Abstract
Autoreactive B cells play a central role in systemic lupus erythematosus (SLE). Characterization of DNA-reactive B cells in the blood of lupus patients has been limited by the low frequency of the population. Using a tetrameric configuration of a peptide mimetope of DNA, we identified peptide-reactive B cells in peripheral blood. Antibodies derived from these B cells bound to peptide and were largely cross-reactive to dsDNA. This methodology enables us to track the development of autoreactive B cells, which recognize peptide and dsDNA, in individual patients with SLE and permits the isolation of autoreactive B cells for further characterization.
Keywords: lupus, B cells, immunoglobulins, peptide
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by the production of a wide variety of autotantibodies, many of which react with nuclear antigens. In particular, autoantibodies against dsDNA are essentially diagnostic of the disease, displaying changes in titer that correlate with disease activity and contributing to lupus nephritis (Vlahakos et al., 1992; Ehrenstein et al., 1995). However, given the rather low frequency of autoreactive B cells it has been difficult to isolate and investigate their functional and molecular properties.
Various transgenic mouse models have been generated to obtain an enhanced number of transgenic autoreactive B cells. In combination with antibodies targetting a specific idiotype, it has been possible to study the effects of different parameters, such as genetic background, hormones and antigen exposure, on the devolpment of autoreactive B cells. However, it has been demonstrated that the fate of the self-reactive repertoire is affected by competition with non-self reactive cells and the maturation of self-reactive B cells is altered in the absence of competition in these transgenic mice (Cyster et al., 1994). In addition, it remains to be elucidated if experiences obtained from autoimmune mouse models are also relevant in patients with SLE.
Human hybridomas and Epstein-Barr virus transformed human B cells have been previously used to generate human antibodies. These techniques are low efficiency and involve a selection bias; therefore, they are unable to permit an analysis of the frequency of autoreactive B cells in individual B cell subsets, although they have permitted the molecular characterization of particular autoantibodies. In recent years, a new strategy has been developed to sample large numbers of antibodies from human peripherl blood B cells. Immunoglobulin (Ig) heavy and light chain genes derived from individual B cells of specific B cell populations are amplified by single cell RT-PCR and expressed in vitro. These expressed human antibodies can be tested for specificity against self and non-self antigens. Based on this new technique, an assessment of the percentage of self- or poly-reactive B cells in early B cell populations has revealed two tolerance checkpoints. In a study of a small number of lupus patients, a breach in the tolerance checkpoint at the transitional to naïve B cell junction could be demonstrated. Although this method allows us to directly analyze the frequency of autoreactive B cells, it would be useful to establish a more convenient and economical method which could be used to enumerate autoreactive B cells in specific B cell subsets in the routine analysis of large numbers of patients.
Our laboratory has previously reported that immunization of BALB/c mice with an octameric form of the peptide DWEYSVWLSN (MAP-peptide) in which DWEYS is a mimetope of dsDNA, results in the production of pathogenic IgG anti-dsDNA antibodies, glomerular immunoglobulin deposition (Putterman and Diamond, 1998) and excitotoxic neuronal loss following a breach in the blood-brain barrier (Huerta et al., 2006; Kowal et al., 2006). To track the peptide-reactive B cell population in this immune response, we generated a fluorochome–labeled tetrameric DWEYS peptide (DWEYS-tetramer), which has higher avidity than monomer for B cells with a peptide-reactive B cell receptor (BCR). Using this reagent, we identified peptide-reactive and dsDNA-cross-reactive B cells in immunized mice (Newman et al., 2003).
Our goal in this study was to test whether we could identify autoreactive B cells reactive to peptide and dsDNA in lupus patients by virtue of their binding to fluorochome-tagged tetramer. For this purpose, human monoclonal antibodies derived from isolated tetramer positive and negative B cells were expressed and analyzed for antigenic specificity using the above in vitro antibody expression methodology.
Materials and Methods
Tetramer generation
DWEYSVWLSN-streptavidin-allophycocyanin tetramers were generated by combining 30µl biotinylated peptide (650µM) (AnaSpec, San Jose, CA) with 90µl allophycocyanin-labeled streptavidin (6.1µM) (Molecular Probes, Eugene, OR). Each mixture was incubated at 4°C overnight. Subsequently, peptide–APC complexes were separated from free peptide by gel filtration using a Bio-Gel P-30 spin column (Bio Rad, Hercules, CA) (Newman et al., 2003).
Single cell sorting
Human blood samples were obtained from 3 female SLE patients with quiescent disease (SLEDAI<4) and one female healthy donor. Informed consent was obtained and the protocol received IRB approved from Columbia University. Patient M55 was 37 years old and on hydroxychloroquine and low dose prednisone. Patient C9 and C15 were 35 and 23 years old, respectively, and were both being treated with mycophenolate mofetil, hydroxychloroquine and prednisone. The female healthy donor was 33 years old with no known autoimmune disease. B cells were enriched by incubation with RosetteSep (Stem Cell Technology, Vancouver, BC Canada) followed by Ficoll-Paque Plus (GE Healthcare) gradient centrifugation. Enriched B cells were stained with anti–human CD19 (Invitrogen) and DWEYS-APC tetramers. 4', 6-Diamidino-2-phenylindole dihydrochloride (DAPI) (Molecular Probes) was used to identify and exclude dead cells. Individual CD19+DWEYS-tetramer+ and CD19+DWEYS-tetramer− B cells were sorted into 96-well PCR plates containing 4 µl of 0.5 × PBS, 10 mM DTT, 8 U RNAsin (Promega, Madison, WI) and 3 U Prime RNase Inhibitor (Eppendorf, Westbury, NY) using a FACSAria (Becton Dickinson). All samples were immediately frozen on dry ice and stored at −70°C.
cDNA Synthesis and RT-PCR
cDNA was synthesized in the original sort plates and Individual IgH (µ only) and IgL chain (kappa or lambda) gene rearrangements were amplified in two rounds of PCR (50 cycles each) before cloning into human Ig γ1, Igκ, or Igλ expression vectors using primers that include restriction sites as described previously (gift of M.C. Nussenzweig, Rockefeller University) (Wardemann et al., 2003). Analysis of the DNA sequences was performed using the BLAST program (www.ncbi.nlm.nih.gov/blast/) and JOINSOLVER program.
Antibody production
Antibodies were produced in vitro as described previously (Wardemann et al., 2003; Yurasov et al., 2005). In brief, human embryonic kidney fibroblast 293A cells were cultured in DMEM supplemented with 10% ultra-low IgG FCS and cotransfected with 12.5 µg/ml of IgH and IgL chain encoding plasmid DNA by calcium phosphate precipitation. Cells were washed with serum-free DMEM 6 hours after transfection and thereafter cultured in DMEM supplemented with 1% Nutridoma SP (Roche, Indianapolis, IN). Supernatants were collected after 6 days of culture.
ELISAs
Antibody concentrations in supernatants were determined by using human IgG1-kappa or lambda as standards (Southern Biotechiology, Birmingham, AL). The capture antibody and detection antibody were unlabelled goat anti-human IgG and alkaline phosphatase conjugated goat anti-human kappa or lambda, respectively. The ELISA assay for dsDNA-binding was performed as described (Putterman and Diamond, 1998). Briefly calf thymus dsDNA, 25 µl per well at 100 µg/ml was adsorbed to 96-well half area plates (Corning Life Science, Pittsburgh, PA), dried overnight at 37°C. DWEYS-MAP-peptide at 20 µg/ml in PBS was adsorbed to plates at 4°C overnight. The following day, plates were blocked with 1% BSA in PBS for 1 h at 37°C. Supernatants at a concentration of 5µg/ml were incubated at 4°C overnight. All ELISAs were developed with alkaline phosphatase conjugated goat anti-human IgG (Southern Biotechnology) and OD405 was measured using a Victor microplate reader (Perkin Elmer, Waltham, MA). Clone #53 from M.C. Nussenzweig’s laboratory was used as a negative control.
Statistical analysis
P-values for analysis of antibody reactivity were calculated by Chi-square test. P values <0.05 were considered to be statistically significant. Data was analyzed using the GraphPad Prism4- (GraphPad, San Diego, CA) software.
Supplemental materials
Tables S1–3 show IgH and IgL chain characteristics and antibody reactivities with DWEYS-peptide and dsDNA. Figure S1 shows Ig gene features in both DNA-binding and DNA-negative antibodies.
Results
Autoreactive antibodies are specifically enriched in the tetramer-binding B cell subsets of SLE patients
To test whether we could identify a peptide- and dsDNA- reactive B cell population in patients with SLE, tetramer positive and negative peripheral blood B cells of 3 patients with SLE were sorted to amplify heavy and light chain rearrangements of individual B cells and subsequently express all antibodies in order to determine their specificities. All patients met the revised ACR criteria for SLE (Hochberg, 1997). Of these patients, M55 exhibited elevated serum titers of both anti-dsDNA and anti-peptide antibodies; C9 exhibited no elevation in serum titers of either of these specificities; C15 displayed weak serum reactivity with dsDNA. The gating strategy used for sorting is shown in Figure 1.
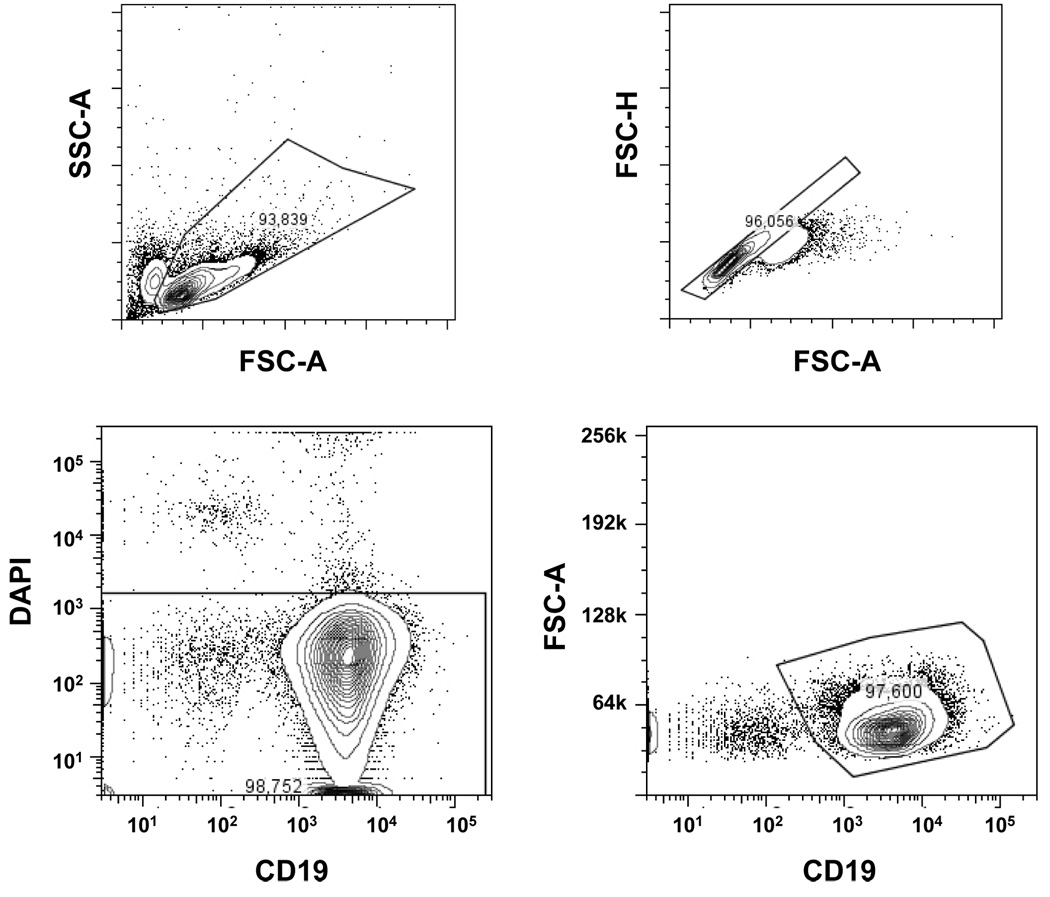
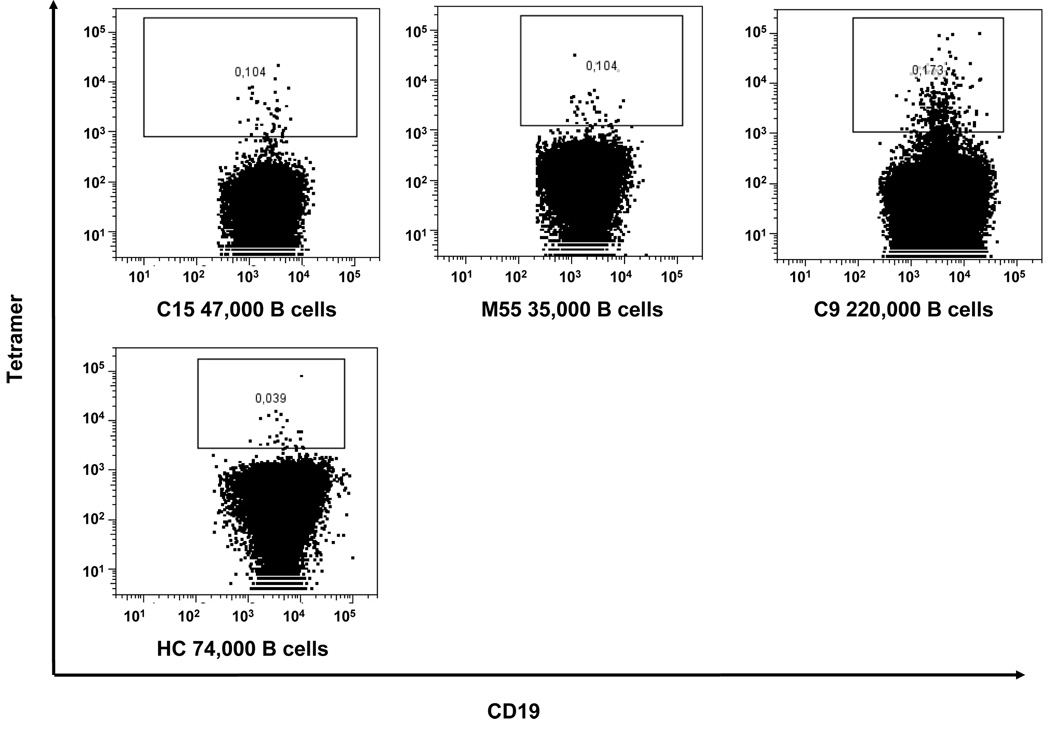
Figure 1. Tetramer-binding B cells in peripheral blood.
(A) Gating strategy used to determine tetramer-binding B cells.
(B) Tetramer staining in patients with SLE and a healthy control is depicted using a B cell gate. The number of total B cells is indicated at the bottom of individual figures.
After sorting, the population of tetramer-positive B cells was 75–80% pure on repeat analysis (data not shown). Compared with low frequency of tetramer-binding B cells (0.10%– 0.17%) before sorting, the population was enriched approximately 750 fold for tetramer-positive B cells (from 0.1% to 75%). Using the single cell RT-PCR methodology previously used to study the B cell repertoire in SLE (Wardemann et al., 2003), IgM heavy chain and both kappa and lambda light chain Ig genes of 155 B cells were cloned and expressed in 293A cells as intact IgG1. We elected to focus on IgM antibodies as the frequency of a successful PCR reaction has been reported to be higher for IgM antibodies, probably due to the lower number of somatic point mutations.
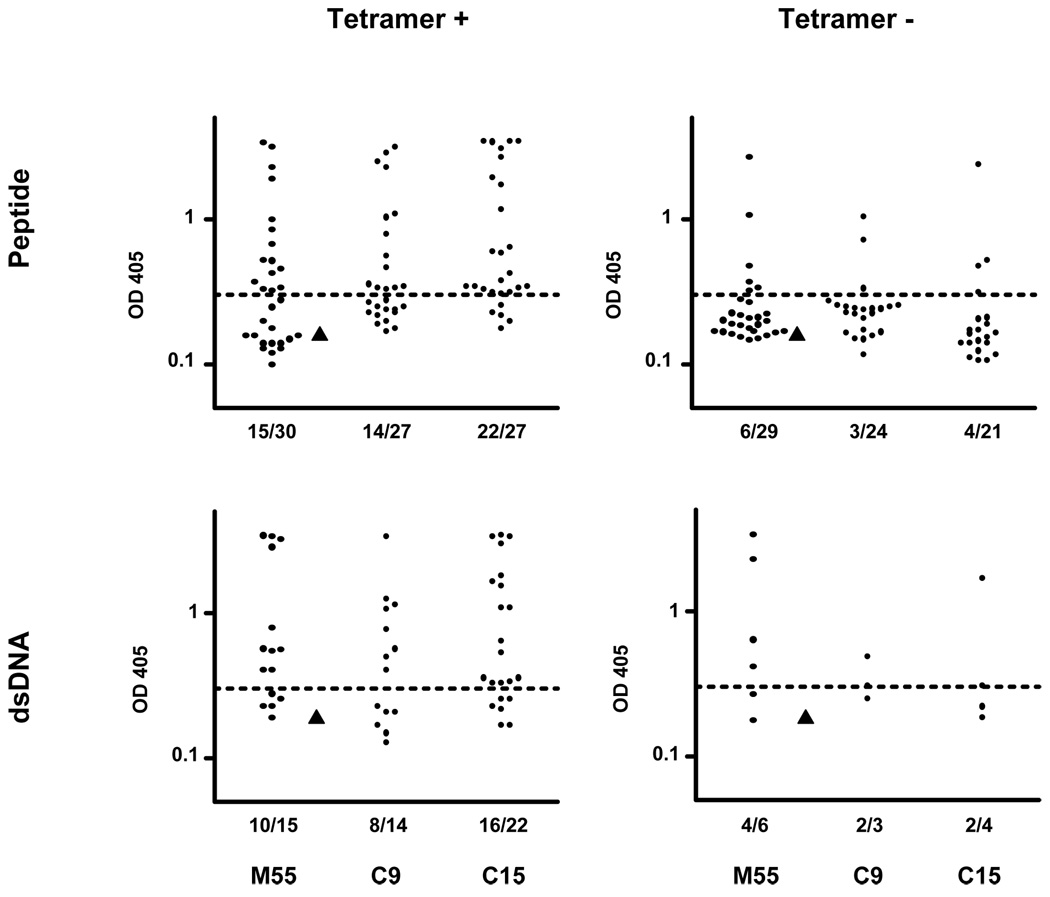
Since we chose this in vitro antibody expression system developed by M.C. Nussenzweig’s laboratory, the cut-off OD405 for positive reactivity was determined by a negative control antibody, # 53, from their lab (> twice the mean value for peptide and dsDNA). In all three patients, peptide- and dsDNA-reactive antibodies were enriched in the tetramer-positive B cell compartment. As shown in Table 1 and Figure 2, the percentage of peptide-reactive IgM antibodies, assayed at 5µg/ml, was 50–81% within the tetramer-positive fraction compared with 12–21% in the tetramer-negative population (p=0.02 for M55, p<0.01 for C9, and p <0.0001 for C15). Since the population from which sequences were obtained was only 75–80% tetramer-positive after sorting and since only IgM sequences, which might display low affinity for antigen and therefore not be deemed peptide binding in the ELISA, were obtained, the majority of tetramer-positive cells appear to be peptide-reactive. Importantly, 57–73% of peptide-reactive antibodies also bound dsDNA by ELISA at a concentration of 5ug/ml (Table 1). In contrast, 7–17% of the antibodies from tetramer-negative B cells were reactive with dsDNA (p=0.05 for M55; p=0.03 for C9; and p<0.01 for C15). Interestingly, even in this population, 50–67% of those antibodies binding peptide also bound dsDNA, confirming this frequent cross-reactivity (Table 1 and Supplemental Tables S1–S3).
Table 1.
IgM peptide and dsDNA-reactive antibodies are enriched in the tetramer-binding B cell compartment.
| Antigen binding | |||||
|---|---|---|---|---|---|
| patients | population | Peptide + | DNA +# | Peptide − | DNA +* |
| M55 | Tetramer + | 15 | 10 | 15 | 2 |
| Tetramer − | 6 | 4 | 23 | 1 | |
| C9 | Tetramer + | 14 | 8 | 13 | 1 |
| Tetramer − | 3 | 2 | 21 | 0 | |
| C15 | Tetramer + | 22 | 16 | 5 | 0 |
| Tetramer − | 4 | 2 | 17 | 1 | |
| Healthy donor | Tetramer + | 0 | 0 | 10 | 0 |
The number of dsDNA reactive antibodies out of peptide-binding antibodies.
The number of dsDNA reactive antibodies out of non peptide-binding antibodies.
Human embryonic kidney fibroblast 293A cells were co-transfected with 12.5 µg/ml of IgH and IgL chain encoding plasmid DNA by calcium phosphate precipitation. Supernatants were collected after 6 days of culture. Abs from tetramer positive or negative B cells were then tested for binding to peptide and dsDNA by ELISA at 5ug/ml. IgM peptide and dsDNA-reactive Abs were enriched in the tetramer-positive B cell compartments. (p=0.02 in M55; p<0.01 in C9; p<0.0001 in C15 for peptide reactivity. p=0.05 in M55; p=0.03 in C9; p<0.01 in C15 for dsDNA reactivity)
Figure 2. Peptide and dsDNA ELISAs.
Expressed antibodies derived from tetramer-binding B cells were tested by ELISA for reactivity with peptide at 5ug/ml. All peptide-reactive antibodies were further tested for specificity against dsDNA at same concentration. A horizontal dotted line shows the cut-off OD405 for positive reactivity. One negative control antibody, #53, from M.C. Nussenzweig’s laboratory was shown as triangle and twice this value was used to determine the cut-off OD405.
We also obtained antibodies from tetramer-binding B cells in the peripheral blood of a normal individual. The frequency of these cells was 3–5 fold lower than in patients (Figure 1B). Ten antibodies were expressed; of those none bound peptide or dsDNA, indicating that in normal donors the proportion of B cells binding tetramer in a non-specific manner outweighs specifically binding cells.
Ig gene features in autoreactive B cells of lupus patients
As we could obtain full length Ig gene sequences in the process of generating recombinant antibodies, it allowed us investigate Ig gene features in human anti-dsDNA antibodies. Compared to the non dsDNA-reactive antibodies, there was no consistent pattern of Ig gene usage in dsDNA-specific antibodies derived from three lupus patients (Supplemental Figure S1A). It has been suggested that a long heavy chain complementarity-determining region 3 (CDR3) and the presence of positively charged amino acids in CDR3 are associated with autoreactive antibodies (Ichiyoshi and Casali, 1994; Shiokawa et al., 1999; Wardemann et al., 2003). When we compared CDR3 length in dsDNA-binding and non DNA-binding antibodies, there was no significant difference. Similarly, there was no correlation between autoreactivity and the presence of positively charged amino acids in the CDR3 (Supplemental Figure S1B). Consistent with previous findings, we could not predict antibody reactivity based on sequence analysis alone because of the diversity of Ig gene usage in human anti-dsDNA antibodies.
Discussion
This study employed a tetrameric fluorochrome-labeled form of a dsDNA mimetope to identify dsDNA-specific B cells within the peripheral blood of patients with SLE. The tetramer was previously successfully used to track the peptide-specific B cell population in peptide-immunized BALB/c mice (Newman et al., 2003; Rice et al., 2005). Using the same approach, we were able to identify and enrich a rare peptide-specific B cell population in patients with SLE, and to demonstrate that the antibodies derived from these B cells bound peptide at high frequency and were largely cross-reactive to dsDNA.
To be noted, the tetramer did not tag all the peptide-reactive B cells in lupus patients. One autoreactive B cell population, plasma cells, will most probably not be included in tetramer-binding B cells as they may not have sufficient surface BCR to bind labelled tetramer to their membrane. Additionally, some B cells making peptide-reactive-antibodies were present in the tetramer non-binding population. Whether these might have been anergic B cells with low expression of membrane Ig is a question worth exploring.
Furthermore, the peptide was developed as a mimetope of dsDNA; DNA reactive-B cells, which do not recognize DWEYS-peptide, will not be tagged by tetramer.
It is also clear that the B cells sorted for reactivity to tetramer include some producing antibodies that do not bind peptide at 5µg/ml. Some of these may bind at higher concentration but some undoubtedly reflect the non-specific binding seen in B cells from the healthy control. Similarly, not all peptide-reactive antibodies bind dsDNA at 5µg/ml although the majority do; from murine studies, it is clear that there are peptide-reactive antibodies that display no cross-reactivity with dsDNA.
Nonetheless, peptide reactive B cells represent an important autoreactive B cell population. As it is relatively simple to generate fluorochrome-labeled peptide tetramer and flow cytometry analyses are commonly used in many laboratories, it may be possible to routinely use this technique in analyses of the number and phenotype of DNA-reactive B cells in patients with SLE.
Obviously, it is important that we continue to pursue the generation of other labeled antigens that might identify a greater percentage of the DNA-reactive B cell population. We have tried to tag both DNA and histone bound to DNA and have been unable to generate a probe specific for DNA-binding B cell receptors. Nonetheless, this technique should have general utility for a large number of antigenic specificities. Importantly, in autoimmune disease, it may assist in monitoring response to therapy; in vaccination studies, it may help determine the extent of specific immune activation. Overall, we suggest it may be a general approach to identify B cells of a particular autoantigenic or foreign antigenic specificity.
Supplementary Material
Ig heavy and light chain genes encoding ds-DNA reactive (white bar) or non dsDNA-reactive antibodies (black bar) from each patient were compared.
Antibodies binding only to peptide were excluded from non dsDNA-reactive antibody population in this analysis. Underneath the axis of each graph the first number represents the number of sequences of dsDNA-reactive antibodies and second number represents the number of sequences of non dsDNA-reactive antibodies.
A: Ig V and J gene family usage
B: Heavy chain CDR3 length and positive charges. Left bar graphs show the percentage of heavy chains whose CDR3 length were ≤ 27bp; 30–42bp; 45–57bp; and ≥ 60bp. Right bar graphs show percentage of heavy chain CDR3s with zero, one, two or three positively charged amino acids (aa).
Acknowledgements
We would like to thank Sergey Yurasov for help with PCR techniques and Kristie M. Gordon and Stella Stefanova for their assistance with cell sorting. This work was supported by the NIH and the Irvington Institute Fellowship Program of the Cancer Research Institute.
Nonstandard abbreviations used
- SLE
systemic lupus erythematosus
- Ig
immunoglobulin
- BCR
B cell receptor
- FR
framework region
- CDR
complementarity-determining region
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cyster JG, Hartley SB, Goodnow CC. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature. 1994;371:389–395. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- Ehrenstein MR, Katz DR, Griffiths MH, Papadaki L, Winkler TH, Kalden JR, Isenberg DA. Human IgG anti-DNA antibodies deposit in kidneys and induce proteinuria in SCID mice. Kidney Int. 1995;48:705–711. doi: 10.1038/ki.1995.341. [DOI] [PubMed] [Google Scholar]
- Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- Huerta PT, Kowal C, DeGiorgio LA, Volpe BT, Diamond B. Immunity and behavior: antibodies alter emotion. Proc Natl Acad Sci U S A. 2006;103:678–683. doi: 10.1073/pnas.0510055103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyoshi Y, Casali P. Analysis of the structural correlates for antibody polyreactivity by multiple reassortments of chimeric human immunoglobulin heavy and light chain V segments. J Exp Med. 1994;180:885–895. doi: 10.1084/jem.180.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal C, Degiorgio LA, Lee JY, Edgar MA, Huerta PT, Volpe BT, Diamond B. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proc Natl Acad Sci U S A. 2006;103:19854–19859. doi: 10.1073/pnas.0608397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J, Rice JS, Wang C, Harris SL, Diamond B. Identification of an antigen-specific B cell population. J Immunol Methods. 2003;272:177–187. doi: 10.1016/s0022-1759(02)00499-4. [DOI] [PubMed] [Google Scholar]
- Putterman C, Diamond B. Immunization with a peptide surrogate for double-stranded DNA (dsDNA) induces autoantibody production and renal immunoglobulin deposition. J Exp Med. 1998;188:29–38. doi: 10.1084/jem.188.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JS, Newman J, Wang C, Michael DJ, Diamond B. Receptor editing in peripheral B cell tolerance. Proc Natl Acad Sci U S A. 2005;102:1608–1613. doi: 10.1073/pnas.0409217102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiokawa S, Mortari F, Lima JO, Nunez C, Bertrand FE, 3rd, Kirkham PM, Zhu S, Dasanayake AP, Schroeder HW., Jr IgM heavy chain complementarity-determining region 3 diversity is constrained by genetic and somatic mechanisms until two months after birth. J Immunol. 1999;162:6060–6070. [PubMed] [Google Scholar]
- Vlahakos DV, Foster MH, Adams S, Katz M, Ucci AA, Barrett KJ, Datta SK, Madaio MP. Anti-DNA antibodies form immune deposits at distinct glomerular and vascular sites. Kidney Int. 1992;41:1690–1700. doi: 10.1038/ki.1992.242. [DOI] [PubMed] [Google Scholar]
- Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, Nussenzweig MC. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201:703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ig heavy and light chain genes encoding ds-DNA reactive (white bar) or non dsDNA-reactive antibodies (black bar) from each patient were compared.
Antibodies binding only to peptide were excluded from non dsDNA-reactive antibody population in this analysis. Underneath the axis of each graph the first number represents the number of sequences of dsDNA-reactive antibodies and second number represents the number of sequences of non dsDNA-reactive antibodies.
A: Ig V and J gene family usage
B: Heavy chain CDR3 length and positive charges. Left bar graphs show the percentage of heavy chains whose CDR3 length were ≤ 27bp; 30–42bp; 45–57bp; and ≥ 60bp. Right bar graphs show percentage of heavy chain CDR3s with zero, one, two or three positively charged amino acids (aa).