Abstract
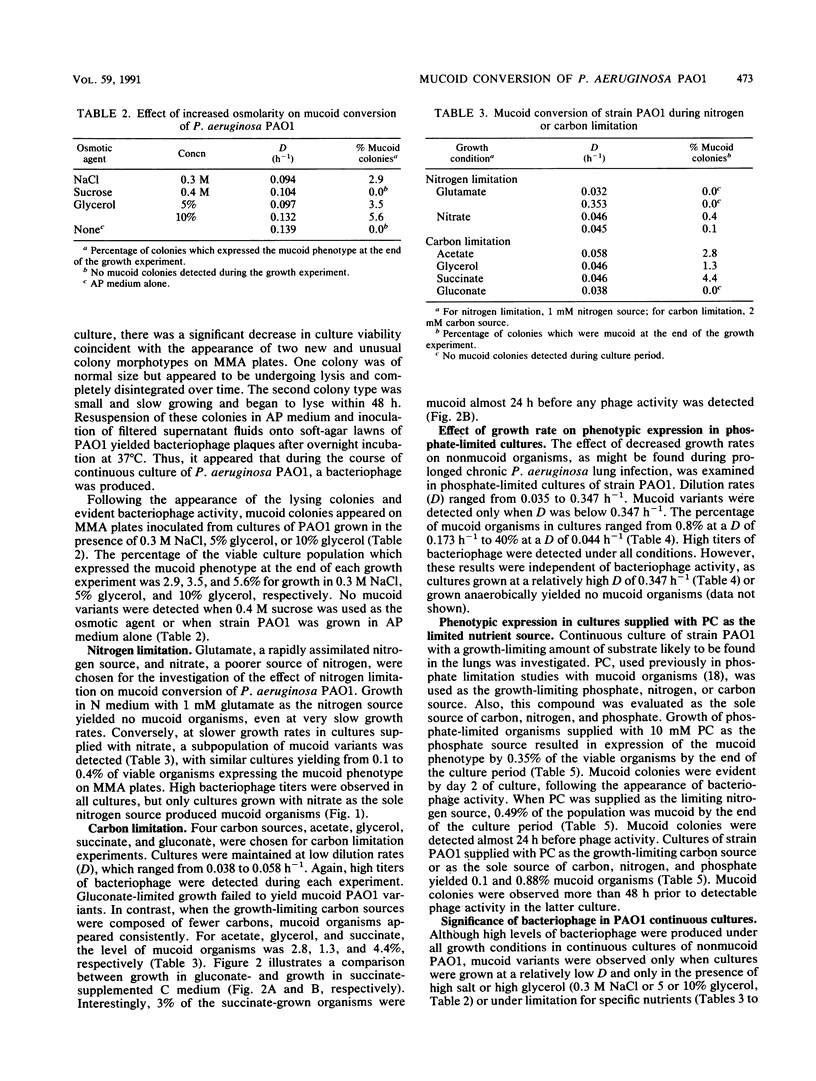
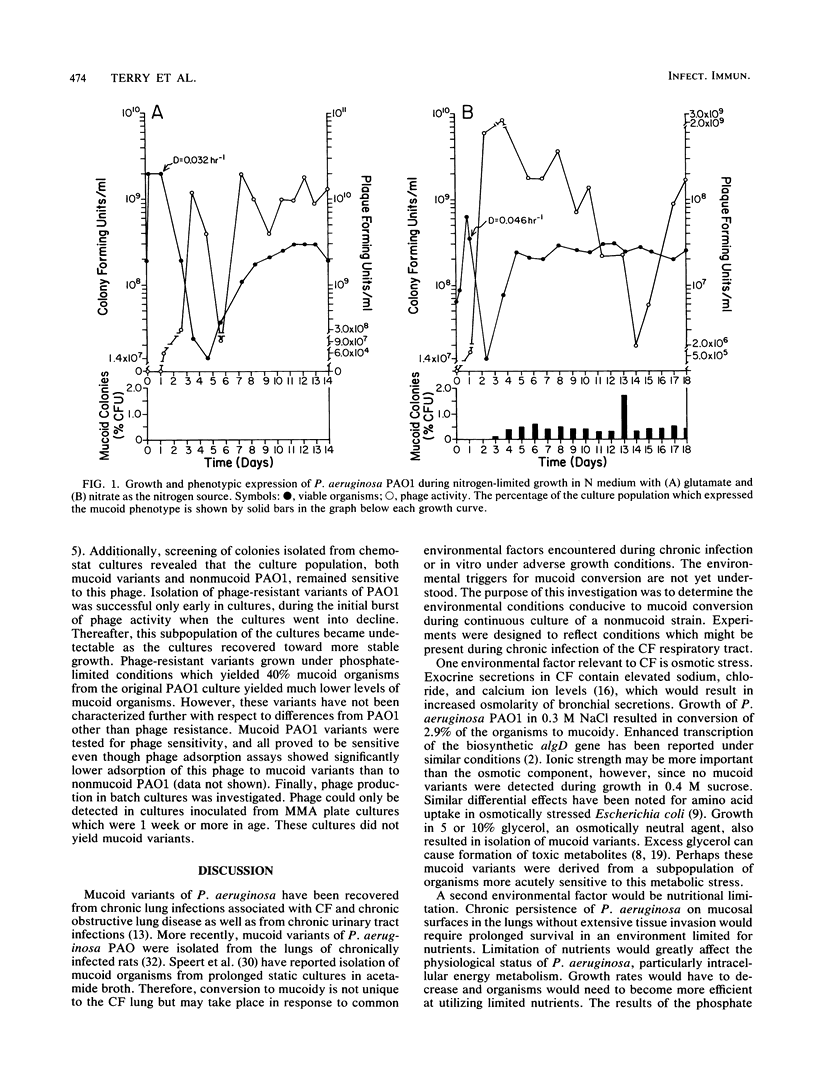
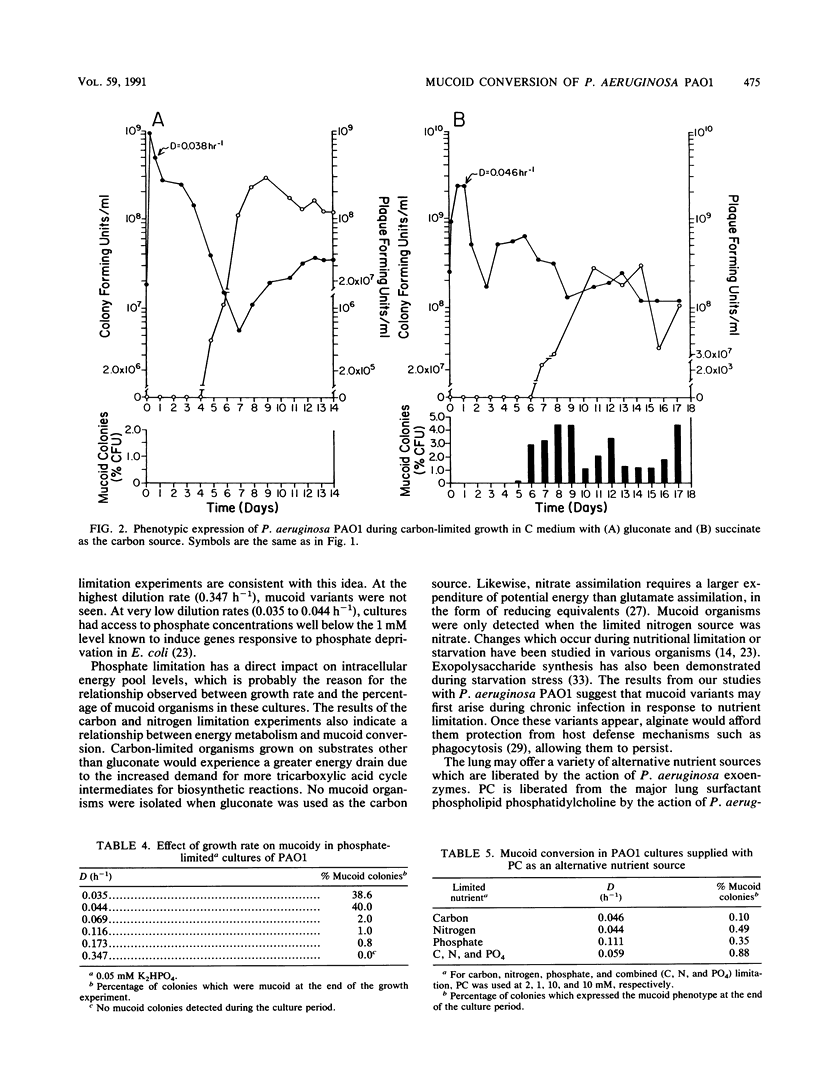
Growth and conversion to the mucoid phenotype by nonmucoid Pseudomonas aeruginosa PAO1 was studied in a chemostat system under conditions designed to reflect those likely to be present during chronic infection in the lung in cystic fibrosis patients. Mucoid variants were consistently isolated during continuous culture in the presence of 0.3 M NaCl or 5 or 10% glycerol. Mucoid subpopulations were also detected under conditions of carbon, nitrogen, or phosphate limitation. During carbon or nitrogen limitation, mucoid conversion was dependent upon the choice of substrate. Phosphate-limited cultures exhibited an inverse relationship between culture growth rate and number of mucoid organisms detected. Mucoid variants were not detected when dilution rates (D) exceeded 0.173 h-1. Conversely, at a D of 0.044 h-1, 40% of the population expressed the mucoid phenotype. Phosphorylcholine, a product of phospholipase C activity on the major lung surfactant phosphatidylcholine, was also used as a growth substrate in nutrient limitation studies. Under all conditions, growth of PAO1 supplied with phosphorylcholine resulted in isolation of mucoid variants, indicating that the lung may provide at least one nutrient source conducive to mucoid conversion. Continuous culture also resulted in detection of a phage associated with strain PAO1. High titers of phage were present under all conditions, including those which yielded no mucoid organisms, suggesting that environmental conditions rather than the phage regulated the appearance of mucoid variants.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berka R. M., Gray G. L., Vasil M. L. Studies of phospholipase C (heat-labile hemolysin) in Pseudomonas aeruginosa. Infect Immun. 1981 Dec;34(3):1071–1074. doi: 10.1128/iai.34.3.1071-1074.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry A., DeVault J. D., Chakrabarty A. M. High osmolarity is a signal for enhanced algD transcription in mucoid and nonmucoid Pseudomonas aeruginosa strains. J Bacteriol. 1989 May;171(5):2312–2317. doi: 10.1128/jb.171.5.2312-2317.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzins A., Chakrabarty A. M. Cloning of genes controlling alginate biosynthesis from a mucoid cystic fibrosis isolate of Pseudomonas aeruginosa. J Bacteriol. 1984 Jul;159(1):9–18. doi: 10.1128/jb.159.1.9-18.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V., Dikshit R., Konyecsni W. M., Chakrabarty A. M., Misra T. K. The algR gene, which regulates mucoidy in Pseudomonas aeruginosa, belongs to a class of environmentally responsive genes. J Bacteriol. 1989 Mar;171(3):1278–1283. doi: 10.1128/jb.171.3.1278-1283.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V., Gill J. F., Chakrabarty A. M. Gene algD coding for GDPmannose dehydrogenase is transcriptionally activated in mucoid Pseudomonas aeruginosa. J Bacteriol. 1987 Jan;169(1):351–358. doi: 10.1128/jb.169.1.351-358.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L. R., Linker A. Production and characterization of the slime polysaccharide of Pseudomonas aeruginosa. J Bacteriol. 1973 Nov;116(2):915–924. doi: 10.1128/jb.116.2.915-924.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedberg W. B., Kistler W. S., Lin E. C. Lethal synthesis of methylglyoxal by Escherichia coli during unregulated glycerol metabolism. J Bacteriol. 1971 Oct;108(1):137–144. doi: 10.1128/jb.108.1.137-144.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring K., Hofnung M., Nikaido H. Stimulation of glutamine transport by osmotic stress in Escherichia coli K-12. J Bacteriol. 1990 Aug;172(8):4741–4743. doi: 10.1128/jb.172.8.4741-4743.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George R. H. Pseudomonas infections in cystic fibrosis. Arch Dis Child. 1987 May;62(5):438–439. doi: 10.1136/adc.62.5.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. B., Ohman D. E. Cloning and expression in Pseudomonas aeruginosa of a gene involved in the production of alginate. J Bacteriol. 1984 Jun;158(3):1115–1121. doi: 10.1128/jb.158.3.1115-1121.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. B., Ohman D. E. Construction and characterization of Pseudomonas aeruginosa algB mutants: role of algB in high-level production of alginate. J Bacteriol. 1987 Apr;169(4):1593–1602. doi: 10.1128/jb.169.4.1593-1602.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder W., Dijkhuizen L. Physiological responses to nutrient limitation. Annu Rev Microbiol. 1983;37:1–23. doi: 10.1146/annurev.mi.37.100183.000245. [DOI] [PubMed] [Google Scholar]
- Hoiby N. Epidemiological investigations of the respiratory tract bacteriology in patients with cystic fibrosis. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Aug;82(4):541–550. [PubMed] [Google Scholar]
- Kilbourn J. P. Bacterial content and ionic composition of sputum in cystic fibrosis. Lancet. 1978 Feb 11;1(8059):334–334. doi: 10.1016/s0140-6736(78)90111-3. [DOI] [PubMed] [Google Scholar]
- Krieg D. P., Bass J. A., Mattingly S. J. Aeration selects for mucoid phenotype of Pseudomonas aeruginosa. J Clin Microbiol. 1986 Dec;24(6):986–990. doi: 10.1128/jcm.24.6.986-990.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg D. P., Bass J. A., Mattingly S. J. Phosphorylcholine stimulates capsule formation of phosphate-limited mucoid Pseudomonas aeruginosa. Infect Immun. 1988 Apr;56(4):864–873. doi: 10.1128/iai.56.4.864-873.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E. C. Glycerol dissimilation and its regulation in bacteria. Annu Rev Microbiol. 1976;30:535–578. doi: 10.1146/annurev.mi.30.100176.002535. [DOI] [PubMed] [Google Scholar]
- Marcus H., Baker N. R. Quantitation of adherence of mucoid and nonmucoid Pseudomonas aeruginosa to hamster tracheal epithelium. Infect Immun. 1985 Mar;47(3):723–729. doi: 10.1128/iai.47.3.723-729.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. R. Mucoid variation in Pseudomonas aeruginosa induced by the action of phage. J Med Microbiol. 1973 Feb;6(1):111–118. doi: 10.1099/00222615-6-1-111. [DOI] [PubMed] [Google Scholar]
- Matin A., Auger E. A., Blum P. H., Schultz J. E. Genetic basis of starvation survival in nondifferentiating bacteria. Annu Rev Microbiol. 1989;43:293–316. doi: 10.1146/annurev.mi.43.100189.001453. [DOI] [PubMed] [Google Scholar]
- Mian F. A., Jarman T. R., Righelato R. C. Biosynthesis of exopolysaccharide by Pseudomonas aeruginosa. J Bacteriol. 1978 May;134(2):418–422. doi: 10.1128/jb.134.2.418-422.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. V., Rubero V. J. Mucoid conversion by phages of Pseudomonas aeruginosa strains from patients with cystic fibrosis. J Clin Microbiol. 1984 May;19(5):717–719. doi: 10.1128/jcm.19.5.717-719.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Pier G. B. Role of Pseudomonas aeruginosa mucoid exopolysaccharide in adherence to tracheal cells. Infect Immun. 1985 Jan;47(1):1–4. doi: 10.1128/iai.47.1.1-4.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzmann S., Boring J. R. Antiphagocytic Effect of Slime from a Mucoid Strain of Pseudomonas aeruginosa. Infect Immun. 1971 Jun;3(6):762–767. doi: 10.1128/iai.3.6.762-767.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speert D. P., Farmer S. W., Campbell M. E., Musser J. M., Selander R. K., Kuo S. Conversion of Pseudomonas aeruginosa to the phenotype characteristic of strains from patients with cystic fibrosis. J Clin Microbiol. 1990 Feb;28(2):188–194. doi: 10.1128/jcm.28.2.188-194.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hartingsveldt J., Marinus M. G., Stouthamer A. H. Mutants of Pseudomonas aeruginosa bblocked in nitrate or nitrite dissimilation. Genetics. 1971 Apr;67(4):469–482. doi: 10.1093/genetics/67.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMANAKA T., OTA A., OKUNUKI K. A nitrite reducing system reconstructed with purified cytochrome components of Pseudomonas aeruginosa. Biochim Biophys Acta. 1961 Oct 28;53:294–308. doi: 10.1016/0006-3002(61)90442-5. [DOI] [PubMed] [Google Scholar]


