax D68R. Bcl-xL mt1, a mutant incapable of binding Bax (23), was also incapable of countering Bax D68R (Fig. 3C).
To confirm that killing by Bax D68R in the absence of Bcl-xL does not require endogenous Bax or Bak, we first showed that an shRNA to Bcl-xL efficiently reduced its level in bax−/−bak−/− MEF (Fig. S3D). That shRNA abolished colony formation by the bax−/−bak−/− MEF reconstituted with Bax D68R but not Bax (Fig. S3E), consistent with the results observed in cells lacking Bcl-xL (Fig. 3).
Thus, whereas each prosurvival protein regulates WT Bax (15), these experiments (Figs. 2D and 3, and Fig. S3 A and E) strongly implicate Bcl-xL as the sole barrier in MEF to Bax D68R activation. When that barrier is lowered, Bax D68R becomes fully active.
Other Cell Types Are also Vulnerable Specifically to Bax D68R.
To investigate whether Bcl-xL was the sole restraint on Bax D68R in other cell types, we first downregulated Bcl-xL with shRNA in FDC-P1 myeloid cells (Fig. S3F). Even the partial reduction of Bcl-xL achieved ablated colony formation specifically in the cells expressing Bax D68R but not Bax (Fig. S3G).
We also tested the impact of Bax D68R in B and T cell blasts. Because survival of mature B and T lymphocytes is unaffected by Bcl-xL deficiency (24), we reasoned that these cells might have insufficient Bcl-xL to restrain Bax D68R. Indeed, both B and T cells tolerated Bax but not Bax D68R (Fig. S3H).
Bax D68R Binds Weakly to the Prosurvival Proteins.
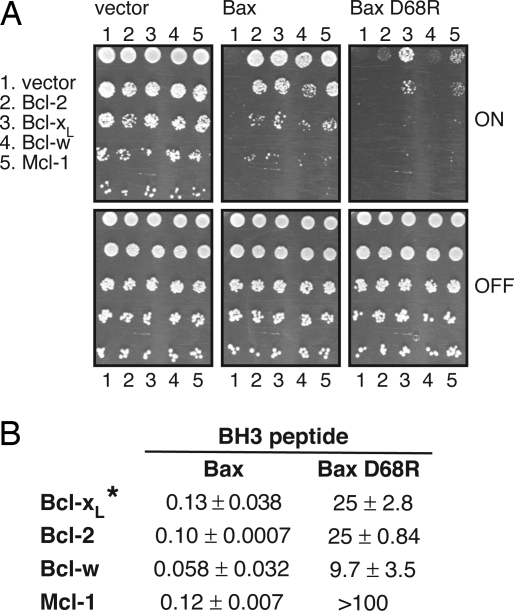
Because Bcl-xL constrained Bax D68R functionally despite their failure to coimmunoprecipitate (Fig. 1B and Fig. S1C), we hypothesized that they might interact with an affinity below the threshold for coimmunoprecipitation. To test interaction of the full-length proteins in the absence of other family members, we used a functional assay in yeast (25), based on the well-established ability of Bax to block yeast growth and of coexpressed Bcl-2 to rescue it (26). Unlike other interaction assays, such as the yeast two-hybrid system, this approach tests whether the native proteins can associate. As expected, yeast expressing Bax alone formed no colonies (Fig. 4A, Center, lane 1), but coexpression of Bcl-2, Bcl-xL, Bcl-w, or Mcl-1 efficiently rescued colony formation (Fig. 4A, Center, lanes 2–5). Although Bax D68R also prevented growth (Fig. 4A, Right, lane 1), colony formation was rescued substantially by Bcl-xL (Fig. 4A, Right, lane 3) and weakly by Mcl-1 (Fig. 4A, Right, lane 5) but not by Bcl-2 or Bcl-w (Fig. 4A, Right, lanes 2 and 4). Because yeast lack the apoptotic machinery of mammalian cells, the ability of Bcl-xL to antagonize Bax D68R in yeast as well as mammalian cells would be consistent with the notion that Bcl-xL protects against it by direct association, even though they do not coimmunoprecipitate (Fig. 1B).
Fig. 4.
Bcl-xL antagonizes Bax D68R in yeast but binds only weakly to a Bax D68R BH3 peptide. (A) Bcl-xL counters growth suppression of yeast by Bax D68R. Yeast cotransformed with constructs encoding the indicated prosurvival proteins and Bax or Bax D68R (all full length), each under the control of an inducible (GAL) promoter were spotted onto repressing glucose (OFF) or inducing galactose (ON) plates as 5-fold serial dilutions. Images are representative of 2 independent experiments. (B) Relative affinity (IC50 in μM) of the C-terminally truncated prosurvival proteins (see Materials and Methods) for WT or D68R Bax BH3 peptides (34-mers), determined by solution competition assays. Data shown represent means ± 1 SD of 2 independent experiments. *Data from experiments using human Bcl-xL Δ45–84 ΔC24; comparable results were obtained with mouse Bcl-xL ΔC24.
To assess the affinities of prosurvival family members for the Bax D68R BH3 domain, we compared their binding in solution competition assays to long (34-mer) peptides spanning the BH3 of WT or D68R Bax. The mutant peptide did not bind detectably to Mcl-1 (IC50 >100 μM); it did bind weakly to Bcl-xL, Bcl-2, and Bcl-w but at affinities (9.7–25 μM) more than 2 orders of magnitude weaker than to the WT Bax BH3 peptide (Fig. 4B). Because Bcl-xL did not bind to the Bax D68R BH3 peptide more tightly than did Bcl-2 or Bcl-w, we infer that its ability to protect against full-length Bax D68R in mammalian cells (Fig. 3) and yeast (Fig. 4A) is unlikely to rely on binding via the Bax BH3 domain (see Discussion).
Enforced Membrane Localization Makes Bax D68R Even More Potent.
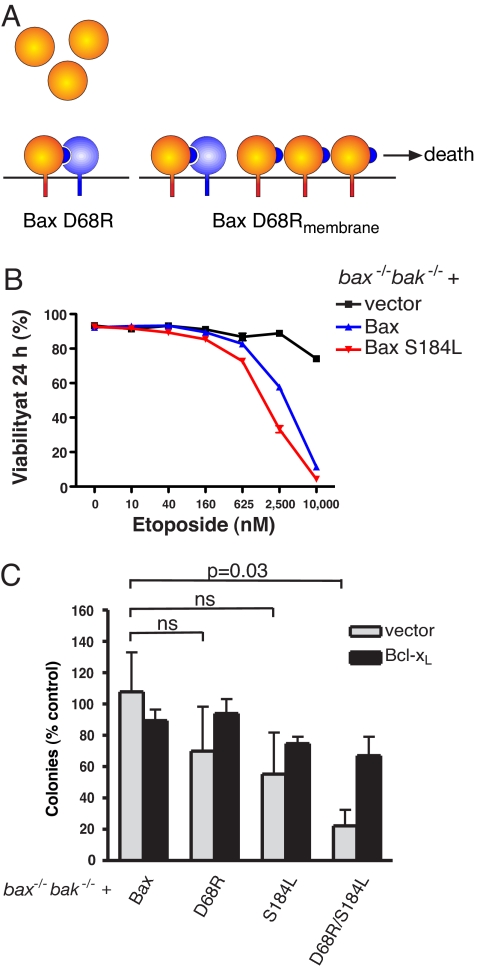
We hypothesized that, in unstressed cells, endogenous Bcl-xL can constrain Bax D68R, despite their weak affinity, because only a very small proportion of the Bax molecules, perhaps that already present on membranes (Fig. 2A and Fig. S2B), must be sequestered to maintain viability (Fig. 5A; see Discussion). If so, shifting Bax D68R to the membranes might allow it to overwhelm endogenous Bcl-xL (Fig. 5A). Replacing serine 184 in the C-terminal anchor (Fig. S4A) with a hydrophobic residue targets Bax to mitochondria but leaves it inactive in the absence of a cytotoxic stimulus (27). Such mutations both render the anchor more lipophilic and prevent the phosphorylation of S184 that, in some cells, keeps Bax cytosolic (28, 29). As expected, Bax S184L localized predominantly to the carbonate-resistant fraction, indicating membrane integration (Fig. S4B), and responded like WT Bax to a death stimulus (Fig. 5B and Fig. S4C).
Fig. 5.
Enhanced killing when Bax D68R is forced onto membranes. (A) MEF may possess sufficient endogenous Bcl-xL (blue) to counter the small fraction of membrane-bound Bax D68R (orange) (Fig. 2A). This capacity might be overwhelmed if the predominantly cytosolic Bax D68R is driven onto membranes. (B) Bax S184L is fully functional. The viability of reconstituted bax−/−bak−/− MEF (described in Fig. 2A) after etoposide treatment (0–10 μM) for 24 h was assessed by propidium iodide exclusion using flow cytometry. (C) Combining the deregulated D68R mutation with S184L enhances Bax-mediated apoptosis. Colony formation was assessed for parental bax−/−bak−/− MEF, or these MEF stably overexpressing Bcl-xL, after infection with retroviruses expressing WT Bax or mutant (D68R, S184L, or D68R/S184L) forms of Bax. Data represent means ± 1 SEM of 3 or more independent experiments. Results were compared using two-tailed unpaired Student's t tests. ns, P > 0.05.
Notably, even though otherwise unstressed bax−/−bak−/− MEF tolerated the Bax D68R or S184L single mutants, Bax D68R/S184L induced apoptosis in the absence of any other stimulus (Fig. S4D) and substantially reduced clonal growth (Fig. 5C). Because neither the Bax D68R nor S184L mutant on their own kill bax−/−bak−/− cells, we suggest that the double mutant increased the proportion of Bax molecules that must be sequestered beyond the capacity of endogenous Bcl-xL (Fig. 5A). Accordingly, Bcl-xL overexpression restored the balance and prevented the death induced by Bax D68R/S184L (Fig. 5C).
Discussion
Prosurvival Constraint Is Essential to Prevent Bax-Mediated Apoptosis.
A major unresolved issue in initiation of apoptosis is whether the prosurvival Bcl-2 proteins must directly bind Bax to prevent it from permeabilizing the mitochondria, or whether Bax freed from their control would remain inert in the absence of an imposed activation signal. By replacing the invariant aspartate in the Bax BH3 domain with the basic arginine, we greatly impaired its association with its prosurvival relatives (Figs. 1B and 4B). Bax D68R closely resembled WT Bax in localization, activation, and ability to kill cells (Fig. 2 A and B and Fig. S2). However, Bcl-xL seems to be the only effective brake on Bax D68R, because its absence, downregulation by RNAi, or selective inactivation rendered several types of mouse cells vulnerable to killing by Bax D68R but not WT Bax (Figs. 2D and 3 and Fig. S3). This mutation thus seems to confine Bax regulation to a single antagonist, and when that barrier is compromised, its prodeath activity is unleashed. Collectively, these findings argue that the prosurvival proteins must keep Bax in check and that this function most likely relies largely on their ability to engage Bax via its BH3 domain.
Intriguingly, our data indicate that Bcl-xL can also restrain Bax in a manner not shared with the other prosurvival family members. Overexpression of Bcl-xL but not the others protected bcl-x−/− cells from killing by Bax D68R (Fig. 3). That result cannot readily be accounted for by residual ability of Bcl-xL to engage the Bax D68R BH3 domain, because Bcl-xL did not bind a Bax D68R BH3 peptide more tightly than Bcl-2 or Bcl-w (Fig. 4B). Nevertheless, the protective function of Bcl-xL may still rely on weak binding to another region of full-length Bax. Consistent with direct binding, Bcl-xL could block the growth inhibition of yeast by Bax D68R (Fig. 4A) and, unlike WT Bcl-xL, a Bcl-xL mutant incapable of binding Bax failed to protect bcl-x−/− cells against Bax D68R (Fig. 3C). Notably, previous structure-based mutagenesis of Bcl-xL also suggested that it could suppress Bax-mediated cell death in a manner not requiring binding via the Bcl-xL groove (30). The step of Bax activation regulated specifically by Bcl-xL is as yet unknown and might involve its conformational change, translocation to the membrane, membrane integration, or oligomerization. Pertinently, very recent studies using recombinant Bcl-xL and Bax in a cell-free system suggest that Bcl-xL can regulate several of these steps (31).
Two recent studies characterizing other Bax BH3 mutants (12, 32) reached divergent conclusions about its regulation. Kim et al. (12) reported that Bax bearing the double mutation L70A/D71A, or Bak with the analogous I82A/N83A mutations, could still induce death in bax−/−bak−/− cells treated with cytotoxic drugs, but neither coimmunoprecipitated with Bcl-xL, Mcl-1, or Bcl-2 (Bcl-w was not examined). They therefore concluded that Bax and Bak remain inactive when the prosurvival proteins cannot bind them (i.e., that activation of Bax and Bak relies on their direct engagement by certain BH3-only proteins). In contrast, Zhou et al. (32) reported that both the L70A and the D71A mutation perturb the Bax structure, drive it to the membrane, and render it fully active, as we confirmed for L70A (Fig. S4D). In any case, because Bax D68R failed to coimmunoprecipitate with any prosurvival protein (Fig. 1B) but was still inhibited by Bcl-xL, Bax L70A/D71A (and the analogous Bak mutant) might be constrained by one or more prosurvival proteins, at an affinity not readily detectable by coimmunoprecipitation.
The proposed ability of certain BH3-only proteins, namely tBid, Bim, and perhaps Puma, to directly engage and activate Bax (see the Introduction) would not readily account for our results. Both Bad and its mimic ABT-737, neither of which bind Bax, selectively killed cells bearing Bax D68R (Fig. 2D and Fig. S3A). Moreover, Bax D68R (but not WT Bax) killed cells lacking Bcl-xL without any additional cytotoxic stimulus for activating BH3-only proteins (Fig. 3 and Fig. S3E). Although inactivation or downregulation of Bcl-xL arguably might free an associated BH3-only “activator” of Bax, the death induced specifically by Bax D68R was not blocked by overexpression of other prosurvival proteins (Fig. 3C), which can bind all of the proposed activator BH3-only proteins (16). Nevertheless, these results do not exclude the possibility that, in other circumstances, BH3-only proteins or nonfamily proteins can directly activate Bax.
Association of Bax with the mitochondrial membrane via its C-terminal anchor is necessary but not sufficient for its proapoptotic activity (27). As expected if the mitochondrial outer membrane is the critical site for control of Bax by its prosurvival relatives (Fig. 5A), targeting Bax D68R to that membrane tipped the balance toward cell death: expression of Bax D68R/S184L but not Bax D68R killed bax−/−bak−/− cells (Fig. 5C and Fig. S4D). We conclude that the membrane localization allows more of the double mutant to form an active conformer (see below) than can be neutralized by endogenous Bcl-xL. Consistent with this idea, increasing the Bcl-xL level rescued long-term cell survival (Fig. 5C).
Although the affinity of Bcl-xL for the Bax D68R BH3 peptide was very weak, the true affinities for the full-length proteins on the mitochondrial membrane probably considerably exceed those measured in vitro with truncated recombinant proteins and peptides (Fig. 4B), which may not fully replicate interactions between the full-length proteins in vivo (33). Localization to the 2D membrane surface should greatly increase their effective local concentrations, and integration of their C-termini into the membrane may both aid docking of the Bax BH3 domain into the receptor groove and promote association of other parts of the proteins.
Model for Bax Regulation.
Our most intriguing result is that release of Bax from all prosurvival control inside cells seems sufficient to unshackle its proapoptotic activity, seemingly without the need for any additional induced activation signal. We infer therefore that a small proportion of Bax must exist in a primed state or readily assume that state. Because the BH3 domains of Bax (6, 8, 34) and Bak (35, 36) are required for homo-oligomerization as well as association with prosurvival relatives, we propose that primed Bax is a minor conformer with its BH3 domain accessible to the prosurvival proteins. Because cytosolic Bax is monomeric and has its BH3 domain buried (9), apparently hydrogen-bonded to the C-terminal helix (32), the primed Bax most likely forms on the membrane. Pertinently, only a small proportion of Bax is required to trigger apoptosis (18, 19).
We suggest that a crucial role of the prosurvival family members is to capture any Bax (or Bak) with its BH3 domain exposed to prevent those molecules from nucleating Bax (or Bak) oligomerization. How primed Bax forms is not clear. It might form in part spontaneously, given that mild heat (37) or detergents (38) can induce an active conformation in Bax, but posttranslational modifications may well also contribute. For example, Bax phosphorylation and dephosphorylation, particularly on S184 (28, 39), may well regulate its translocation to membranes. New reconstitution systems (e.g., ref. 31), should help to clarify the mechanisms.
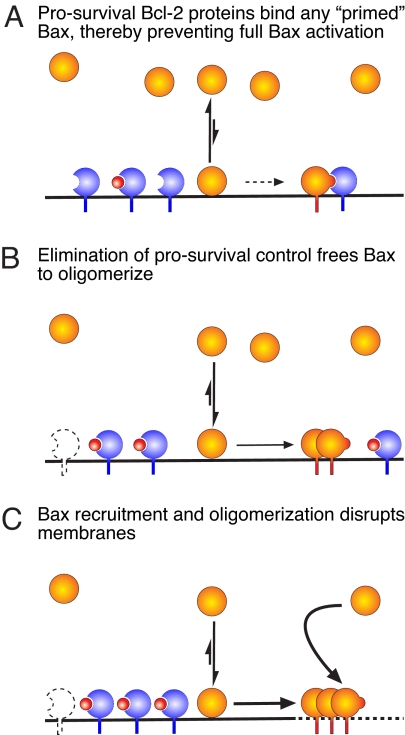
Our working model for Bax regulation (Fig. 6) thus envisions that, on the mitochondrial outer membrane, there is a minor Bax conformer with its BH3 exposed, formed by a mechanism yet to be defined. Any prosurvival relative with an unoccupied groove captures this primed form (Fig. 6A). However, if cellular insults have blocked synthesis of the prosurvival proteins, induced their degradation, or activated BH3-only proteins that inactivate them (Fig. 6B), the primed Bax can now self-associate, as shown recently for Bak (36). Autoactivation by recruitment of more Bax from the cytosol (31, 40) may then propagate the larger multimers thought to permeabilize the outer membrane and thereby launch the apoptotic program (Fig. 6C). In this model, prosurvival constraint is essential to preclude induction of cell death by Bax and presumably also by Bak.
Fig. 6.
Model for Bax regulation. (A) In healthy cells, Bax (orange) is predominantly cytosolic but is in equilibrium with minor populations on the mitochondrial membrane. Posttranslational modifications, physical stimuli, and certain BH3-only proteins (e.g., tBid) may enhance its translocation to membranes. However, any membrane-bound Bax conformers with the BH3 domain exposed (“primed Bax”) are sequestered by available prosurvival Bcl-2 proteins (blue), preventing full Bax activation and apoptosis. (B) Upon cellular stress, the capacity of the prosurvival proteins to bind Bax can be overwhelmed because of their inactivation by BH3-only proteins (red), or their depressed synthesis or degradation (prosurvival ghost). Left unchecked, the membrane-bound Bax can begin to self-associate and oligomerize. (C) Further translocation of Bax molecules, perhaps by direct recruitment from the cytosol, stimulates the Bax oligomerization that is thought to permeabilize the outer mitochondrial membrane, leading to activation of the caspases that dismantle the cell.
Because our results suggest that tight binding of Bax by the prosurvival proteins is not required to restrain it, the threshold for apoptosis may be determined in large part by the proportion of prosurvival relatives that remain unoccupied by BH3-only proteins. The BH3-only proteins appear to titrate the prosurvival proteins to initiate apoptosis. In certain situations Bim or Puma seems to be rate-limiting, given that loss of a single bim or puma allele impairs apoptosis of certain cells (41, 42). Thus, the balance between BH3-only and prosurvival proteins may largely determine how readily Bax and Bak become activated.
Other evidence supports our conclusion that the ability of Bcl-2 homologues to bind Bax or Bak is essential to prevent apoptosis. Bak, which interacts with both Mcl-1 and Bcl-xL, drove cell death when both Mcl-1 and Bcl-xL were eliminated (17). Likewise, because all of the prosurvival proteins bind WT Bax (Fig. 1B), Bax provoked apoptosis if and only if all were neutralized, but Bcl-2 could not restrain a Bax BH3 mutant (K64A) lacking detectable Bcl-2 binding (15). Furthermore, the prosurvival function of the viral distant Bcl-2 orthologue M11L relies on its binding Bax and Bak rather than BH3-only proteins (43). Finally, the lifespan of anucleate platelets is limited by the availability of Bcl-xL to engage Bak (44).
The findings reported here, together with these other recent observations, rule out sequestration of BH3-only proteins as the sole function of the prosurvival Bcl-2 family members. Instead, we suggest that the Bcl-2-like proteins form a crucial barrier to apoptosis, in part by their ability to bind the BH3-exposed conformers of Bax and Bak, thereby preventing full activation of these critical cell death mediators (Fig. 6). However, once levels of the relevant prosurvival proteins fall below a certain threshold, owing to their elimination or neutralization by BH3-only proteins, Bax and Bak apparently become free to launch the apoptotic program.
Materials and Methods
Expression Constructs.
N-terminally HA- or FLAG-tagged mammalian expression vectors for WT or mutant human Bax, human or mouse Bcl-2, Bcl-xL, Bcl-w, or Mcl-1 were made by subcloning into pEF PGKpuro or pEF pGKhygro vectors (16, 45, 46). Retroviral expression constructs were made by subcloning into pMSCV-IRES-GFP (47) or vectors with the GFP cassette replaced with hygromycin (15) or puromycin resistance genes. Those expressing BH3-only proteins have been described in ref. 16. The mir30-style shRNA pMSCV-LMP retroviral vectors (48) were kindly provided by Michael Hemann (Massachusetts Institute of Technology); the hairpin target sequences are 5′-ATACTCAGTCATCCACAGGGCGA for bcl-2 and 5′-ATTAGATCACTGAACGCTCTCCG for bcl-x. Yeast expression vectors were made by subcloning the cDNAs for the full-length prosurvival proteins or Bax, respectively, into the pGALL(TRP1) and pGALS(LEU2) vectors (25).
The inserts and mutations were verified by sequencing; details of oligonucleotides and constructs are available from the authors.
Structural Analysis.
Structural coordinates were from the RCSB Protein Data Bank (http://www.rcsb.org/pdb/), and representations were produced using PyMOL (49).
Flow Cytometric Analysis.
Transgene or retroviral gene expression was confirmed by flow cytometric analysis of fixed, permeabilized cells, as described previously (45, 50), as was flow cytometric detection of activated Bax with the mouse monoclonal anti-Bax antibody (clone 3; BD Biosciences) (17, 51). The samples were analyzed using a FACScan (BD Biosciences).
Binding Assays.
Relative binding affinity was determined in solution competition assays using the Biacore 3000, as previously described (16).
Other Methods.
Details of cell lines and cell viability assays, assays on T and B cell blasts, yeast colony assays, immunoprecipitation and immunoblotting, subcellular fractionation, and recombinant proteins and peptides are provided with the SI.
Supplementary Material
Acknowledgments.
We thank J. Blyth, D. Cooper, H. Ierino, T. Pham, K. Pioch, G. Siciliano, and the Walter and Eliza Hall Institute of Medical Research Flow Cytometry Laboratory for excellent technical assistance and animal husbandry; and Abbott Laboratories, R. Anderson, P. Bouillet, L. Chen, P. Colman, S. Cory, P. Czabotar, C. Day, G. Dewson, P. Ferrer, M. Hardwick, M. Hemann, R. Kluck, P. Kelly, the late S. Korsmeyer, M. Kvansakul, D. Loh, N. Motoyama, L. O'Reilly, A. Strasser, C. Thompson, M. van Delft, W. Welch, S. Willis, and M. Yabal for gifts of essential reagents, discussions, and technical advice. This work is supported by grants and fellowships from The Cancer Council Victoria (Project Grant 461239, studentship to D.T.R., scholarship to E.F.L.), the Australian National Health and Medical Research Council (Project Grant 433007, Program Grant 461221, and fellowships to J.I.F., C.J.H., W.D.F., J.M.A., and D.C.S.H.), the U.S. National Cancer Institute (CA80188), and the Leukemia and Lymphoma Society (SCOR 7015–02).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808691105/DCSupplemental.
References
- 1.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Youle RJ, Strasser A. The Bcl-2 protein family: Opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 3.Korsmeyer SJ, Shutter JR, Veis DJ, Merry DE, Oltvai ZN. Bcl-2/Bax: A rheostat that regulates an anti-oxidant pathway and cell death. Semin Cancer Biol. 1993;4:327–332. [PubMed] [Google Scholar]
- 4.Hsu Y-T, Wolter KG, Youle RJ. Cytosol-to-membrane redistribution of Bax and Bcl-XL during apoptosis. Proc Natl Acad Sci USA. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu Y-T, Youle RJ. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J Biol Chem. 1998;273:10777–10783. doi: 10.1074/jbc.273.17.10777. [DOI] [PubMed] [Google Scholar]
- 6.Zha H, Aimé-Sempé C, Sato T, Reed JC. Proapoptotic protein Bax heterodimerizes with Bcl-2 and homodimerizes with Bax via a novel domain (BH3) distinct from BH1 and BH2. J Biol Chem. 1996;271:7440–7444. doi: 10.1074/jbc.271.13.7440. [DOI] [PubMed] [Google Scholar]
- 7.Sattler M, et al. Structure of Bcl-xL-Bak peptide complex: Recognition between regulators of apoptosis. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 8.Wang K, Gross A, Waksman G, Korsmeyer SJ. Mutagenesis of the BH3 domain of Bax identifies residues critical for dimerization and killing. Mol Cell Biol. 1998;18:6083–6089. doi: 10.1128/mcb.18.10.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki M, Youle RJ, Tjandra N. Structure of Bax: Coregulation of dimer formation and intracellular localization. Cell. 2000;103:645–654. doi: 10.1016/s0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]
- 10.Leber B, Lin J, Andrews DW. Embedded together: The life and death consequences of interaction of the Bcl-2 family with membranes. Apoptosis. 2007;12:897–911. doi: 10.1007/s10495-007-0746-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fletcher JI, Huang DCS. Controlling the cell death mediators Bax and Bak: Puzzles and conundrums. Cell Cycle. 2008;7:39–44. doi: 10.4161/cc.7.1.5178. [DOI] [PubMed] [Google Scholar]
- 12.Kim H, et al. Hierarchical regulation of mitochondrion-dependent apoptosis by Bcl-2 subfamilies. Nat Cell Biol. 2006;8:1348–1358. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- 13.Letai A, et al. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 14.Kuwana T, et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Willis SN, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 17.Willis SN, et al. Pro-apoptotic Bak is sequestered by Mc1–1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lakhani SA, et al. Caspases-3 and -7: Key mediators of mitochondrial events of apoptosis. Science. 2006;311:847–851. doi: 10.1126/science.1115035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu Y, et al. Bax does not have to adopt its final form to drive T cell death. J Exp Med. 2006;203:1147–1152. doi: 10.1084/jem.20051736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Dai S, Zhu Y, Marrack P, Kappler JW. The structure of a Bcl-xL/Bim fragment complex: Implications for Bim function. Immunity. 2003;19:341–352. doi: 10.1016/s1074-7613(03)00234-6. [DOI] [PubMed] [Google Scholar]
- 21.Oltersdorf T, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 22.van Delft MF, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng EH-Y, Levine B, Boise LH, Thompson CG, Hardwick JM. Bax-independent inhibition of apoptosis by Bcl-xL. Nature. 1996;379:554–556. doi: 10.1038/379554a0. [DOI] [PubMed] [Google Scholar]
- 24.Motoyama N, et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x deficient mice. Science. 1995;267:1506–1510. doi: 10.1126/science.7878471. [DOI] [PubMed] [Google Scholar]
- 25.Hawkins CJ, Wang SL, Hay BA. A cloning method to identify caspases and their regulators in yeast: Identification of Drosophila IAP1 as an inhibitor of the Drosophila caspase DCP-1. Proc Natl Acad Sci USA. 1999;96:2885–2890. doi: 10.1073/pnas.96.6.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zha H, et al. Structure-function comparisons of the proapoptotic protein Bax in yeast and mammalian cells. Mol Cell Biol. 1996;16:6494–6508. doi: 10.1128/mcb.16.11.6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nechushtan A, Smith CL, Hsu YT, Youle RJ. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 1999;18:2330–2341. doi: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardai SJ, et al. Phosphorylation of Bax Ser184 by Akt regulates its activity and apoptosis in neutrophils. J Biol Chem. 2004;279:21085–21095. doi: 10.1074/jbc.M400063200. [DOI] [PubMed] [Google Scholar]
- 29.Xin M, Gao F, May WS, Flagg T, Deng X. Protein kinase Czeta abrogates the proapoptotic function of Bax through phosphorylation. J Biol Chem. 2007;282:21268–21277. doi: 10.1074/jbc.M701613200. [DOI] [PubMed] [Google Scholar]
- 30.Minn AJ, et al. Bcl-xL regulates apoptosis by heterodimerization-dependent and -independent mechanisms. EMBO J. 1999;18:632–643. doi: 10.1093/emboj/18.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Billen LP, Kokoski CL, Lovell JF, Leber B, Andrews DW. Bcl-XL inhibits membrane permeabilization by competing with Bax. PLoS Biol. 2008;6:1268–1280. doi: 10.1371/journal.pbio.0060147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou H, Hou Q, Hansen JL, Hsu YT. Complete activation of Bax by a single site mutation. Oncogene. 2007;26:7092–7102. doi: 10.1038/sj.onc.1210517. [DOI] [PubMed] [Google Scholar]
- 33.Liu X, et al. Bcl-xL does not have to bind Bax to protect T cells from death. J Exp Med. 2006;203:2953–2961. doi: 10.1084/jem.20061151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.George NM, Evans JJ, Luo X. A three-helix homo-oligomerization domain containing BH3 and BH1 is responsible for the apoptotic activity of Bax. Genes Dev. 2007;21:1937–1948. doi: 10.1101/gad.1553607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chittenden T, et al. A conserved domain in Bak, distinct from BH1 and BH2, mediates cell death and protein binding functions. EMBO J. 1995;14:5589–5596. doi: 10.1002/j.1460-2075.1995.tb00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dewson G, et al. To trigger apoptosis, Bak exposes its BH3 domain and homo-dimerizes via BH3:groove interactions. Mol Cell. 2008;30:369–380. doi: 10.1016/j.molcel.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Pagliari LJ, et al. The multidomain proapoptotic molecules Bax and Bak are directly activated by heat. Proc Natl Acad Sci USA. 2005;102:17975–17980. doi: 10.1073/pnas.0506712102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsu YT, Youle RJ. Nonionic detergents induce dimerization among members of the Bcl-2 family. J Biol Chem. 1997;272:13829–13834. doi: 10.1074/jbc.272.21.13829. [DOI] [PubMed] [Google Scholar]
- 39.Xin M, Deng X. Protein phosphatase 2A enhances the proapoptotic function of Bax through dephosphorylation. J Biol Chem. 2006;281:18859–18867. doi: 10.1074/jbc.M512543200. [DOI] [PubMed] [Google Scholar]
- 40.Tan C, et al. Auto-activation of the apoptosis protein Bax increases mitochondrial membrane permeability and is inhibited by Bcl-2. J Biol Chem. 2006;281:14764–147675. doi: 10.1074/jbc.M602374200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bouillet P, Cory S, Zhang L-C, Strasser A, Adams JM. Degenerative disorders caused by Bcl-2 deficiency are prevented by loss of its BH3-only antagonist Bim. Dev Cell. 2001;1:645–653. doi: 10.1016/s1534-5807(01)00083-1. [DOI] [PubMed] [Google Scholar]
- 42.Villunger A, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins Puma and Noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 43.Kvansakul M, et al. A structural viral mimic of prosurvival Bcl-2: A pivotal role for sequestering proapoptotic Bax and Bak. Mol Cell. 2007;25:933–942. doi: 10.1016/j.molcel.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 44.Mason KD, et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–1186. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 45.Huang DCS, Cory S, Strasser A. Bcl-2, Bcl-xL and adenovirus protein E1B19kD are functionally equivalent in their ability to inhibit cell death. Oncogene. 1997;14:405–414. doi: 10.1038/sj.onc.1200848. [DOI] [PubMed] [Google Scholar]
- 46.O'Connor L, et al. Bim: A novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998;17:384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Parijs L, et al. Uncoupling IL-2 signals that regulate T cell proliferation, survival, and Fas-mediated activation-induced cell death. Immunity. 1999;11:281–288. doi: 10.1016/s1074-7613(00)80103-x. [DOI] [PubMed] [Google Scholar]
- 48.Dickins RA, et al. Probing tumor phenotypes using stable and regulated synthetic microRNA precursors. Nat Genet. 2005;37:1289–1295. doi: 10.1038/ng1651. [DOI] [PubMed] [Google Scholar]
- 49.DeLano WL. The PyMOL molecular graphics system. [Accessed 2006];2002 Available at http://www.pymol.org.
- 50.Huang DCS, O'Reilly LA, Strasser A, Cory S. The anti-apoptosis function of Bcl-2 can be genetically separated from its inhibitory effect on cell cycle entry. EMBO J. 1997;16:4628–4638. doi: 10.1093/emboj/16.15.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dewson G, Snowden RT, Almond JB, Dyer MJ, Cohen GM. Conformational change and mitochondrial translocation of Bax accompany proteasome inhibitor-induced apoptosis of chronic lymphocytic leukemic cells. Oncogene. 2003;22:2643–2654. doi: 10.1038/sj.onc.1206326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.