Preface
Blood vessels promote tumor growth, while both blood and lymphatic vessels facilitate tumor metastasis by serving as conduits for the transport of tumor cells to new sites. Angiogenesis and lymphangiogenesis are regulated by integrins, which are members of a family of cell surface receptors whose ligands are extracellular matrix proteins and immunoglobulin superfamily molecules. Select integrins promote endothelial cell migration and survival during angiogenesis and lymphangiogenesis, while other integrins promote pro-angiogenic macrophage trafficking to tumors. Several integrin-targeted therapeutic agents are currently in clinical trials for cancer therapy. Here we review the evidence implicating integrins as a family of fundamental regulators of angiogenesis and lymphangiogenesis.
Introduction
Angiogenesis, the development of new blood vessels, is a fundamental physiological process that promotes embryonic development, tissue repair and fertility, yet that also promotes chronic inflammation, tumor growth and tumor metastasis1. New blood vessels in tumors can grow by sprouting from preexisting vessels or by recruitment of rare, circulating bone marrow-derived endothelial progenitor cells1-3. Tumor cells, macrophages, and fibroblasts within tumors can secrete factors, such as vascular endothelial growth factor (VEGF), that induce blood vessel growth in tumors2,4-5. Basic and clinical studies indicate that suppression of angiogenesis can inhibit tumor progression and metastasis6. Many lines of investigation implicate integrins, which are key regulators of endothelial cell migration and survival, as key regulators of tumor angiogenesis (Figure 1).
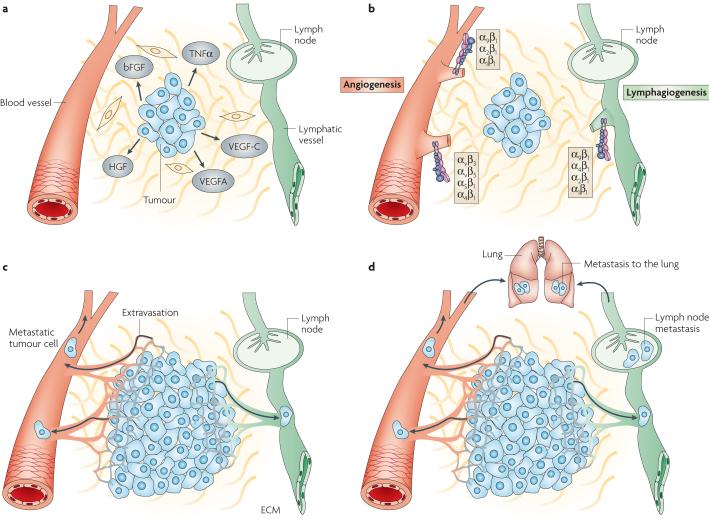
Figure 1. Mechanisms regulating angiogenesis and lymphangiogenesis.
(A) Tumor cells near pre-existing blood vessels secrete growth factors and chemokines such as VEGF-A, bFGF, and TNFα that stimulate quiescent vascular endothelium to enter the cell cycle. Tumors also secrete factors such as VEGF-C, VEGF-A or HGF that stimulate the growth of new lymphatic vessels in the peritumoral space. (B) These growth factors activate or upregulate expression of integrins such as α1β1, α2β1, α4β1, α5β1 and αvβ3 on blood vessels and α4β1, α9β1, α2β1 and α1β1 on lymphatic vessels. Tumor derived VEGF-C also promotes new lymphatic vessel growth in draining lymph nodes. (C) These integrins then promote endothelial cell migration and survival during invasion of tumor tissue, resulting in the creation of new vessels sprouts. The new blood vessels promote tumor growth by removing waste products and providing nutrients. These new blood and lymphatic vessels also provide an avenue for tumor metastasis. (D) Lymphangiogenesis promotes metastasis to lymph nodes and, sometimes, more distant tissues such as lung, while angiogenesis promotes metastasis to local and distant sites, such as lung.
Like angiogenesis, lymphangiogenesis - the growth of new lymphatic vessels - promotes tumor metastasis2,7-9. The lymphatic system is comprised of a network of blind-ended, thin walled lymphatic capillaries, collecting vessels and specialized secondary immune organs, including lymph nodes, tonsils, Peyer’s Patches [G] and the spleen. Lymphatic vessels drain protein-rich interstitial fluids and immune cells through the lymph nodes and return fluids back to the circulation at the thoracic duct. After entering lymph nodes, antigen-presenting cells such as dendritic cells may activate B- and T-cell immune responses. Tumors induce the growth of new lymphatic vessels within tumors and draining lymph nodes, enhancing dendritic cell trafficking to lymph nodes; increased lymphatic vessel density in tumors is also associated with increased metastasis to lymph nodes7-9. New findings indicate that select integrins can modulate lymphangiogenesis and may thereby affect tumor metastasis (Figure 1).
Angiogenesis and lymphangiogenesis can be stimulated by tumor-associated macrophages. Circulating bone marrow-derived cells (monocytes) migrate into tumors in response to tumor-secreted chemokines and differentiate into macrophages. Pro-angiogenic tumor macrophages release a number of potent pro-angiogenic cytokines, such as VEGF-A, VEGF-C, tumor necrosis factor alpha (TNF-α), interleukin-8 (IL-8) and basic fibroblast growth factor (bFGF)4-5 and express a broad array of extracellular matrix degrading proteases, including urokinase-type plasminogen activator (uPA), the matrix metalloproteinases MMP-2, MMP-7, MMP-9 and MMP12 and elastase4. Importantly, new evidence suggests that macrophage infiltration can activate the angiogenic switch [G] in spontaneous tumors5. Select integrins play key roles in regulating the trafficking of circulating monocytes and progenitor cells to tumors.
The Integrin Family of Adhesion Receptors
The integrin family is an extensive group of structurally related receptors for extracellular matrix (ECM) proteins and immunoglobulin superfamily molecules. Integrins are divalent cation-dependent heterodimeric membrane glycoproteins comprised of non-covalently associated α and β subunits that promote cell attachment and migration on the surrounding extracellular matrix. Eighteen α and eight β subunits can associate to form twenty-four unique integrin heterodimers. Each integrin subunit consists of an extracellular domain, a single transmembrane region, and a short (approximately thirty to forty amino acids) cytoplasmic region10. While some integrins, such as α5β1, primarily recognize a single ligand, others, such as αvβ3 can bind several ligands. Many integrins, including αvβ3, α5β1, αIIbβ3, αvβ6, and α3β1 recognize the tripeptide Arg-Gly-Asp (RGD) in their ligands11. Sequences flanking the RGD peptide are also important for integrin selectivity12. Other integrins recognize alternative short peptide sequences; for example, integrin α4β1 recognizes Glu-Ile-Leu-Asp-Val (EILDV) and Arg-Glu-Asp-Val (REDV) in the alternatively spliced fibronectin domain known as IIICS13. Some integrins, such as α4β1, can also bind cell surface receptors, such as Vascular Cell Adhesion Molecule-1 (VCAM-1), to promote cell-cell adhesion (Text Box 1).14
Text Box 1: Regulation of Integrin Activation
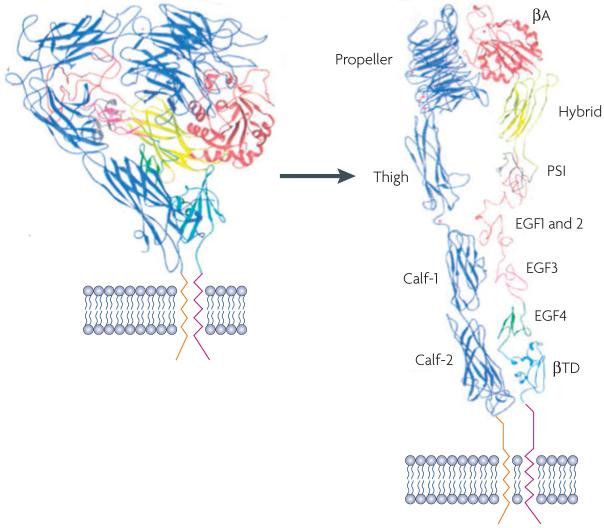
Integrin activity during angiogenesis can be modulated by integrin expression or by growth factor or chemokine receptor signaling, which alters integrin conformation (“inside-out” signaling). Crystallography, nuclear magnetic resonance and electron microscopy studies suggest that the globular region formed by the N-termini of the α and β chains is bent towards the membrane and the cytoplasmic regions of the two subunits are closely associated with one another in an inactive integrin116-119. Integrin activation is associated with an unbending and elongation of the dimer and separation of the cytoplasmic domains116-119. This allows interaction of integrin cytoplasmic domains with intracellular proteins and permits integrin-mediated signal transduction. Once activated, integrins bind ligands, cluster and initiate their own signaling cascades that lead to cell migration and survival. The activity of many endothelial cell integrins during angiogenesis is regulated primarily at the transcriptional level. The expression of integrins α1β1, α2β1, α4β1, α5β1 and αvβ3 is strongly induced by pro-angiogenic growth factors or chemokines20-23, 67,73. However, other integrins are activated by receptor-mediated signaling. Integrin αvβ5 is constitutively expressed on vascular endothelium, yet is only functionally activated by VEGFR2 mediated signal transduction31. In circulating cells such as monocytes and other leukocytes, integrins are generally inactive until cells are stimulated by chemokines, hormones or other factors. For example, integrin α4β1 activity on leukocytes can be stimulated by chemokine signaling127. Additionally, integrin αIIbβ3 is inactive on resting platelets but becomes activated from within when an external stimulus such as thrombin or epinephrine binds a cell surface receptor and induces the conformational change in the integrin cytoplasmic domains. It then binds its ligand fibrinogen, leading to platelet aggregation128. Thus, integrins roles in angiogenesis can be controlled either by expression or by intracellular signaling (inside-out signaling). Thus in tumors, the expression of VEGF and other pro-angiogenic factors can continuously stimulate integrin expression and activity and stimulate unregulated angiogenesis. A schematic representation of the structure of an inactive and active integrin is shown (adapted with permission from ref. 123).
Textbox 2: Integrin regulation of cell migration and focal adhesions
Integrins “integrate” signals from the extracellular matrix to the intracellular cytoskeleton in focal adhesions. Integrins have short cytoplasmic tails and no intrinsic enzymatic or kinase activities. To integrate signals and activate intracellular signaling pathways, integrins co-cluster with serine, threonine and tyrosine kinases, phosphatases and adaptor proteins in focal adhesions. Focal adhesion complexes are comprised of integrins, protein kinases such as focal adhesion kinase (FAK), Src and many other kinases, adaptor proteins such as Shc, signaling intermediates such as PI-3-kinase, Rho and Rac GTPases and actin binding cytoskeletal proteins such as talin, α-actinin, paxillin, tensin and vinculin15-16. Integrin signaling promotes cell migration by providing traction along the extracellular matrix and by promoting actin remodeling through the Rho family small GTPases. This actin remodeling leads to cytoplasmic flow in the direction of cell migration and cell body retraction at the trailing end of the cells. Individual components of integrin-mediated signaling cascades, such as FAK, Shc and Raf, play essential roles in angiogenesis. For example, focal adhesion kinase (FAK) is a mediator of signal transduction by integrins and growth factor receptors in endothelial cells. Overexpression of FAK in mice promoted angiogenesis122. In contrast, conditional deletion of focal adhesion kinase in endothelial cells led to defective angiogenesis in the embryos, yolk sac, and placenta, impaired vasculature and associated hemorrhage, edema, and developmental delay, and late embryonic lethality123. In addition, in vitro deletion of FAK in ECs isolated from floxed FAK mice exhibited reduced tube formation, cell survival, proliferation, and migration in vitro123. These results indicate that FAK plays a critical role in angiogenesis and vascular development. In addition, Shc, an important adaptor protein that potentiates MAP kinase pathway signaling is activated by both integrins and growth factor receptors and plays critical roles in early vascular development. Shc is primarily expressed during early mouse development in the developing heart and endothelium.124 Shc null animals died between E10.5 and 11.5 with defects in heart and blood vessel development and isolated Shc null cells exhibited migration deficiencies.124 Like Shc, Raf-1 is an integral component of the MAP kinase signaling pathway. This signaling intermediate is activated by integrins and is critical for vascular morphogenesis.125 Mice null for Raf-1 die during early development by E11.5 with vascular defects in the yolk sac, placenta and head.125 Thus, integrin mediated signaling likely plays important roles in vascular development adult angiogenesis. A schematic representation of integrin signaling and focal adhesion components is shown.
Textbox 3: The links between inflammation, wound-healing and cancer
Inflammation is increasingly thought to play important roles in tumor initiation, progression and metastasis126. Inflammation is caused by tissue injury, ischemia, toxins and other damaging stimuli. Injured tissues release chemokines and growth factors that attract leukocytes and upregulate adhesion molecules on the surface of endothelium in injured tissues to promote wound-healing. When the release of these factors is chronic, invasion of leukocytes into tissues is sustained and the further release of chemokines and factors can induce oxidative damage, DNA mutations, tumor development, tumor angiogenesis and lymphangiogenesis and subsequent metastasis. A key example is the increased risk of developing colon carcinoma in patients with chronic inflammation such as inflammatory bowel disease126. Substantial evidence supports the role of inflammation in tumor angiogenesis. The number of macrophages in tumor tissues is significantly greater than in normal tissues127-128. Importantly, high TAM densities indicate poor prognoses in breast, prostate, ovarian and cervical cancer129-134. Tumor associated macrophages promote neovascularization by releasing potent pro-angiogenic cytokines and growth factors, such as VEGF, tumor necrosis factor -α (TNF-α), interleukin-8 (IL-8) and bFGF135-137. Additionally, TAMs also express a broad array of proteases known to play roles in the angiogenic process. These proteases include urokinase-type plasminogen activator (uPA) and the matrix metalloproteinases MMP-2, MMP-7, MMP-9 and MMP12138-141 that remodel and breaking down the extracellular matrix (ECM). Tumors produce factors such as monocyte chemoattractant protein-1 (MCP-1, or CCL2) and RANTES (CCL5) that increase the infiltration of TAMs in several primary tumors including breast, ovarian, melanoma, and glioblastoma142-144. Besides stimulating tumor angiogenesis and lymphangiogenesis, inflammatory chemokines, such as IL-6, or the genes that regulate them, such as IKKbeta and IKKalpha, also promote tumor progression to malignancy by directly stimulating tumor cell activation or cancer stem cell proliferation146-150.
FIG. Box 1.
FIG. Box 2.
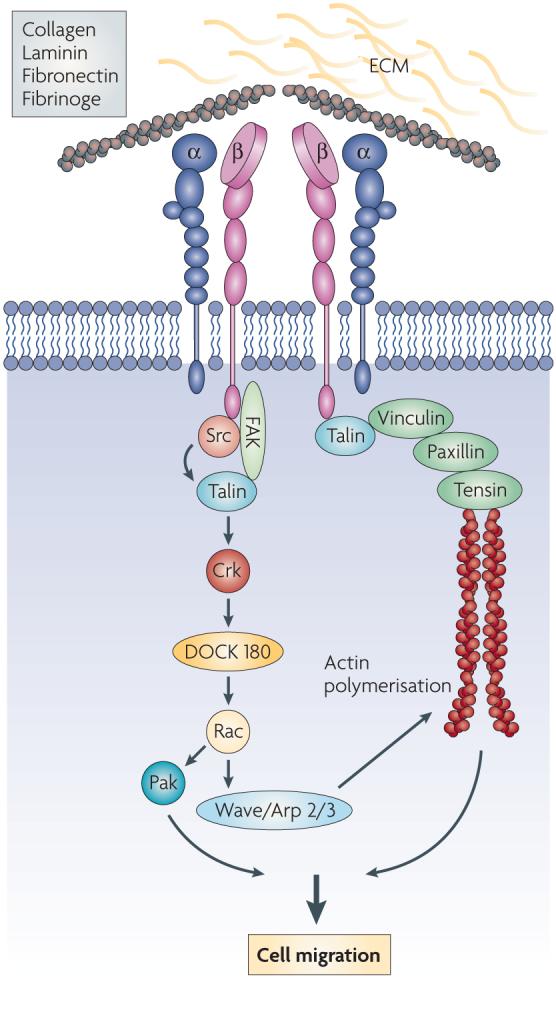
Integrin ligation promotes integrin clustering and subsequent integrin-mediated intracellular signal transduction. Unlike growth factor receptors, integrins have no intrinsic enzymatic or kinase activities, but activate complex signaling pathways by coclustering with kinases and adaptor proteins in focal adhesion complexes. A number of signaling moieties are activated by integrins and many of these are found within focal adhesion complexes. Focal adhesion complexes are comprised of integrins, protein kinases - such as focal adhesion kinase (FAK) and Src - adaptor proteins such as Shc, signaling intermediates such as Rho family GTPases, actin binding cytoskeletal proteins such as talin, α-actinin, paxillin, tensin and vinculin15-16 and other signaling proteins. Integrin signaling promotes cell migration, proliferation and survival (Text Box 2). Loss of integrin ligation inhibits these events and unligated integrins can actively initiate apoptosis, even without loss of cell attachment. This form of death is stress response- and death-receptor-independent, but caspase 8-dependent, and has been called ‘integrin mediated death’17.
In the last several years, some of the key molecular mechanisms that regulate angiogenesis and lymphangiogenesis have been delineated. Many lines of investigation implicate integrins as key regulators of endothelial cell migration and survival during these events. In this Review, we will discuss the evidence that supports roles for various integrins in the regulation of angiogenesis and lymphangiogenesis and we discuss the status of integrin-based therapeutics for the treatment of cancer.
Integrins in Vascular Endothelium and Angiogenesis
In vitro and in vivo data have implicated a number of endothelial cell integrins in the regulation of cell growth, survival and migration during angiogenesis. These integrins include the heterodimers α1β1, α2β1, α4β1, α5β1, α6β1, α6β4, α9β1, αvβ3 and αvβ5, (Table 1). Additionally, the glial cell integrin αvβ8 regulates brain blood vessel development.18 Distinct experimental approaches, such as inhibition of angiogenesis by antagonists, knockin mutations and genetic deletions in ovo have led to conflicting theories of the roles of several integrins in angiogenesis, including αv integrins and α2β1 integrin. However, increasing molecular evidence in vivo points to major roles key integrins in tumor angiogenesis.
Alpha v Integrins
The αv integrin subunit can heterodimerize with several different beta subunits (β1, β3, β5, β6 and β8) to achieve unique ligand-binding profiles. The integrin αvβ3, a receptor for RGD-containing proteins such as vitronectin, fibronectin, fibrinogen and osteopontin (which are components of the ECM), was the first of the alpha v integrins to be characterized19. Integrin αvβ3 was the first αv integrin to be shown to regulate angiogenesis.20 This integrin is widely expressed on blood vessels in human tumor biopsies but not on vessels in normal human tissues. Its expression on endothelial cells is stimulated by angiogenic growth factors such as bFGF, TNFα, and IL-8 and it is upregulated on endothelium in tumors, wounds and sites of inflammation20. While angiogenesis during wound healing is tightly regulated and self-limiting, angiogenesis associated with chronic inflammation and cancer is often persistent and abnormal1. However, many of the same molecules that regulate angiogenesis in wound healing, such as integrin αvβ3, also regulate pathological angiogenesis. Increasing evidence points to important causative links between inflammation and cancer and some evidence suggests that inflammation promotes the angiogenic switch [G] in tumors4-5 (Textbox 3).
Several experimental approaches indicate that αvβ3 plays a key role in endothelial cell survival and migration during angiogenesis20-21. Integrin αvβ3 is expressed in response to angiogenic growth factors and tumors in chick chorioallantoic membrane [G], mouse, rabbit and human models of angiogenesis and tumor growth20-23. Antagonists of αvβ3 inhibited angiogenesis and tumor growth in a variety of animal models of cancer and blocked corneal as well as choroidal [G] angiogenesis in animal models of ocular disease20-25. Inhibition of αvβ3 function in quail embryos disrupted vasculogenesis by blocking lumen formation and disturbing vascular patterning26.
Integrin αvβ3 antagonists induce endothelial cell apoptosis, increase the activity of the tumour suppressor p53, increase levels of the cell cycle inhibitor p21WAF1/CIP1 and decrease levels of the anti-apoptotic protein bax27. Further studies show that αvβ3 antagonists activate a caspase 8-dependent cell death program28. Thus, a sizeable body of evidence indicates that integrin αvβ3 promotes angiogenesis and endothelial cell survival and that antagonism of this integrin suppresses angiogenesis by inducing endothelial cell apoptosis in vitro and in vivo. Ligation of endothelial αvβ3 integrin has also been shown to activate MAP kinase, focal adhesion kinase (FAK) and Src, among other kinases, resulting in cell proliferation, differentiation and migration29.
The related integrin, αvβ5, promotes an angiogenic pathway that is distinct from that regulated by αvβ3. Anti-αvβ3 antibodies blocked angiogenesis induced by bFGF while antibodies that target αvβ5 blocked angiogenesis induced by VEGF in both the rabbit corneal eye pocket and the chick chorioallantoic membrane (CAM) assay23. The VEGF/αvβ5 pathway, but not the bFGF/αvβ3 pathway, is dependent on Src kinase and protein kinase C30. In vivo angiogenesis assays showed that bFGF and TNF-α depend on αvβ3 to initiate angiogenesis while αvβ5 is required for TGF-α and VEGF mediated angiogenesis. These data indicate unique roles for αvβ3 and αvβ5 integrins in angiogenesis and suggest that both could be important therapeutic targets. Some pathological conditions may depend on αvβ5 while other may depend on αvβ3. For example, integrin αvβ5 is more prevalent on cerebral cavernous malformations than is integrin αvβ331. Additionally, only VEGF, which acts through αvβ5, rather than bFGF, which acts through αvβ3, promotes survival of developing retinal vessels32 as well as vascular permeability33-34. As vascular permeability promotes tumor metastasis33-34, these results suggest that integrins that promote vascular permeability, such as αvβ5, may also promote tumor metastasis.
In contrast to the roles that are indicated by expression and function analysis, studies of integrin β3 null mice suggest that this integrin is absolutely required for vascular development35. While half of integrin β3 null mice survive embryogenesis, the other half die in utero, exhibiting intrauterine bleeding or defective placental development35. These mice exhibit apparently normal vessels in the brain and gut, as well as normal postnatal retinal neovascularization. However, male mice lacking β3 integrin exhibit coronary capillaries of irregular endothelial thickness, with endothelial protrusions into the lumen, and expanded cytoplasmic vacuoles36. These defective coronary vessels can be normalized by administration of inhibitors of VEGF or Flk-1, suggesting that enhanced VEGF signaling may compensate for the loss of β3 integrin36. In fact, β3 null mice exhibit enhanced tumor angiogenesis compared with normal mice37, with strongly upregulated VEGFR2 expression and signaling.38 Taken together, these studies suggest that compensatory VEGFR2 signaling changes may play a role in the survival of β3 deficient animals. In contrast to β3 null mice, integrin αvβ5 null mice exhibit completely normal development and normal angiogenesis39, indicating that this integrin is not required for vascular development.
Genetic ablation of the alpha v subunit (which eliminates expression of integrins αvβ1, αvβ3, αvβ5, αvβ6 and αvβ8) suggest that in some mice, αv integrins are not required for blood vessel development in most tissues. Twenty percent of embryos lacking αv integrins survive to birth with normal blood vessels in many tissues. However, eighty percent of mice die in utero between E10.5 and E11.5 with defective placental blood vessels.40 The remaining twenty percent of αv null mice die shortly after birth with severe brain and intestinal hemorrhage, with distended and leaky vessels in these tissues.40 Thus, αv integrins appear to play key roles in embryonic development of blood vessels in tissues such as placenta and brain. Surprisingly, brain blood vessels developed normally in mice with Tie2-Cre-mediated endothelial cell specific deletion of αv integrins, but not in mice with Nestin-Cre and GFAP-Cre mediated embryonic central nervous system deletion of αv integrins41. Mice null for integrin β8 also exhibited brain blood vessel defects similar to αv null mice.18 Together, these studies indicate that glial cell αvβ8 integrin is required for proper brain blood vessel development. The brain blood vessels of mice lacking integrin αv or β8 subunits exhibit enlarged, disrupted blood vessels, with defective apposition of glial and endothelial cells18,42. Interestingly, these studies suggest that glial cell integrins may play roles in establishing or maintaining the blood-brain barrier.
Results from studies of integrin antagonists indicate that αv integrins promote angiogenesis, while genetic deletion studies indicate that αv integrins are not required for angiogenesis. One hypothesis to explain this conflict is that αv integrins act as negative regulators of angiogenesis; once deleted in development, angiogenesis occurs at an accelerated rate. An alternative hypothesis is that animals lacking αv integrins develop compensatory changes in VEGF signaling that permit angiogenesis to occur during embryogenesis. New studies bring clarification to the much-disputed role of this integrin in angiogenesis. Importantly, knockin mutant animals expressing the point mutations Y747F and Y759F in the integrin β3 cytoplasmic tail developed normally, but exhibit reduced growth factor and tumor induced angiogenesis in vivo.43 Mutant endothelial cells exhibit impaired adhesion, spreading, migration and tube formation, as well as impaired complex formation between VEGF receptor-2 and β3 integrin43. These results provide genetic evidence that integrin β3 plays an important role in promoting angiogenesis43. Together, these diverse results can be interpreted to indicate that integrin αvβ3 plays an important role in angiogenesis and that loss of expression of this integrin in development can be partially compensated for by upregulation of other angiogenesis signaling pathways.
How does integrin αvβ3 modulate angiogenesis? Unligated or antagonized αvβ3 integrin can inhibit cell survival. In a three-dimensional collagen matrix, αvβ3-bearing cells undergo apoptosis while cells deficient in αvβ3 survive longer.44 As intact collagen is not a ligand for αvβ3, these studies indicated that unligated integrin αvβ3 provides a stimulus for apoptosis. This unligated integrin mediated cell death is caspase-8 dependent and is sometime called ‘integrin-mediated death.’28 Functioning as a biosensor, αvβ3 may promote angiogenesis in the ligated state, but when ligands are absent or ligand-binding is antagonized, αvβ3 may activate a death pathway to inhibit angiogenesis.
Thus, depending on the microenvironment and the ligation state, integrins promote or inhibit angiogenesis by positively or negatively regulating cell survival. Integrin αvβ3 appears to function as a regulator of angiogenesis by balancing opposing signals in the tumor microenvironment.
Fibronectin-binding Integrins
Fibronectin is key extracellular matrix protein that is deposited by endothelial cell during normal45 and tumor angiogenesis46. Short fibronectin peptide loops containing the sequence Arginine-Glycine-Aspartic acid (RGD) interact with integrins such as α5β1, αvβ5 and αvβ311. Fibronectin secreted by endothelial cells also contains the alternatively spliced EIIIA, EIIIB and IIICS domains, which bind to integrins α4β1 and α9β1.13, 47 Fibronectin is essential for developmental angiogenesis as deletion of all fibronectin isoforms is early embryonic lethal, with yolk sac and other mesodermal tissue defects48. Deletion of only EIIIA and EIIIB alternatively spliced variants is also embryonic lethal with sever vascular defects that suppress placentation, yolk sac vessel formation and heart formation49. Thus fibronectin plays a key role in angiogenesis, as do many of its receptors, the β1 integrins.
Genetic ablation studies have demonstrated the critical role of β1 integrins in angiogenesis and vascular development. β1-integrin-null embryos die by E9.5-10.5 in utero due to implantation defects50-51. However, animals with an endothelial cell specific deletion of β1 integrin (Tie2Creβ1 floxed mice) die by E10.5 with severe vascular defects.52 Integrin β1-null teratomas [G] grown in wild type hosts develop small host-derived vessels but no β1-null vessels53. Furthermore, endothelial cell proliferation and vessel branching is absent in β1-null embryoid bodies53. Thus, the β1 integrin family clearly plays a key role in angiogenesis.
Integrin α5β1 is poorly expressed on quiescent endothelium but its expression is significantly upregulated on endothelium during tumor angiogenesis in both mice and humans.46 Expression of this integrin is also upregulated during corneal angiogenesis.54 Integrin α5β1 expression is induced in response to a variety of angiogenic stimuli, such as bFGF, IL-8, and the ECM protein, Del-1, but not by VEGF46,55. Current evidence suggests that the activity of this integrin in angiogenesis is regulated by transcription55. Indeed, expression of α5β1 in endothelial cells (ECs) is regulated by a homeobox family transcription factor, HoxD3 which itself is induced in response to bFGF and other stimuli but not by VEGF55. Hox D3 antisense suppresses integrin α5β1 expression in vitro and in vivo while overexpression of Hox D3 upregulates α5β1 expression55.
Antagonists of α5β1 inhibited tumor46, corneal54 and choroidal56 angiogenesis in chicks and mice, thus leading to inhibition of tumor growth or tumor regression. Integrin α5β1 mediated adhesion promotes endothelial cell migration and survival in vivo and in vitro by suppressing the activity of Protein Kinase A (PKA)57-58. Activation of PKA by expression of the catalytic subunit of PKA or by exposure of cells to cAMP or forskolin [G] directly inhibits cell migration and stimulates apoptosis in vitro and in vivo. Thus, integrin α5β1 promotes endothelial cell survival during angiogenesis.
Embryonic deletion of the integrin α5 subunit induces early mesenchymal abnormalities, leading to lethality of α5-null embryos59. Embryos lacking integrin α5 have a truncated posterior and lack posterior somites [G]. These embryos also present with abnormal organization of the emerging extra embryonic and embryonic vasculature, and reduced complexity of the emerging vasculature59. Further studies using α5 null ES cells to grow teratocarcinomas showed decreased proliferation, increased apoptosis and decreased vascularization in teratocarcinomas derived from α5-null ES cells compared with controls60. In vitro growth of embryoid bodies lacking α5 integrins showed a delay and reduction in the formation of the early vascular plexus and formation of complex vascular structures61. Together, these data indicate a key role for α5β1 in vasculogenesis and angiogenesis.
Integrin α4β1, another important fibronectin receptor, is best known as a lymphocyte integrin involved in adhesion and extravasation of lymphocytes by binding to VCAM-1 expressed on inflamed endothelial cells. This integrin can bind both IIICS fibronectin and VCAM-1, a member of the immunoglobulin superfamily. Loss of integrin α4 during development leads to defects in placentation, heart development and coronary artery development, causing lethality between E10.0-E12.062. However, due to the severe heart defects, it is not clear whether α4β1 plays a role in developmental angiogenesis.
Nevertheless, recent studies showed that integrin α4β1 is expressed on neovessels in murine and human tumors and in response to VEGF, bFGF, IL-1β and TNF-α63. Antagonists of this integrin blocked tumor neovascularization and decreased tumor growth in chick and murine models of tumor growth63. This integrin promotes adhesion of endothelium with VCAM-1 expressing vascular smooth muscle cells during blood vessel formation and antagonists of α4β1 induced cell death of both endothelial cells and pericytes [G]63. Transient, but direct, contact between endothelial cells and pericytes during angiogenesis appears to play an important role in cell survival signaling in each cell type, although the exact signaling mechanisms remain unknown. As endothelial cells express the pericyte chemoattractant PDGF and pericytes express VEGF, it is possible that close proximity of the two cell types facilitated by integrin α4β1-VCAM interactions allows each cell type to respond to growth and survival signals emanating from the other cell type63. Thus, integrin α4β1-VCAM-1 dependent cell-cell attachment promotes the survival of both endothelial cells and pericytes during angiogenesis.
The most recent fibronectin-binding integrin to be found to play a role in angiogenesis is integrin α9β164-67. This interesting integrin is structurally similar to integrin α4β1 but unlike α4β1, it is a receptor for a number of ECM proteins and cell surface receptors including tenascin-C, thrombospondin, osteopontin, IIICS fibronectin, VCAM and other ligands64-67. Integrin α9β1 is expressed on epithelia, osteoclasts, smooth muscle cells and also endothelial cells. Recent studies show that this integrin promotes VEGF-A stimulated angiogenesis by a unique mechanism; it directly binds VEGF-A 121 and blocking antibodies to α9β1 suppress VEGF-A induced angiogenesis64. However, other studies found that α9β1 bind directly to the N-terminus of thrombospondin and blocking antibodies to α9β1 inhibited angiogenesis induced by this thrombospondin fragment65. Integrin α9β1 null mice die 8-12 days after birth due to lethal defects in development of the lymphatic system; however, they do not exhibit obvious defects in development of blood vessels68.
Fibronectin and several of its receptors clearly play central roles in promoting angiogenesis during development and during tumor growth. However, additional integrin subfamilies, such as the laminin family also regulate angiogenesis as described below.
Laminin-binding integrins
Similar to integrins αvβ3/αvβ5, results of studies of the expression and function of the laminin and collagen binding integrins α1β1 and α2β1 differ from in ovo genetic deletions. In normal animals, VEGF-A treatment upregulates expression of both α1β1 and α2β1 on vascular endothelial cells69. Function-blocking antibodies directed against both integrins reduced VEGF-1 induced angiogenesis in vivo and reduced tumor growth and angiogenesis69. However, integrin α1-null mice and α2-null mice are viable and fertile, indicating that these integrins are not required for angiogenesis in developing embryos. Tumors grown in α1β1 deficient mice grew more slowly and exhibited less angiogenesis, indicating an important role for this integrin in angiogenesis70. However, mice lacking α2β1 integrins exhibit enhanced B16F10 melanoma tumor growth and angiogenesis when compared to wildtype mice71. In contrast, LLC tumors did not exhibit increased angiogenesis or growth in alpha 2 null mice. Enhanced angiogenesis was attributed to an increase in VEGFR1 expression and function on α2 null endothelial cells. While B16F10 melanoma cells expressed high levels of the VEGFR1 binding growth factor PLGF, LLC cells expressed low levels of PLGF. Upon transfection with PLGF, LLC tumors grew more rapidly in α2β1 null animals compared with wildtype, suggesting that compensatory changes in growth factor expression and signaling can occur in animals with null mutations71. Integrins α1β1 and α2β1 appear to play important roles in tumor angiogenesis, although complete understanding of their roles is still developing.
The α6 integrin subunit can form heterodimers with either the β1 or the β4 subunit. Integrin α6β1 is expressed at high levels in capillary endothelial cells in vivo72. Antibody inhibitors of α6 integrin prevented endothelial cell tube formation in vitro, suggesting a role for α6 in the angiogenic process72. Endothelial cell migration and tube formation was also blocked by down-regulation of α6 expression in brain microvascular endothelial cells with siRNA72. Histological analysis reveals that the β4 subunit is expressed on human and murine tumor endothelium73. In addition, the α6β4 ligand, laminin 5, is also expressed by tumor blood vessels73. However, mice that are null for the β4 or the α6 subunit do not exhibit overt vascular defects but die immediately after birth in part due to severe skin blistering caused by passage through the birth canal74-76.
In stably adherent cells, the β4 integrin mediates hemidesmosome [G] assembly76. However, in growth factor stimulated cells, hemidesmosomes do not form and the β4 subunit cytoplasmic tail is tyrosine phosphorylated in response to receptor tyrosine kinase activation of the Src family kinases. Upon phosphorylation of Tyr(1440) and Tyr(1422), the β4 subunit interacts with the SH2 domain of Shc, promoting Raf-ERK and Rac-JNK signaling and immediately early gene expression77. Mice with a targeted deletion of the C-terminal portion of the β4 subunit cytoplasmic tail (Δ1355) developed normally, but adult mice exhibited a reduced angiogenic response to bFGF and VEGF73. Spontaneous tumor growth in these animals was also suppressed, as was tumor angiogenesis. Integrin α6β4 did not affect proliferation of endothelial cells but was required for endothelial cell adhesion and migration. In addition, endothelial cell specific loss of β4 integrin suppressed nuclear translocation of phosphoErk and NFκB73. These studies suggest that integrin α6β4 is a novel target for anti-angiogenic therapies.
Integrin α6β1 is also expressed on endothelium; its expression can be confirmed by immunoprecipitation76. Unlike α6β4, which shows a preference for laminin-5, integrin α6β1 binds most laminin isoforms as well as other extracellular matrix proteins including Cyr61, TSP-1 and TSP-278. As α6 integrin antagonists and α6 siRNA constructs inhibit angiogenesis, it is possible that integrin α6β1 promotes angiogenesis. However, as these agents can block the function of both integrins α6β1 and α6β4, it is not yet clear what role integrin α6β1 plays in tumor angiogenesis.
The studies described here show that endothelial cell integrins that bind to diverse extracellular matrix ligands promote angiogenesis during embryonic development and tumor growth. Embryonic deletion of many of these integrins does not lead to an embryonic lethal phenotype, indicating that many integrins have overlapping functions during development. However, as antagonists or deletion of these integrins suppresses tumor angiogenesis, each integrin can contribute to angiogenesis in at least some tumor microenvironments.
Integrins on bone marrow derived cells
Integrins on bone marrow-derived immune cells can also promote angiogenesis by facilitating myeloid cell and endothelial progenitor cell homing to tumors (Figure 2). Circulating bone marrow derived cells migrate into tumors in response to secreted chemokines and cytokines. Monocytes can then differentiate into pro-angiogenic secreting macrophages. Myeloid cells express a number of functional integrins (α2β1, α4β1, α5β1, αvβ3, αvβ5, αMβ2 (CD11b) and αXβ2 (CD11c). A subset of these integrins regulate extravasation of these cells from the blood stream and their migration within neovascular microenvironments.
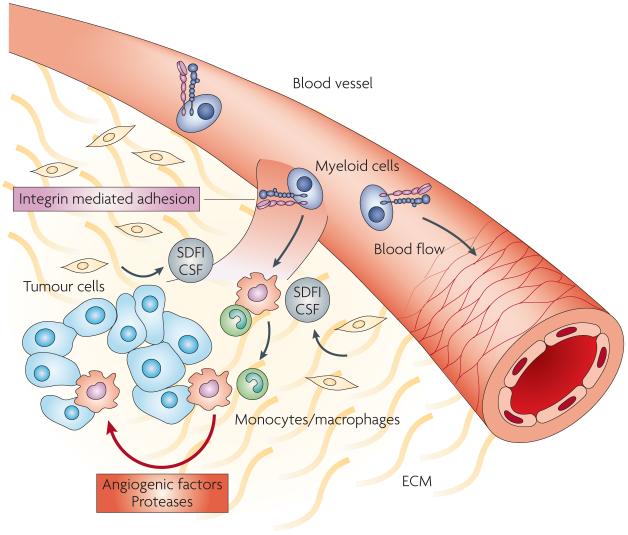
Figure 2. Myeloid cells promote angiogenesis.
Myeloid precursor cells adhere to angiogenic endothelium via activated α4β1 or β2 integrins. A variety of tumor or stromal derived factors, including stromal derived factor 1 (SDF-1), colony stimulating factors (CSF), and others mobilize myeloid cells and activate integrins, promoting extravasation into the tumor environment. In the presence of other cytokines and growth factors, these cells differentiate into macrophages, which support tumor growth by expressing VEGF, other pro-angiogenic factors and proteases.
Recent studies indicate that integrin α4β1, a receptor for VCAM and CS-1 fibronectin, selectively promotes the homing of both endothelial progenitor cells and monocytes to neovascular tissue79-80. Human CD34+ and murine Lin-Sca1+ progenitor cells as well as human and murine bone marrow-derived myeloid cells (CD14+ CD11b+) adhered to endothelial cells in vitro and to tumor endothelium in vivo via integrin α4β179-80. Treatment of mice bearing Lewis lung carcinoma tumors with antagonists of integrin α4β1 significantly suppressed the number of monocytes and progenitor cells found within tumors and reduced blood vessel density86. These studies suggest that the suppression of monocyte and progenitor cell homing to tumors by integrin α4β1 antagonists could be a useful supplementary approach to suppress tumor angiogenesis and growth.
Other integrins can also mediate homing of precursor cells to sites of neovascularization. The adhesion of circulating endothelial precursor cells to endothelial monolayers was shown to be integrin β2 dependent. Hematopoietic progenitor cells (Sca+ Lin-cells) from β2-integrin deficient mice exhibited reduced homing to sites of ischemia81-82. Together, these studies identified key functions for integrins during precursor cell/monocyte homing to angiogenic sites.
The platelet integrins αIIbβ3 and α2β1 may indirectly regulate angiogenesis by promoting platelet deposition within tumors and damaged tissues. Platelets release several pro-angiogenic factors, which are stored in alpha granules [G], such as VEGF-A, SDF-1, and sphingosine-1-phosphate, suggesting that integrins on platelets may also play roles in angiogenesis83-84. Thus, integrins on bone marrow derived cells can play key roles in angiogenesis.
Integrins in Lymphangiogenesis
Lymphatic vessels form a network that drains fluid and cells from tissues; these vessels are required for tissue homeostasis. Lymphatic capillaries are lined by loosely associated endothelial cells without a covering of pericytes or vascular smooth muscle. This structure allows ready passage of immune cells and tumor cells into the lymphatic system. Indeed, lymph nodes are typically the first organ to exhibit tumor metastasis, and sentinel node monitoring is used extensively to detect metastases.
Analysis of the role of lymphatics in tumor growth and metastasis had been hindered until recently by the absence of lymphatic markers. Recent identification of specific lymphatic markers, such as the transcription factor Prox-1 and the CD44 homolog lymphatic vessel hyaluronan receptor-1 (LYVE-1)85-86, has made it possible to study mechanisms regulating lymphangiogenesis. The growth factors VEGF-C and VEGF-D, which can be expressed by tumor cells or macrophages in tumors, promote growth of the lymphatic vessel network by activating the lymphatic endothelial cell receptor VEGFR-387.
To date, evidence is mounting regarding roles for integrins in lymphangiogenesis (Figure 1). Integrin α9β1 is required for development of the fully functional lymphatic system because mice deficient in α9β1 integrin die 6 to 12 days after birth due to chylothorax, an accumulation of lymph in the pleural cavity68. Integrin α9β1 plays a role in growth factor mediated lymphangiogenesis as Prox-1, a lymphatic endothelial cell selective transcription factor coordinately upregulates integrin α9β1 and VEGFR3 expression and endothelial cell motility in vivo88. Recent studies show that this integrin promotes VEGF-C and D stimulated cell migration by directly binding these growth factors89. Importantly, antagonism of α9β1 suppresses VEGF-C induced motility. Taken together, these studies indicate that α9β1 plays unique yet critical roles in lymphangiogenesis.
Other studies have shown that integrins α1β1 and α2β1 are expressed on lymphatic endothelium in healing wounds in response to VEGF-A. Inhibition of these integrins blocked lymphangiogenesis in these wounds90. Integrin α5β1 is expressed by a subpopulation of lymphatic vessels in the inflamed cornea and small molecule antagonists of this integrin inhibited inflammatory lymphangiogenesis91. Integrin α4β1 is highly expressed on tumor lymphatic endothelium92 and antagonists of this integrin can block lymphangiogenesis and tumor metastasis. In contrast, αv integrins appear to play little or no role in lymphangiogenesis and integrin α5β1 appears to play no role in tumor lymphangiogenesis92. Thus, several integrins appear to play important roles in lymphangiogenesis yet the profile of integrins regulating lymphangiogenesis distinct from those regulating angiogenesis. Importantly, antagonists of these integrins may be useful in preventing tumor metastasis by blocking lymphangiogenesis.
Potential clinical applications
Preclinical studies suggested that antagonists of several integrins might be useful to suppress tumor angiogenesis and growth either alone or in combination with current cancer therapeutics. While antagonists of several integrins are undergoing preclinical evaluation and development, only a handful of integrin-based drugs have so far been tested in the clinic. Integrin antagonists include function-blocking antibodies, peptides and organic small molecules. Function-blocking anti-integrin monoclonal antibodies typically have high affinity and specificity and have been well characterized for years. These were the first integrin antagonists to reach the clinic. Cyclic peptide antagonists have also been evaluated in clinical trials. Small organic inhibitors, particularly orally active drugs, are the most cost effective therapeutics but the development of selective and high affinity integrin inhibitors is time consuming. Hence, no organic small molecules antagonists of integrins have yet entered clinical trials.
Of the several integrin antagonists undergoing clinical evaluation for cancer treatment, all have proven nontoxic, including Abegrin (Medi-522), a humanized [G] anti-αvβ3 antibody, CNTO95 a human αvβ3/αvβ5 antibody, Volociximab, a chimeric mouse/human anti-α5β1 antibody, Cilengitide, a cyclic peptide inhibitor of integrins αvβ3/αvβ5 and ATN161, a non-RGD based peptide inhibitor of α5β1 (Table 2). These agents are likely nontoxic because the targeted integrins are only expressed or activated in remodeling tissues such as tumors.
Abegrin, or MEDI-522, was the first anti-integrin therapeutic to be tested in clinical trials for cancer. It is a humanized version of the anti-integrin αvβ3 monoclonal antibody LM609, which has been shown to block tumor angiogenesis by inducing apoptosis in newly formed endothelial cells. A Phase I study showed that the early version of this drug, Vitaxin, had very low toxicity and was well tolerated93-94. When tested on patients with metastatic cancer who had failed other treatments, Vitaxin led to disease stabilization without toxicity. However, in a second clinical trial, use of Vitaxin on patients with leiomyosarcoma [G] did not suggest anti-tumor activity. In 2001, Medimmune began clinical trials using Vitaxin (renamed MEDI-522/Abegrin) and in 2003 initiated Phase II trials in patients with advanced metastatic melanoma and in patients with metastatic prostate cancer95. In one melanoma trial, 112 patients with stage IV melanoma received 8 mg/kg MEDI-522 each week. Of these patients, 57 also received 1000 mg/m2 dacarbazine [G] once every 3 weeks while the rest (n=55) did not. The overall survival was 12.7 months for MEDI-522 + dacarbazine and 9.4 months for MEDI-522 alone. Both groups showed prolonged survival when compared with a historical control95. A recent study in patients showed that MEDI-522 showed functional efficacy by reducing focal adhesion kinase activity in patients’ blood vessels96. Based on these results, Phase III cancer clinical trials are in the planning stages.
On the basis of preclinical studies showing both integrins αvβ3 and αvβ5 regulate angiogenesis, a human monoclonal antibody directed against both αvβ3 and αvβ5 integrins, CNTO 95, was developed by Centocor. CNTO 95 reduced angiogenesis and tumor growth in human melanoma xenografts in nude mice and rats97. Preclinical safety studies on cynomolgus macaques (Macaca fascicularis) showed no toxicity98. This antibody has completed phase I safety trials with a favorable safety profile, extended treatment offered to one third of patients and one patient exhibiting a partial response99. CNTO95 is now under evaluation in Phase I/II clinical trial for the treatment of melanoma (http://clinicaltrials.gov/show/NCT00246012). As CNTO95 inhibits both integrins αvβ3 and αvβ5, two of the integrins that promote tumor angiogenesis, it may have wide-spread clinical utility. Additionally, most carcinoma cells express integrin αvβ5, which has been shown to promote tumor cell invasion100. Targeting the alpha v integrins may thus block both tumor cell invasion and metastasis and tumor angiogenesis.
For these reasons, a cyclic RGD-peptide antagonist of αvβ3/αvβ5, Cilengitide (EMD 121974) has been developed as a cancer therapeutic. Phase I clinical trials showed favorable safety profiles and no dose-limiting toxicities101-102. In addition, Cilengitide was shown to enhance radiotherapy in cancer patients103. This drug was evaluated in Phase I/IIa clinical trials for glioblastoma and significantly enhanced progression free survival was observed101. On this basis, as of 2007, E. Merck was planning to evaluate Cilengitide in Phase III trials for glioblastoma. Cilengitide is currently in phase II trials for glioblastoma, non-small cell lung cancer, melanoma and pancreatic cancer104-106. Therefore, three distinct alpha v integrin targeting drugs offer promise as cancer therapeutics.
Since beta 1 integrins also play significant roles in angiogenesis, targeting these integrins in addition to alpha v integrins may be provide useful benefit in suppressing angiogenesis and tumor growth. Antagonists of one integrin, the alpha 5 beta 1 integrin have undergone clinical testing. A chimeric mouse/human anti-α5β1 antibody, M200 (volociximab), developed by Protein Design Lab and now partnered with Biogen-Idec Pharmaceuticals, has shown low toxicity in Phase I studies. M200 was evaluated in Phase II trials for metastatic melanoma, renal cell carcinoma and non-small cell lung cancer107-108. In renal cell carcinoma studies M200 was well-tolerated at a dose of 10 mg/kg every 2 weeks and stable disease was noted in 87% of patients. In a melanoma trial in combination with DTIC [G], the antibody was well-tolerated and anti-tumor activity was noted in 62% of patients
Another drug in clinical trials, ATN-161, is a peptide inhibitor of integrin α5β1. In animal models of colon cancer, ATN-161 reduced metastases and improved survival when combined with chemotherapy109. In Phase I safety trials, ATN161 was well tolerated and several patients exhibited stable disease. ATN-161 is currently in Phase II clinical trials for multiple myeloma and other tumors110. Thus, these two integrin α5β1 inhibiting drugs may offer future benefit to cancer patients.
However, as many integrins can promote angiogenesis, it is not yet clear whether targeting one or more than one will have the most significant impact on tumor angiogenesis and growth. It is likely also that integrin antagonists may be combined with other angiogenesis inhibitors such as VEGF inhibitors like Avastin.
Integrin Targeted Tumor Imaging and Treatment
Integrin ligands are also under investigation as tumor endothelium targeted diagnostic agents. Both peptide and antibody based diagnostic agents targeting RGD binding integrins or αv integrins have been evaluated in animal models of cancer. PEGylated [G] cyclic RGD peptides labeled with 18F or 64Cu were effective in imaging xenograft brain and breast tumors with a low signal to noise ratio. These imaging approaches have been able to identify xenograft tumors as small as 1.5mm in diameter111-112. Paramagnetic polymerized liposomes conjugated to an anti-αvβ3 antibody were also used to detect neovascular tumors in experimental rabbit tumors113. Integrin αv-targeted ultrasound microbubbles [G] also preferentially bound neovasculature at the periphery of experimental tumors114. Thus integrin targeting is proving useful in tumor imaging. It is possible that agents targeting distinct integrins may be useful in pre-classifying patients for receipt of anti-integrin drugs.
Integrin targeting agents may also be useful in selectively delivering chemotherapeutics or gene therapeutic agents to tumors, thus minimizing global toxicities. For example, an integrin αvβ3 and αvβ5 targeted liposome (nanoparticles) was used to deliver a mutant Raf construct to tumors in animals; this Raf construct induced endothelial cell apoptosis and subsequent tumor suppression115. Integrins have thus also proven to be effective targets for delivery of imaging and therapeutic agents.
Conclusions/Perspectives
Blood and lymphatic vessels play critical roles in promoting tumor growth and metastasis. A substantial body of experimental evidence indicates that integrins regulate endothelial cell migration and survival during angiogenesis and lymphangiogenesis. In addition, integrins promote monocyte trafficking to tumors and subsequent angiogenesis and lymphangiogenesis. As angiogenesis promotes tumor growth and metastasis and lymphangiogenesis promotes tumor metastasis by providing conduits for tumor escape through the lymphatic system, antagonists of these integrins may be useful in blocking tumor metastasis in patients.
Although there is much experimental evidence supporting roles for integrins in tumor angiogenesis and lymphangiogenesis, conflicts between integrin knockout and in vivo tumor studies need to be resolved in the future. Why do many integrin deletion mutants develop normally yet exhibit altered tumor angiogenesis? Future comparisons of embryonic and tumor angiogenesis will address whether compensatory molecular changes occur in surviving mutant animals or whether there are quantitative but non-lethal changes in blood vessel densities in mutant animals.
As several integrin-targeted therapeutic agents are in clinical trials for cancer therapy, future clinical studies will likely determine whether integrin inhibitors will be best used against select tumors, such as those in which tumor cells themselves express the targeted integrin. In addition, integrin targeted nanoparticles will continue to be developed for the imaging of tumor vasculature and delivery of gene therapy or chemotherapeutic drugs. As integrins are clearly a family of critical and fundamental regulators of angiogenesis and lymphangiogenesis, the future of integrin antagonists in cancer therapy is promising.
Acknowledgments
This work was supported by NIH grants R01CA83133 and R01CA126820 to Judith Varner and a postdoctoral research fellowship from the California Breast Cancer Research Program to Barbara Garmy-Susini.
GLOSSARY
- Peyer’s Patches
Secondary lymphoid organs named after the 17th century Swiss anatomist Joseph Conrad Peyer that are comprised of round lymphoid follicles in the mucosa of the small intestine
- angiogenic switch
The induction of new blood vessel sprouting at an early time point in tumor development that leads to rapid, exponential tumor growth
- chick chorioallantoic membrane
A thin, highly vascularized fetal membrane formed by fusion of the chorion and allantois in fertilized chicken eggs that is often used to evaluate pro- and anti-angiogenic agents
- choroidal angiogenesis
The development of new blood vessels in the highly vascular area of the eye that lies between the retina and the sclera
- teratomas
Germ cell tumors comprised of undifferentiated and differentiated cells derived from the three germ layers, mesoderm, ectoderm and endoderm; teratomas may include hair, teeth and other complex structures
- forskolin
A diterpene derived from the Indian Coleus plant that raises cAMP levels in cells by activating adenyl cyclase
- posterior somites
Cuboidal, segmented masses of mesoderm organized in pairs and distributed along the developing neural tube. Posterior somites give rise to the thoracic, lumbar, and sacral vertebrae
- pericytes
A mesenchymal cell precursor to vascular smooth muscle that associates with endothelial cells during angiogenesis and provides support to small capillaries
- hemidesmosome
An organized adhesive structure on the surface of epithelial cells comprised of integrin α6β4 attached on the exterior of the cell to laminin and on the interior of the cells to plectin and cytosolic keratins
- alpha granules
Endosomes or granules in platelets that contain growth factors such as VEGF, TGFbeta and PDGF
- humanized antibody
A synthetic monoclonal antibody comprised of a human antibody backbone fused with the antigen recognition regions of a mouse monoclonal antibody through recombinant DNA techniques, which is developed to eliminate immunogenic sequences
- leiomyosarcoma
A neoplasm comprised of tumor cells arising from smooth muscle (sarcoma) and frequently found in the stomach and small intestines
- Dacarbazine
A chemotherapeutic, DNA alkylating agent used in the treatment of malignant melanoma and Hodgkin’s disease
- DTIC
An abbreviation for Dacarbazine, a chemotherapeutic agent
- PEGylated
Covalently modified with poly(ethylene glycol) to make a hydrophobic drug more soluble and to mask a drug from the host immune system
- Microbubbles
Small (3 μm or less) gas-filled bubbled that serve as contrast enhancing agents in diagnostic medical ultrasound imaging
Footnotes
-Links to web sites:
http://www.cancerpublications.com/newsletter/colorectal/AIO/v1n4/Bukowski/index.htm Ronald M. Bukowsi: Integrins and inhibitors of angiogenesis
References
- 1.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;42:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 2.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 3.Lyden D, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat. Med. 2001;11:1194–1201. doi: 10.1038/nm1101-1194.This article established the concept that bone marrow derived cells in lung and other tissues could help create an environment that attracts metastatic tumor cells.
- 4.Schmid MC, Varner JA. Myeloid cell trafficking and tumor angiogenesis. Cancer Lett. 2007;250:1–8. doi: 10.1016/j.canlet.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin EY, Pollard JW. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 2007;67:5064–5066. doi: 10.1158/0008-5472.CAN-07-0912.This article shows that macrophages play critical roles in altering the fate of tumors by secreting pro-angiogenic growth factors.
- 6.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 7.Roma AA, et al. Peritumoral lymphatic invasion is associated with regional lymph node metastases in prostate adenocarcinoma. Mod. Pathol. 2006;19:392–398. doi: 10.1038/modpathol.3800546. [DOI] [PubMed] [Google Scholar]
- 8.Dadras SS, et al. Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph nodes. Mod. Pathol. 2005;18:1232–1242. doi: 10.1038/modpathol.3800410. [DOI] [PubMed] [Google Scholar]
- 9.Hirakawa S, et al. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J. Exp. Med. 2005;201:1089–1099. doi: 10.1084/jem.20041896.This article showed for the first time that tumors induce lymphangiogenesis not only in the peritumoral space but also in draining lymph nodes.
- 10.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 11.Plow EF, et al. Ligand binding to integrins. J. Biol. Chem. 2000;275:21785–21788. doi: 10.1074/jbc.R000003200. [DOI] [PubMed] [Google Scholar]
- 12.Haas TA, Plow EF. Integrin-ligand interactions: a year in review. Curr Opin Cell Biol. 1994;6:656–662. doi: 10.1016/0955-0674(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 13.Komoriya A, et al. The minimal essential sequence for a major cell type-specific adhesion site (CS1) within the alternatively spliced type III connecting segment domain of fibronectin is leucine-aspartic acid-valine. J. Biol. Chem. 1991;266:15075–15079. [PubMed] [Google Scholar]
- 14.Jin H, Varner J. Integrins: roles in cancer development and as treatment targets. Br. J. Cancer. 2004;90:561–565. doi: 10.1038/sj.bjc.6601576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat. Rev. Mol. Cell. Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 16.Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Stupack DG. Integrins as a distinct subtype of dependence receptors. Cell Death Differ. 2005;12:1021–1030. doi: 10.1038/sj.cdd.4401658. [DOI] [PubMed] [Google Scholar]
- 18.Zhu J, et al. beta8 integrins are required for vascular morphogenesis in mouse embryos. Development. 2002;129:2891–2903. doi: 10.1242/dev.129.12.2891.This article shows that integrin αvβ8 is required for the formation of normal brain blood vessels.
- 19.Cheresh DA. Human endothelial cells synthesize and express an Arg-Gly-Asp-directed adhesion receptor involved in attachment to fibrinogen and von Willebrand factor. Proc. Natl. Acad. Sci. U S A. 1987;84:6471–6475. doi: 10.1073/pnas.84.18.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alphav beta3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751.This is the first article demonstrating a role for an integrin in angiogenesis.
- 21.Brooks PC, et al. Integrin alphav beta3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 22.Brooks PC, et al. Antiintegrin alphav beta3 blocks human breast cancer growth and angiogenesis in human skin. J. Clin. Invest. 1995;96:1815–1822. doi: 10.1172/JCI118227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedlander M, et al. Definition of two angiogenic pathways by distinct alphav integrins. Science. 1995;270:1500–1502. doi: 10.1126/science.270.5241.1500.This article established that two unique pathways of angiogenesis are regulated by two distinct alpha v integrins.
- 24.Friedlander M, et al. Involvement of integrins alphav beta3 and alphav beta5 in ocular neovascular diseases. Proc. Natl. Acad. Sci. USA. 1996;93:9764–9769. doi: 10.1073/pnas.93.18.9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedlander M, et al. Angiogenesis inhibition and choroidal neovascularization suppression by sustained delivery of an integrin antagonist, EMD478761. Invest Ophthalmol Vis Sci. 2007;48:5184–5190. doi: 10.1167/iovs.07-0469. [DOI] [PubMed] [Google Scholar]
- 26.Drake CJ, Cheresh DA, Little CD. An antagonist of integrin alpha v beta 3 prevents maturation of blood vessels during embryonic neovascularization. J. Cell Sci. 1995;108:2655–2661. doi: 10.1242/jcs.108.7.2655. [DOI] [PubMed] [Google Scholar]
- 27.Strömblad S, Becker JC, Yebra M, Brooks PC, Cheresh DA. Suppression of p53 activity and p21WAF1/CIP1 expression by vascular cell integrin alphaVbeta3 during angiogenesis. J. Clin. Invest. 1996;98:426–433. doi: 10.1172/JCI118808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stupack DG, Puente XS, Boutsaboualoy S, Storgard CM, Cheresh DA. Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J. Cell Biol. 2001;155:459–470. doi: 10.1083/jcb.200106070.This article established the concept of integrin mediated death by showing that unligated integrins promote cell death.
- 29.Eliceiri BP, Klemke R, Strömblad S, Cheresh DA. Integrin alphavbeta3 requirement for sustained mitogen-activated protein kinase activity during angiogenesis. J. Cell Biol. 1998;140:1255–1263. doi: 10.1083/jcb.140.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eliceiri BP, et al. Src-mediated coupling of focal adhesion kinase to integrin alpha(v)beta5 in vascular endothelial growth factor signaling. J. Cell Biol. 2002;157:149–160. doi: 10.1083/jcb.200109079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seker A, et al. Expression of integrins in cerebral arteriovenous and cavernous malformations. Neurosurgery. 2006;58:159–168. doi: 10.1227/01.neu.0000192174.55131.09. [DOI] [PubMed] [Google Scholar]
- 32.Alon T, et al. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat. Med. 1995;1:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- 33.Weis S, Cui J, Barnes L, Cheresh D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J. Cell Biol. 2004;167:223–232. doi: 10.1083/jcb.200408130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Criscuoli ML, Nguyen M, Eliceiri BP. Tumor metastasis but not tumor growth is dependent on Src-mediated vascular permeability. Blood. 2005;105:1508–1514. doi: 10.1182/blood-2004-06-2246. [DOI] [PubMed] [Google Scholar]
- 35.Hodivala-Dilke KM, et al. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J. Clin. Invest. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weis SM, et al. Cooperation between VEGF and beta3 integrin during cardiac vascular development. Blood. 2007;109:1962–1970. doi: 10.1182/blood-2005-10-038893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reynolds LE, et al. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nature Med. 2002;8:27–34. doi: 10.1038/nm0102-27. [DOI] [PubMed] [Google Scholar]
- 38.Reynolds AR, et al. Elevated Flk1 (vascular endothelial growth factor receptor 2) signaling mediates enhanced angiogenesis in beta3-integrin-deficient mice. Cancer Res. 2004;64:8643–8650. doi: 10.1158/0008-5472.CAN-04-2760.This study established the concept that developmental loss of an integrin could lead to enhanced angiogenesis through compensatory mechanisms.
- 39.Huang X, Griffiths M, Wu J, Farese RV, Jr., Sheppard D. Normal development, wound healing, and adenovirus susceptibility in beta5-deficient mice. Mol. Cell Biol. 2000;20:755–759. doi: 10.1128/mcb.20.3.755-759.2000.This article showed that loss of αvβ5 during development has no significant effect on angiogenesis.
- 40.Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell. 1998;95:507–519. doi: 10.1016/s0092-8674(00)81618-9.This study shows that αv integrins are essential for development in most animals but that some animals can survive in ovo loss of αv integrins until the early postnatal period.
- 41.McCarty JH, et al. Selective ablation of alpha v integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development. 2005;132:165–176. doi: 10.1242/dev.01551. [DOI] [PubMed] [Google Scholar]
- 42.McCarty JH, et al. Defective associations between blood vessels and brain parenchyma lead to cerebral hemorrhage in mice lacking alphav integrins. Mol. Cell. Biol. 2002;22:7667–7677. doi: 10.1128/MCB.22.21.7667-7677.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahabeleshwar GH, Feng W, Phillips DR, Byzova TV. Integrin signaling is critical for pathological angiogenesis. J. Exp. Med. 2006;203:2495–2507. doi: 10.1084/jem.20060807.This study shows that animals with an intact but non-functional β3 integrin exhibit defective angiogenesis.
- 44.Montgomery AM, Reisfeld RA, Cheresh DA. Integrin alpha v beta 3 rescues melanoma cells from apoptosis in three-dimensional dermal collagen. Proc. Natl. Acad. Sci. U. S. A. 1994;91:8856–8860. doi: 10.1073/pnas.91.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clark RA, et al. Blood vessel fibronectin increases in conjunction with endothelial cell proliferation and capillary ingrowth during wound healing. J. Invest. Dermatol. 1982;79:269–276. doi: 10.1111/1523-1747.ep12500076. [DOI] [PubMed] [Google Scholar]
- 46.Kim S, Bell K, Mousa SA, Varner JA. Regulation of angiogenesis in vivo by ligation of integrin alpha5beta1 with the central cell-binding domain of fibronectin. Am. J. Pathol. 2000;156:1345–1362. doi: 10.1016/s0002-9440(10)65005-5.This is the first article showing a role for integrin α5β1 in angiogenesis.
- 47.Liao YF, Gotwals PJ, Koteliansky VE, Sheppard D, Van De Water L. The EIIIA segment of fibronectin is a ligand for integrins alpha 9beta 1 and alpha 4beta 1 providing a novel mechanism for regulating cell adhesion by alternative splicing. J. Biol. Chem. 2002;277:14467–14474. doi: 10.1074/jbc.M201100200. [DOI] [PubMed] [Google Scholar]
- 48.George EL, Baldwin HS, Hynes RO. Fibronectins are essential for heart and blood vessel morphogenesis but are dispensable for initial specification of precursor cells. Blood. 1997;90:3073–3081. [PubMed] [Google Scholar]
- 49.Astrof S, Crowley D, Hynes RO. Multiple cardiovascular defects caused by the absence of alternatively spliced segments of fibronectin. Dev. Biol. 2007;311:11–24. doi: 10.1016/j.ydbio.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fassler R, Meyer M. Consequences of lack of beta1 integrin gene expression in mice. Genes Dev. 1995;9:1896–1908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- 51.Stephens LE, et al. Deletion of beta1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9:1883–1895. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- 52.Tanjore H, Zeisberg EM, Gerami-Naini B, Kalluri R. Beta1 integrin expression on endothelial cells is required for angiogenesis but not for vasculogenesis. Dev. Dyn. 2007;237:75–82. doi: 10.1002/dvdy.21385. [DOI] [PubMed] [Google Scholar]
- 53.Bloch W, et al. Beta1 integrin is essential for teratoma growth and angiogenesis. J. Cell Biol. 1997;139:265–278. doi: 10.1083/jcb.139.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muether PS, et al. The role of integrin alpha5beta1 in the regulation of corneal neovascularization. Exp. Eye Res. 2007;85:356–365. doi: 10.1016/j.exer.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 55.Boudreau NJ, Varner JA. The homeobox transcription factor Hox D3 promotes integrin alpha5beta1 expression and function during angiogenesis. J. Biol. Chem. 2004;279:4862–4868. doi: 10.1074/jbc.M305190200. [DOI] [PubMed] [Google Scholar]
- 56.Umeda N, et al. Suppression and regression of choroidal neovascularization by systemic administration of an alpha5beta1 integrin antagonist. Mol. Pharmacol. 2006;69:1820–1828. doi: 10.1124/mol.105.020941. [DOI] [PubMed] [Google Scholar]
- 57.Kim S, Harris M, Varner JA. Regulation of integrin alphavbeta 3-mediated endothelial cell migration and angiogenesis by integrin alpha5beta1 and protein kinase A. J. Biol. Chem. 2000;275:33920–33928. doi: 10.1074/jbc.M003668200. [DOI] [PubMed] [Google Scholar]
- 58.Kim S, Bakre M, Yin H, Varner JA. Inhibition of endothelial cell survival and angiogenesis by protein kinase A. J. Clin. Invest. 2002;110:933–941. doi: 10.1172/JCI14268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang JT, Rayburn H, Hynes RO. Embryonic mesodermal defects in alpha5 integrin-deficient mice. Development. 1993;119:1093–1105. doi: 10.1242/dev.119.4.1093.This article shows that integrin α5 is required during embryonic development of early blood vessels and other tissues.
- 60.Taverna D, Hynes RO. Reduced blood vessel formation and tumor growth in alpha5-integrin-negative teratocarcinomas and embryoid bodies. Cancer Res. 2001;61:5255–5261. [PubMed] [Google Scholar]
- 61.Francis SE, et al. Central roles of alpha5beta1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arterioscler. Thromb. Vasc. Biol. 2002;22:927–933. doi: 10.1161/01.atv.0000016045.93313.f2. [DOI] [PubMed] [Google Scholar]
- 62.Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development. 1995;121:549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]
- 63.Garmy-Susini B, et al. Integrin alpha4beta1-VCAM-1-mediated adhesion between endothelial and mural cells is required for blood vessel maturation. J. Clin. Invest. 2005;115:1542–1551. doi: 10.1172/JCI23445.This article demonstrates that integrin α4β1 on endothelium promotes endothelial cell motility and angiogenesis as well as a transient association of pericytes with endothelium.
- 64.Vlahakis NE, et al. Integrin alpha9beta1 directly binds to vascular endothelial growth factor (VEGF)-A and contributes to VEGF-A-induced angiogenesis. J. Biol. Chem. 2007;282:15187–15196. doi: 10.1074/jbc.M609323200. [DOI] [PubMed] [Google Scholar]
- 65.Staniszewska I, et al. Interaction of alpha9beta1 integrin with thrombospondin-1 promotes angiogenesis. Circ. Res. 2007;100:1308–1316. doi: 10.1161/01.RES.0000266662.98355.66. [DOI] [PubMed] [Google Scholar]
- 66.Liao YF, et al. The EIIIA segment of fibronectin is a ligand for integrins alpha 9beta 1 and alpha 4beta 1 providing a novel mechanism for regulating cell adhesion by alternative splicing. J. Biol. Chem. 2002;277:14467–14474. doi: 10.1074/jbc.M201100200. [DOI] [PubMed] [Google Scholar]
- 67.Marcinkiewicz C, et al. Inhibitory effects of MLDG-containing heterodimeric disintegrins reveal distinct structural requirements for interaction of the integrin alpha 9beta 1 with VCAM-1, tenascin-C, and osteopontin. J. Biol. Chem. 2000;275:31930–31937. doi: 10.1074/jbc.M003209200. [DOI] [PubMed] [Google Scholar]
- 68.Huang XZ, et al. Fatal bilateral chylothorax in mice lacking the integrin alpha9beta1. Mol. Cell. Biol. 2000;20:5208–5215. doi: 10.1128/mcb.20.14.5208-5215.2000.This study demonstrates that integrin α9β1 is required for proper development of the lymphatic system.
- 69.Senger DR, et al. Angiogenesis promoted by vascular endothelial growth factor: regulation through alpha1beta1 and alpha2beta1 integrins. Proc. Natl. Acad. Sci. U. S. A. 1997;94:13612–13617. doi: 10.1073/pnas.94.25.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pozzi A, et al. Elevated matrix metalloprotease and angiostatin levels in integrin alpha 1 knockout mice cause reduced tumor vascularization. Proc. Natl. Acad. Sci. U. S. A. 2000;97:2202–2207. doi: 10.1073/pnas.040378497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Z, et al. {alpha}2{beta}1 integrin expression in the tumor microenvironment enhances tumor angiogenesis in a tumor-cell specific manner. Blood. 2008;111:1980–1988. doi: 10.1182/blood-2007-06-094680.This paper shows that integrin α2β1 null mice exhibit distinct tumor growth patterns that are dependent upon the growth factors that are intrinsically expressed by individual tumor cells.
- 72.Lee TH, et al. Integrin regulation by vascular endothelial growth factor in human brain microvascular endothelial cells: role of alpha6beta1 integrin in angiogenesis. J. Biol. Chem. 2006;281:40450–40460. doi: 10.1074/jbc.M607525200. [DOI] [PubMed] [Google Scholar]
- 73.Nikolopoulos SN, Blaikie P, Yoshioka T, Guo W, Giancotti FG. Integrin beta4 signaling promotes tumor angiogenesis. Cancer Cell. 2004;6:471–483. doi: 10.1016/j.ccr.2004.09.029.This paper shows the important role of integrin α6β4 in angiogenesis.
- 74.van der Neut R, Krimpenfort P, Calafat J, Niessen CM, Sonnenberg A. Epithelial detachment due to absence of hemidesmosomes in integrin beta 4 null mice. Nat. Genet. 1996;13:366–369. doi: 10.1038/ng0796-366. [DOI] [PubMed] [Google Scholar]
- 75.Georges-Labouesse E, et al. Absence of integrin alpha 6 leads to epidermolysis bullosa and neonatal death in mice. Nat. Genet. 1996;13:370–373. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- 76.Dowling J, Yu QC, Fuchs E. Beta4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J. Cell Biol. 1996;134:559–572. doi: 10.1083/jcb.134.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dans M, et al. Tyrosine phosphorylation of the beta 4 integrin cytoplasmic domain mediates Shc signaling to extracellular signal-regulated kinase and antagonizes formation of hemidesmosomes. J. Biol. Chem. 2001;276:1494–502. doi: 10.1074/jbc.M008663200. [DOI] [PubMed] [Google Scholar]
- 78.Leu SJ, et al. Identification of a novel integrin alpha 6 beta 1 binding site in the angiogenic inducer CCN1 (CYR61) J. Biol. Chem. 2003;278:33801–33808. doi: 10.1074/jbc.M305862200. [DOI] [PubMed] [Google Scholar]
- 79.Jin H, et al. A homing mechanism for bone marrow-derived progenitor cell recruitment to the neovasculature. J. Clin. Invest. 2006;116:652–662. doi: 10.1172/JCI24751.This paper shows that integrin α4β1 on bone marrow derived cells promotes monocyte and endothelial precursor cell homing to tumors.
- 80.Jin H, Su J, Garmy-Susini B, Kleeman J, Varner J. Integrin alpha 4 beta 1 promotes monocyte trafficking and angiogenesis in tumors. Cancer Res. 2006;66:2146–2152. doi: 10.1158/0008-5472.CAN-05-2704. [DOI] [PubMed] [Google Scholar]
- 81.Wu Y, et al. Essential role of ICAM-1/CD18 in mediating EPC recruitment, angiogenesis, and repair to the infarcted myocardium. Circ. Res. 2006;99:315–322. doi: 10.1161/01.RES.0000235986.35957.a3. [DOI] [PubMed] [Google Scholar]
- 82.Chavakis E, et al. Role of beta2-integrins for homing and neovascularization capacity of endothelial progenitor cells. J. Exp. Med. 2005;1:63–72. doi: 10.1084/jem.20041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Varner JA. The sticky truth about angiogenesis and thrombospondins. J. Clin. Invest. 2006;116:3111–3113. doi: 10.1172/JCI30685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kopp HG, Rafii S. Thrombopoietic cells and the bone marrow vascular niche. Ann. N .Y. Acad Sci. 2007;1106:175–179. doi: 10.1196/annals.1392.004. [DOI] [PubMed] [Google Scholar]
- 85.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 86.Banerji S, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J. Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Breiteneder-Geleff S, et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am. J. Pathol. 1999;154:385–394. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mishima K, et al. Prox1 induces lymphatic endothelial differentiation via integrin alpha9 and other signaling cascades. Mol. Biol. Cell. 2007;18:1421–1429. doi: 10.1091/mbc.E06-09-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vlahakis NE, Young BA, Atakilit A, Sheppard D. The lymphangiogenic vascular endothelial growth factors VEGF-C and -D are ligands for the integrin alpha9beta1. J. Biol. Chem. 2005;280:4544–4552. doi: 10.1074/jbc.M412816200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hong YK, et al. VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the alpha1beta1 and alpha2beta1 integrins. FASEB J. 2004;18:1111–1113. doi: 10.1096/fj.03-1179fje.This article established the role of integrins α1β1 and α2β1 in lymphangiogenesis.
- 91.Dietrich T, et al. Inhibition of inflammatory lymphangiogenesis by integrin alpha5 blockade. Am. J. Pathol. 2007;171:361–372. doi: 10.2353/ajpath.2007.060896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Garmy-Susini B, Makale M, Fuster M, Varner JA. Methods to study lymphatic vessel integrins. Methods Enzymol. 2007;426:415–438. doi: 10.1016/S0076-6879(07)26018-5. [DOI] [PubMed] [Google Scholar]
- 93.Gutheil JC, et al. Targeted antiangiogenic therapy for cancer using Vitaxin: a humanized monoclonal antibody to the integrin alphavbeta3. Clin. Cancer Res. 2000;6:3056–3061. [PubMed] [Google Scholar]
- 94.McNeel DG, et al. Phase I trial of a monoclonal antibody specific for alphavbeta3 integrin (MEDI-522) in patients with advanced malignancies, including an assessment of effect on tumor perfusion. Clin Cancer Res. 2005;11:7851–7860. doi: 10.1158/1078-0432.CCR-05-0262. [DOI] [PubMed] [Google Scholar]
- 95.Hersey P, et al. A phase II, randomized, open-label study evaluating the antitumor activity of MEDI-522, a humanized monoclonal antibody directed against the human alpha v beta 3 (αvβ3) integrin, ± dacarbazine (DTIC) in patients with metastatic melanoma. Journal of Clinical Oncology, 2005 ASCO Annual Meeting Proceedings Part I. 2005;23:7507. [Google Scholar]
- 96.Zhang D, Pier T, McNeel DG, Wilding G, Friedl A. Effects of a monoclonal anti-alphavbeta3 integrin antibody on blood vessels - a pharmacodynamic study. Invest New Drugs. 2007;25:49–55. doi: 10.1007/s10637-006-9013-8. [DOI] [PubMed] [Google Scholar]
- 97.Trikha M, et al. CNTO 95, a fully human monoclonal antibody that inhibits alphav integrins, has antitumor and antiangiogenic activity in vivo. Int. J. Cancer. 2004;110:326–335. doi: 10.1002/ijc.20116. [DOI] [PubMed] [Google Scholar]
- 98.Martin PL, et al. Absence of adverse effects in cynomolgus macaques treated with CNTO 95, a fully human anti-alphav integrin monoclonal antibody, despite widespread tissue binding. Clin. Cancer Res. 2005;11:6959–6965. doi: 10.1158/1078-0432.CCR-04-2623. [DOI] [PubMed] [Google Scholar]
- 99.Mullamitha SA, et al. Phase I evaluation of a fully human anti-alphav integrin monoclonal antibody (CNTO 95) in patients with advanced solid tumors. Clin. Cancer Res. 2007;13:2128–2135. doi: 10.1158/1078-0432.CCR-06-2779. [DOI] [PubMed] [Google Scholar]
- 100.Brooks PC, et al. Insulin-like growth factor receptor cooperates with integrin alpha v beta 5 to promote tumor cell dissemination in vivo. J. Clin. Invest. 1997;99:1390–1398. doi: 10.1172/JCI119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nabors LB, et al. Phase I and correlative biology study of cilengitide in patients with recurrent malignant glioma. J. Clin. Oncol. 2007;25:1651–1657. doi: 10.1200/JCO.2006.06.6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. www.bio-medicine.org/medicine-technology/Merck-at-ASCO-2007.
- 103.Albert JM, et al. Integrin alpha v beta 3 antagonist Cilengitide enhances efficacy of radiotherapy in endothelial cell and non-small-cell lung cancer models. Int. J. Radiat. Oncol. Biol. Phys. 2006;65:1536–1543. doi: 10.1016/j.ijrobp.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 104.Friess H, et al. A randomized multi-center phase II trial of the angiogenesis inhibitor Cilengitide (EMD 121974) and gemcitabine compared with gemcitabine alone in advanced unresectable pancreatic cancer. BMC Cancer. 2006;6:285. doi: 10.1186/1471-2407-6-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Beekman KW, et al. Phase II evaluations of cilengitide in asymptomatic patients with androgen-independent prostate cancer: scientific rationale and study design. Clin. Genitourin Cancer. 2006;4:299–302. doi: 10.3816/CGC.2006.n.012. [DOI] [PubMed] [Google Scholar]
- 106.Bradley DA, et al. EMD121974 (NSC 707544, cilengitide) in asymptomatic metastatic androgen independent prostate cancer (AIPCa) patients (pts): A randomized trial by the Prostate Cancer Clinical Trials Consortium (NCI 6372) Journal of Clinical Oncology, 2007 ASCO Annual Meeting Proceedings Part I. 2007;25:5137. [Google Scholar]
- 107.Figlin RA, Kondagunta GV, Yazji S, Motzer RJ, Bukowski RM. Phase II study of volociximab (M200), an {alpha}5ß1 anti-integrin antibody in refractory metastatic clear cell renal cell cancer (RCC) Journal of Clinical Oncology, ASCO Annual Meeting Proceedings. 2006;24:4535. [Google Scholar]
- 108.Kuwada SK. Drug evaluation: Volociximab, an angiogenesis-inhibiting chimeric monoclonal antibody. Curr. Opin. Mol. Ther. 2007;9:92–98. [PubMed] [Google Scholar]
- 109.Stoeltzing O, et al. Inhibition of integrin alpha5beta1 function with a small peptide (ATN-161) plus continuous 5-FU infusion reduces colorectal liver metastases and improves survival in mice. Int. J. Cancer. 2003;104:496–503. doi: 10.1002/ijc.10958. [DOI] [PubMed] [Google Scholar]
- 110.Cianfrocca ME, et al. Phase 1 trial of the antiangiogenic peptide ATN-161 (Ac-PHSCN-NH(2)), a beta integrin antagonist, in patients with solid tumours. Br. J. Cancer. 2006;94:1621–1626. doi: 10.1038/sj.bjc.6603171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lim EH, Danth N, Bednarski M, Li KC. A review: Integrin alphavbeta3-targeted molecular imaging and therapy in angiogenesis. Nanomedicine. 2005;1:110–114. doi: 10.1016/j.nano.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 112.Garanger E, Boturyn D, Dumy P. Tumor targeting with RGD peptide ligands-design of new molecular conjugates for imaging and therapy of cancers. Anticancer Agents Med. Chem. 2007;7:552–558. doi: 10.2174/187152007781668706. [DOI] [PubMed] [Google Scholar]
- 113.Sipkins DA, et al. Detection of tumor angiogenesis in vivo by alphaVbeta3-targeted magnetic resonance imaging. Nat. Med. 1998;4:623–626. doi: 10.1038/nm0598-623. [DOI] [PubMed] [Google Scholar]
- 114.Leong-Poi H, et al. Noninvasive assessment of angiogenesis by ultrasound and microbubbles targeted to alpha(v)-integrins. Circulation. 2003;107:455–460. doi: 10.1161/01.cir.0000044916.05919.8b. [DOI] [PubMed] [Google Scholar]
- 115.Hood JD, et al. Tumor regression by targeted gene delivery to the neovasculature. Science. 2002;296:2404–2407. doi: 10.1126/science.1070200.This landmark paper established the potential of integrin targeted nanoparticles in cancer therapy.
- 116.Arnaout MA, Mahalingam B, Xiong JP. Integrin structure, allostery, and bidirectional signaling. Annu. Rev. Dev. Biol. 2005;21:381–410. doi: 10.1146/annurev.cellbio.21.090704.151217. [DOI] [PubMed] [Google Scholar]
- 117.Lu C, Takagi J, Springer TA. Association of the membrane proximal regions of the alpha and beta subunit cytoplasmic domains constrains an integrin in the inactive state. J. Biol. Chem. 2001;276:14642–14648. doi: 10.1074/jbc.M100600200. [DOI] [PubMed] [Google Scholar]
- 118.Beglova N, Blacklow SC, Takagi J, Springer TA. Cysteine-rich module structure reveals a fulcrum for integrin rearrangement upon activation. Nat. Struct. Biol. 2002;9:282–287. doi: 10.1038/nsb779. [DOI] [PubMed] [Google Scholar]
- 119.Vinogradova O, et al. A structural mechanism of integrin alpha(IIb)beta(3) “inside-out” activation as regulated by its cytoplasmic face. Cell. 2002;110:587–597. doi: 10.1016/s0092-8674(02)00906-6. [DOI] [PubMed] [Google Scholar]
- 120.Grabovsky V, et al. Subsecond induction of alpha4 integrin clustering by immobilized chemokines stimulates leukocyte tethering and rolling on endothelial vascular cell adhesion molecule 1 under flow conditions. J. Exp. Med. 2000;192:495–506. doi: 10.1084/jem.192.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.O’Toole TE, et al. Integrin cytoplasmic domains mediate inside-out signal transduction. J. Cell Biol. 1994;124:1047–1059. doi: 10.1083/jcb.124.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Peng X, et al. Overexpression of focal adhesion kinase in vascular endothelial cells promotes angiogenesis in transgenic mice. Cardiovasc. Res. 2004;64:421–430. doi: 10.1016/j.cardiores.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 123.Shen TL, et al. Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis. J. Cell Biol. 2005;169:941–952. doi: 10.1083/jcb.200411155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lai KM, Pawson T. The ShcA phosphotyrosine docking protein sensitizes cardiovascular signaling in the mouse embryo. Genes Dev. 2000;14:1132–1145. [PMC free article] [PubMed] [Google Scholar]
- 125.Hüser M, et al. MEK kinase activity is not necessary for Raf-1 function. EMBO J. 2001;20:1940–1951. doi: 10.1093/emboj/20.8.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lin EY, et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 128.Lin EY, Pollard JW. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 2007;67:5064–5066. doi: 10.1158/0008-5472.CAN-07-0912. [DOI] [PubMed] [Google Scholar]
- 129.Bolat F, et al. Microvessel density, VEGF expression, and tumor-associated macrophages in breast tumors: correlations with prognostic parameters. J. Exp. Clin. Cancer Res. 2006;25:365–372. [PubMed] [Google Scholar]
- 130.Tsutsui S, et al. Macrophage infiltration and its prognostic implications in breast cancer: the relationship with VEGF expression and microvessel density. Oncol. Rep. 2005;14:425–431. [PubMed] [Google Scholar]
- 131.Valković T, et al. Correlation between vascular endothelial growth factor, angiogenesis, and tumor-associated macrophages in invasive ductal breast carcinoma. Virchows Arch. 2002;440:583–588. doi: 10.1007/s004280100458. [DOI] [PubMed] [Google Scholar]
- 132.Esposito I, et al. Inflammatory cells contribute to the generation of an angiogenic phenotype in pancreatic ductal adenocarcinomas. J. Clin. Pathol. 2004;6:630–636. doi: 10.1136/jcp.2003.014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Leek RD, Harris AL. Tumor-associated macrophages in breast cancer. J. Mammary Gland Biol. Neoplasia. 2002;2:177–189. doi: 10.1023/a:1020304003704. [DOI] [PubMed] [Google Scholar]
- 134.Nishie A. Macrophage infiltration and heme oxygenase-1 expression correlate with angiogenesis in human gliomas. Clin. Cancer Res. 1999;5:1107–1113. [PubMed] [Google Scholar]
- 135.Yamashiro S, et al. Tumor-derived monocyte chemoattractant protein-1 induces intratumoral infiltration of monocyte-derived macrophage subpopulation in transplanted rat tumors. Am. J. Pathol. 1994;4:856–867. [PMC free article] [PubMed] [Google Scholar]
- 136.Lewis JS, et al. Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J. Pathol. 2000;2:150–158. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH687>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 137.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J. Clin. Invest. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jodele S, et al. The contribution of bone marrow-derived cells to the tumor vasculature in neuroblastoma is matrix metalloproteinase-9 dependent. Cancer Res. 2005;65:3200–3208. doi: 10.1158/0008-5472.CAN-04-3770. [DOI] [PubMed] [Google Scholar]
- 139.Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J. Clin. Invest. 2004;5:623–633. doi: 10.1172/JCI22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ueno T, et al. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin. Cancer Res. 2000;8:3282–3289. [PubMed] [Google Scholar]
- 141.Niwa Y, et al. Correlation of tissue and plasma RANTES levels with disease course in patients with breast or cervical cancer. Clin. Cancer Res. 2001;2:285–289. [PubMed] [Google Scholar]
- 142.Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;8:2224–2234. doi: 10.1182/blood-2004-03-1109. [DOI] [PubMed] [Google Scholar]
- 143.Gerszten RE, et al. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–723. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 144.Goswami S, et al. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005;12:5278–5283. doi: 10.1158/0008-5472.CAN-04-1853. [DOI] [PubMed] [Google Scholar]
- 145.Luo JL, et al. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature. 2007;446:690–694. doi: 10.1038/nature05656. [DOI] [PubMed] [Google Scholar]
- 146.Greten FR, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 147.Schafer ZT, Brugge JS. IL-6 involvement in epithelial cancers. J. Clin. Invest. 2007;117:3660–3663. doi: 10.1172/JCI34237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sansone P, et al. Il-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J. Clin. Invest. 2007;117:3988–4002. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gao SP, et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas J. Clin. Invest. 2007;117:3846–3856. doi: 10.1172/JCI31871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J. Exp. Med. 2001;6:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lu H, et al. LFA-1 is sufficient in mediating neutrophil emigration in Mac-1-deficient mice. J. Clin. Invest. 1997;99:1340–1350. doi: 10.1172/JCI119293. [DOI] [PMC free article] [PubMed] [Google Scholar]