Abstract
The development of exceptionally potent inhibitors of fatty acid amide hydrolase (FAAH), the enzyme responsible for the degradation of oleamide (an endogenous sleep-inducing lipid), and anandamide (an endogenous ligand for cannabinoid receptors) is detailed. The inhibitors may serve as useful tools to clarify the role of endogenous oleamide and anandamide and may prove to be useful therapeutic agents for the treatment of sleep disorders or pain. The combination of several features—an optimal C12–C8 chain length, π-unsaturation introduction at the corresponding arachidonoyl Δ8,9/Δ11,12 and oleoyl Δ9,10 location, and an α-keto N4 oxazolopyridine with incorporation of a second weakly basic nitrogen provided FAAH inhibitors with Kis that drop below 200 pM and are 102–103 times more potent than the corresponding trifluoromethyl ketones.
Oleamide (1) and anandamide are prototypical members of a class of endogenous fatty acid amides that serve as chemical messengers. Oleamide was found to accumulate in the cerebrospinal fluid under conditions of sleep deprivation (2–4) and to induce physiological sleep in animals (2). In a structurally specific manner, it modulates serotonergic systems (5–9), benzodiazepine-sensitive GABAA receptors (10), blocks glial gap junction cell–cell communication (11, 12), and exhibits the characteristic in vivo analgesic and cannabinoid behavioral effects of anandamide in mice, albeit without cannabinoid receptor binding (9, 13). Most exciting of its activities are its sleep-inducing properties (2, 14) where it reduces mobility, shortens the sleep induction period (14), and lengthens the time spent in slow wave sleep 2 at the expense of wakening (2). Unlike many endogenous sleep-inducing molecules and typical sleep aids that act as central nervous system (CNS) depressants, oleamide induces sleep in a manner indistinguishable from physiological sleep (2, 14). Its endogenous concentrations and temporal associations are consistent with those required of serotonergic and GABAergic neurotransmission, which may be involved in sleep induction (1, 2, 14, 15). In addition to suggesting that oleamide may play a central role in sleep, the studies indicate the potential of developing sleep aids that lack the side effects of sedatives and hypnotics and the suicide-abuse potential of CNS depressants.
Anandamide (16) is an endogenous fatty acid ethanolamide that binds to the central CB1 and peripheral CB2 cannabinoid receptors through which it is thought to exhibit its analgesic and cannabinoid effects (17–20). It blocks glial gap junction communication (11, 12, 21, 22), differentially modulates the serotonergic system (7, 23, 24), modulates sleep and memory in rats analogous to oleamide (25), and exhibits a range of biological properties (17, 26, 27). Most exciting of these properties is the demonstration that endogenous anandamide levels increase on pain stimulation, implicating its role in suppressing pain neurotransmission and in behavioral analgesia (28). Most recently, anandamide has been shown to activate the vanilloid receptor (VR1) analogous to capsaicin and olvanil (N-vanillyloleamide), providing what may be a common site of action for the oleamide and anandamide analgesic effects (29).
Other endogenous fatty acid primary amides and ethanolamides have been described and their biological properties defined (30, 31). These amides include erucamide (32), an angiogenic factor stimulating new blood vessel formation, and palmitoyl ethanolamide (33), which may act as an endogenous ligand for the peripheral CB2 receptor (28, 34).
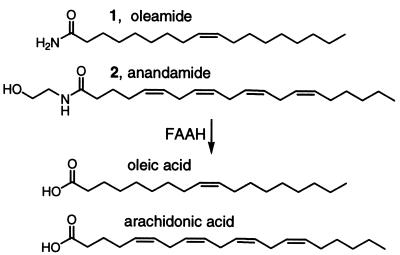
Fatty acid amide hydrolase (FAAH), referred to as oleamide hydrolase and anandamide amidohydrolase in early studies, is an integral membrane protein that degrades fatty acid primary amides and ethanolamides including oleamide (35–37) and anandamide (Fig. 1) (35–44). The distribution of FAAH in the central nervous system suggests that it degrades neuromodulating fatty acid amides at their sites of action and is intimately involved in their regulation (45). FAAH hydrolyzes a wide range of oleoyl and arachidonoyl amides (46) and esters (47–50), illustrating a range of fatty acid substrates, and it appears to work most effectively on arachidonoyl and oleoyl substrates (35, 36).
Figure 1.
FAAH substrates.
Despite this important role, only a select set of FAAH inhibitors have been disclosed (51–58). These include the discovery of the endogenous inhibitor 2-octyl γ-bromoacetoacetate (51), which was reported earlier as an endogenous sleep-inducing compound (52). The remaining inhibitors consist of reversible electrophilic carbonyl inhibitors (53–55) (trifluoromethyl ketones, α-keto esters and amides, and aldehydes) or irreversible inhibitors (56–59) (sulfonyl fluorides and fluorophosphonates) incorporated into the fatty acid structures. A select set of the reversible electrophilic carbonyl FAAH inhibitors were shown to be sleep-inducing agents (1). These results, along with the demonstration that the endogenous sleep-inducing compound 2-octyl γ-bromoacetoacetate is a potent inhibitor of FAAH, suggest it may be a useful target for the development of sleep aids that act in part by preserving endogenous oleamide. Similarly, FAAH inhibitors may prove to be effective analgesics by virtue of preserving endogenous levels of oleamide and anandamide; the latter is released on pain stimulation (28).
Herein, we disclose the development of exceptionally potent α-keto heterocycle inhibitors of FAAH that are 102–103 times more potent than the corresponding trifluoromethyl ketones. Since Edwards' disclosure of α-keto heterocycles as effective protease inhibitors, a number of enzyme inhibitors have been described on the basis of analogous design principles (60–70). The inhibitors developed herein define additional features that may contribute independently to binding affinity which, when combined with the electrophilic carbonyl of the α-keto heterocycle, provides extraordinarily potent inhibitors.
Methods
Inhibitor Synthesis.
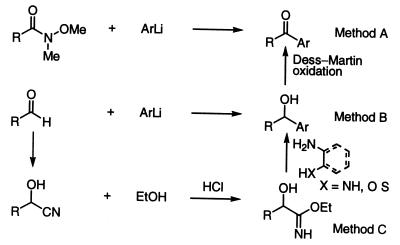
The α-keto heterocycles were prepared by addition of the heteroaryl lithium reagent to the Weinreb amide (Method A) or indirectly from the aldehyde proceeding through the α-hydroxy heterocycles followed by Dess–Martin oxidation via addition of the heteroaryl lithium reagent (Method B) or by cyanohydrin formation, acid-catalyzed conversion to the imidate (HCl–EtOH, CHC13), and condensation with a 2-aminoalcohol, 2-aminoaniline, 2-aminophenol, or o-amino-hydroxypyridine (Method C, Scheme S1). Full details are provided in supplemental data (see www.pnas.org).
Scheme 1.
Inhibition Studies.
All enzyme assays were performed at 20–23°C by using either solubilized rat liver plasma membrane extracts (54) or solubilized COS-7 membrane extracts from cells transiently transfected with human FAAH cDNA (36) in a reaction buffer of 125 mM Tris/1 mM EDTA/0.2% glycerol/0.02% Triton X-100/0.4 mM Hepes, pH 9.0 buffer (51). The initial rates of hydrolysis (≤10–20% reaction) were monitored by following the breakdown of 14C-oleamide to oleic acid as described (2, 51). The inhibition was competitive by a Lineweaver–Burke analysis, linear least squares fits were used for all reaction progress curves, and R2 values were consistently >0.97. IC50 values were determined from the inhibition observed at three to five different inhibitor concentrations (from three or more trials at each inhibitor concentration) by using the formula IC50 = [I]/[(υ0/υi)–1] (71), where υ0 is the control reaction rate without inhibitor and υi is the rate with inhibitor at concentration [I]. Ki values were determined by the Dixon method (x-intercepts of weighted linear fits of [I] vs. 1/rate plots at constant substrate concentration, which were converted to Ki values by using the formula Ki = −xint/[1 + [S]/Km]).
Results
Nature of the Heterocycle.
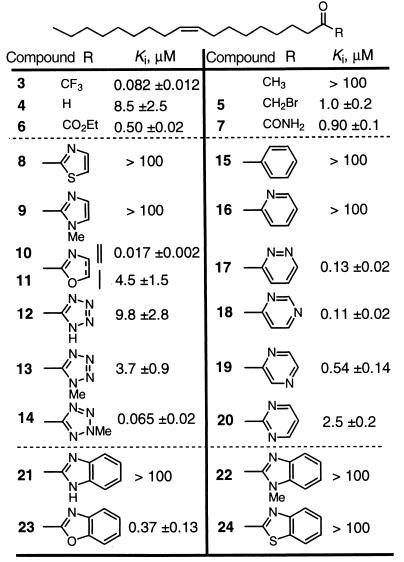
A range of five- and six-membered monocyclic heterocycles and the three most prevalent bicyclic heterocycles (benzthiazole, benzimidazole, and benzoxazole) were incorporated into the oleoyl α-keto heterocycles 8–24. The results of their examination are summarized in Table 1 along with the comparison data for the trifluoromethyl ketone 3 and the related inhibitors 4–7 (54). The inhibitors contain the oleyl chain possessing a 9-Z double bond and a carbonyl at the site of the oleamide carboxamide and adjacent to the electron-deficient heterocycle. Although many of the inhibitors were more potent than oleyl aldehyde (4) and comparable to the α-keto ester 6 and carboxamide 7, only two (14 and 10) matched the potency of the trifluoromethyl ketone 3. Many of the observations made by Edwards on the relative potencies of α-keto heterocycles against elastase were also observed with FAAH. These observations include the unique potency of the benzoxazole vs. benzthiazole and benzimidazole, the more potent activity of the oxazole 10 vs. the thiazole or imidazole, and the substantially more potent behavior of the 2-methyl vs. 1-methyl tetrazoles 14 and 13. In contrast to the observations of Edwards and unique to the studies with FAAH, the oxazole 10 proved substantially more potent than the oxazoline 11, and the six-membered heterocycles containing two nitrogen atoms, one of which remains weakly basic (17–19 vs. 20), were unusually potent, exceeding the activity of the α-keto ester and carboxamide 6 and 7 and approaching that of trifluoromethyl ketone 3. Although there are many potential explanations for this behavior, one that proved consistent with subsequent observations is the enhancement of the inhibitor potency by incorporation of a weakly basic nitrogen.
Table 1.
α-Keto heterocycle inhibitors of FAAH
• Potency approaches that of trifluoromethyl ketone. • Potency increases with additional basic nitrogen.
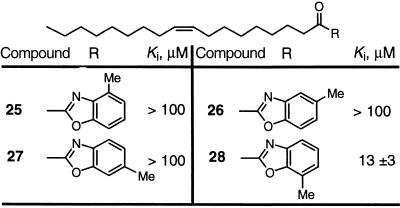
Steric Requirements Surrounding the Benzoxazole.
The benzoxazole 23 was chosen for further examination because it provided the greatest opportunity for functionalization. The 4-, 5-, 6-, and 7-methylbenzoxazoles were prepared to define sites available for functionalization without adversely affecting the inhibitor potency (Table 2). Substitution of any available position on the benzoxazole results in a greatly diminished (28) or complete loss of activity (25–27). This behavior defines precise limits to the size and depth of the FAAH active site, which in turn has implications for its substrate specificity or selectivity.
Table 2.
Substituted α-keto benzoxazole inhibitors of FAAH
• Sensitive to steric interactions surrounding active site. • Defines limits to depth and width of FAAH active site.
Oxazolopyridines: Incorporation of Nitrogen into the Benzoxazole.
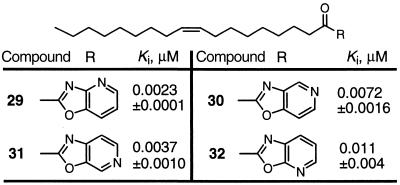
On the basis of the observation that incorporation of an additional basic nitrogen seemed to correlate with enhanced inhibitor potency, the four oxazolopyridines 29–32 were examined and were found to be more potent inhibitors (Table 3). The introduction of a nitrogen into the benzoxazole enhanced the potency 50–200 times, providing inhibitors that are 10–50 times more potent than the trifluoromethyl ketone 3. Although N4 incorporation provided the most potent inhibitor 29, N5–N7 incorporation also provided effective inhibitors (N4 > N6 > N5 > N7), and there is only a 4- to 5-fold difference in the most and least potent agent in the series. Although it is tempting to invoke an active-site dual interaction of a single residue with N3 and N4, the comparable activity of 29–32 suggests the interaction of the second nitrogen is more flexible.
Table 3.
α-Keto oxazolopyridine inhibitors of FAAH
• Potency increases with introduction of basic nitrogen. • Potency increases ca. × 200 and N4 > N6 > N5 > N7. • Relatively insensitive to location of additional nitrogen.
Impact of the Double Bond.
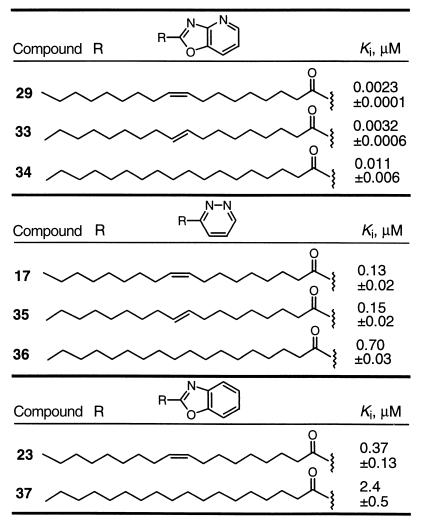
The importance of the oleyl double bond was examined with three of the initial potent inhibitors (Table 4). Identical to observations made with the trifluoromethyl ketone and α-keto ester inhibitors (54, 55), 29 and 17 containing the cis double bond were more potent than 33 and 35, respectively, containing the trans double bond, which in turn were more potent than 34 and 36 in which the double bond was removed. Similarly, 23 was more potent than 37.
Table 4.
Impact of double bond in the C18 α-keto heterocycle inhibitors of FAAH
• C18 Δ9,10: Z (cis) > E (trans) > saturated.
Arachidonyl-Based Inhibitors.
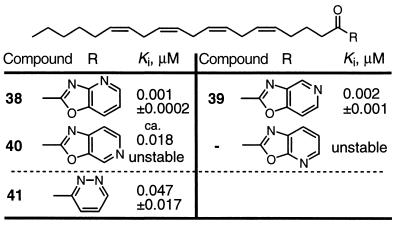
Because two of the best substrates for FAAH are arachidonamide and oleamide (36), we examined five of the potent α-keto heterocycles incorporated into the arachidonoyl skeleton (Table 5). In each instance, the inhibitors were unstable and decomposed rapidly under typical working conditions. Several proved too unstable to purification to assess accurately their inhibitor potency, and that of 40 could only be approximated (about 50% purity). Where this could be assessed accurately, the arachidonoyl inhibitors were two to five times more potent than the oleoyl-based inhibitors. Despite this enhancement, which is consistent with the FAAH substrate preference for arachidonamide vs. oleamide (relative rate of hydrolysis, 1:0.7), their instability precludes effective utility.
Table 5.
Arachidonyl-based α-keto heterocycle FAAH inhibitors
• Potency: arachidonyl > oleyl inhibitors (×2–5). • Stability: oleyl >> arachidonyl inhibitors.
In studies with conformationally restricted trifluoromethyl ketone inhibitors, a well-defined trend favoring a bound bent, but not hairpin, conformation was observed and defined the shape characteristics of the active site (55). The enhanced potency of the arachidonoyl-based inhibitors is likely to be related to this shape characteristic of the FAAH active site and their enhanced preference for adoption of the required bound conformation.
The Fatty Acid Chain.
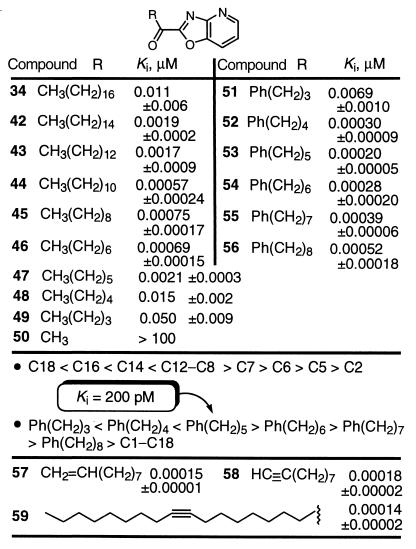
Well-behaved trends were observed with modifications in the fatty acid chain (Table 6). A very well-defined effect of the chain length was observed, and the greatest potency was found with saturated straight chain lengths of C12–C8. This is a chain length that terminates at the location of the Δ9,10 double bond of oleamide, and the Δ8,9/Δ11,12 double bonds of arachidonamide, and corresponds to the location of the bend in the bound conformation identified in studies with trifluoromethyl ketone inhibitors (55). The inhibitor potency progressively increased as the chain length was shortened from C18 to C12 (Ki, 11 → 0.6 nM), leveled off at C12–C8 with subnanomolar Kis (0.57–0.73 nM), and subsequently diminished sharply as the chain length was shortened from C8 to C2, ultimately providing inactive inhibitors (Ki = 0.7 → >100,000 nM). This behavior indicates that each of the first C1–C8 carbons in the chain contributes significantly to inhibitor and substrate binding and that C10–C12 contribute nominally to binding. More importantly, these results indicate that the terminal carbons of the longer C14–C18 inhibitors may actually diminish inhibitor binding affinity relative to the substrate or may not be involved in substrate binding.
Table 6.
Modifications in the fatty acid side chain of α-keto heterocycle inhibitors of FAAH
Incorporating unsaturation into the fatty acid chain increases inhibitor potency (Table 6). The incorporation of a benzene ring provided inhibitors with subnanomolar Kis with the most potent inhibitor 53 possessing a Ki of 200 pM. Again, the inhibitors exhibited a well defined trend, with the optimal C5 methylene spacer corresponding to incorporation of both the Δ9,10 and Δ8,9/Δ11,12 double bonds of oleamide and anandamide. This extraordinary potency was observed with the structurally simple inhibitors 51–56 amendable to further modification. These observations, like those of the straight chain inhibitors 42–50, are analogous to those made with a series of trifluoromethyl ketones (55). The distinction is that the α-keto oxazolopyridines are 102–103 times more potent than the corresponding trifluoromethyl ketones.
Even more significantly, introduction of a double or triple bond at the oleamide Δ9,10 position provided even more potent inhibitors, 57 and 58 with Kis of 150 and 180 pM, respectively. Thus, 57 proved to be 5 times more potent than 45, precisely following the trends seen with 29 vs. 34 (×5), 17 vs. 36 (×5), and 23 vs. 37 (×6) (Table 4). Remarkably, the incorporation of a Δ9,10 triple bond into the oleoyl-based inhibitor 59 provided the most potent inhibitor observed to date, Ki 140 pM.
The Electrophilic Carbonyl.
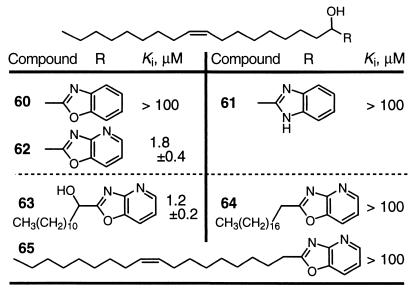
Key to the design of the inhibitors was the electrophilic carbonyl, which is required for potent enzyme inhibition. A set of the α-hydroxy precursors to the initial inhibitors were examined and typically proved inactive as FAAH inhibitors (Table 7). Significantly, the α-hydroxy precursors 62 and 63 to the potent α-keto oxazolopyridines 29 and 44 retained significant FAAH inhibition with Kis of 1.8 and 1.2 μM, respectively. Although this is 103 times less potent than the corresponding keto derivative, they approximate the potency of the initial series of α-keto heterocycles and 4–7 (Table 1). This behavior indicates that the pyridine nitrogen of the N4 oxazolopyridine, and presumably that of the N5–N7 oxazolopyridines, in conjunction with the α-hydroxy group contributes substantially to FAAH active site binding independent of the contributions of the electrophilic carbonyl. The agents 64 and 65 lacking the α-hydroxy groups were inactive, losing at least an additional 102-fold binding affinity with removal of the alcohol or at least 105-fold binding affinities with respect to removal of the keto group.
Table 7.
Comparison with α-hydroxy heterocycle FAAH inhibitors
• Ketone >> alcohol (×103) >> alkane (×105).
Notably, the α-keto heterocycles do not exist predominantly in the hydrated state. Thus, 16, 17, 21, and 23 showed no detectable hydrate or hemiacetal formation in CD3OD, 7% D2O–acetone-d6, or 5% D2O–DMSO-d6, 29 exhibited only 2–4% hydrate or 9% hemiacetal (CD3OD) formation (1H NMR), and 63 showed no hydrate formation (10% D2O in acetone-d6 or DMSO-d6) and slow time-dependent hemiacetal formation (10–20%, 1–24 h, CD3OD). Under identical conditions, the trifluoromethyl ketone 3 was completely (CD3OD) or predominately hydrated (>90%, 7% D2O–acetone-d6) (54).
Inhibition of Human FAAH.
Previous work demonstrated the rat (35) and human enzyme (36) are very homologous (84% sequence identity), exhibit near identical substrate specificities, and incorporate an identical amidase signature sequence and SH3-binding domain suggesting the observations made with rat FAAH will be similar to those of human FAAH. The additive design principles elucidated with rat liver FAAH were also observed for recombinant human FAAH (Table 8). Addition of a weakly basic N4 nitrogen into the benzoxazole ring led to an increase in potency of nearly 100-fold for compound 29 over 23. Incorporation of a shortened alkyl chain with π unsaturation (53) increased potency by another order of magnitude and resulted in an inhibitor with a Ki of less than 100 pM. Overall, the relative and absolute potencies of these inhibitors against human FAAH were very similar to rat liver FAAH.
Table 8.
Inhibition of recombinant human FAAH
| Compound | Ki, μM (human) | Ki, μM (rat) |
|---|---|---|
| 23 | 0.073 | 0.37 |
| 29 | 0.0013 | 0.0023 |
| 53 | 0.000094 | 0.00020 |
• Relative and absolute potencies against rat and human FAAH not distinguishable.
Discussion.
A potent class of competitive inhibitors of FAAH was developed on the basis of complementary binding interactions provided by the electrophilic carbonyl of an α-keto heterocycle and that of heterocycles (oxazolopyridines) incorporating a weakly basic nitrogen. FAAH belongs to a new class of amidases that has not been extensively studied and possess a distinct combination of active site residues involved in catalysis. Mutagenesis studies have characterized FAAH as a possible serine–lysine dyad amidase that lacks a participating active site histidine (72). It utilizes a serine nucleophile (Ser-241) and incorporates two additional active site serines (Ser-217 and -218) that enhance catalysis (73). Key to its enhanced amide vs. ester bond cleavage is its enlistment of Lys-142 with an apparently perturbed pKa (7.8) as a base for Ser-241 deprotonation and for subsequent protonation of the amine leaving group (72). It is possible that the impact of the second weakly basic nitrogen of the oxazolopyridines is derived from hydrogen bonding to one or more of these active site residues and that the positioning of this residue is sufficiently flexible to interact with a weakly basic nitrogen in a range of locations.
Well-defined relationships were observed in the development of the inhibitors. Several oleyl α-keto heterocycles exhibit FAAH inhibition comparable to the corresponding α-keto ester and carboxamide. The more potent include six-membered heterocycles incorporating a second weakly basic nitrogen as well as benzoxazole. Substitution at any of the available sites on the α-keto benzoxazole inhibitor eliminated activity, defining limits to the depth and width of the FAAH active site. Incorporation of an additional basic nitrogen into the benzoxazole providing the four isomeric oxazolopyridines (N4–N7) afforded exceptionally potent inhibitors 50–200 times more active than the benzoxazole and 8–40 times more active than the corresponding trifluoromethyl ketone. Arachidonoyl-based inhibitors were found to be two to three times more potent than the oleoyl-based inhibitors, consistent with the relative rates of FAAH hydrolysis of arachidonamide vs. oleamide, but are sufficiently unstable so as to preclude their use. The removal of the oleoyl Δ9,10 cis double bond or the incorporation of a trans olefin reduced inhibitor potency consistent with prior observations (54, 55). The inhibitor potency exhibited a smooth dependency on the fatty acid chain length, C18 < C16 < C14 < C12–C8 > C7 > C6 > C5 > C2, exhibiting the maximum potency at C12–C8, which corresponds to the location of the oleoyl Δ9,10 cis double bond and the arachidonoyl Δ8,9/Δ11,12 double bonds. This optimal length appears to correspond to the location of a bend, but not hairpin conformation, in the bound conformation identified with conformationally restrained inhibitors (55). Incorporation of π-unsaturation into the medium length (C12–C8) inhibitors at the sites of oleoyl or arachidonoyl unsaturation further enhances the inhibitor potency, and this may be accomplished with incorporation of a double bond, triple bond, or phenyl ring. The combination of these features: C8–C12 chain length, π unsaturation incorporation at the arachidonoyl Δ8,9/Δ11,12 and oleoyl Δ9,10 location, and an α-keto N4-oxazolopyridine provides inhibitors with potencies that drop below Kis of 200 pM and are 102–103 times more potent than the corresponding trifluoromethyl ketones. With these inhibitors, removal of the keto group reduces potency >105 times, and its reduction to an alcohol reduces potency 103 times. Although the α-hydroxy oxazolopyridines are 103 times less potent than the corresponding ketones, they exhibit inhibition comparable to many of the initial α-keto heterocycle or related α-keto ester and carboxamide inhibitors.
It is likely that similar design principles may be explored with α-keto heterocycle protease inhibitors that will extend their potencies beyond that achieved by introduction of the electrophilic carbonyl. The in vivo properties of the inhibitors detailed herein and their effects on the oleamide and anandamide sites of action are under investigation and will be disclosed in due time. Such inhibitors should prove to be useful tools to probe FAAH and related amidase mechanisms of catalysis and the biological role of oleamide and anandamide and may prove useful therapeutic agents in their own right with applications as sleep aids or analgesics that act by preserving endogenous levels of oleamide and anandamide.
Supplementary Material
Acknowledgments
We gratefully acknowledge the financial support of the Skaggs Institute for Chemical Biology, National Institutes of Health (CA42056, D.L.B.; MH58542, B.F.C.), fellowships for R.A.F. (American Cancer Society 576211) and M.P.P. (National Science Foundation), and the postdoctoral sabbatical leaves of H.S. and H.M., sponsored by Chugai Pharmaceutical and Sumitomo Pharmaceutical, respectively. Supplemental data: full experimental details for the inhibitor syntheses, full characterization of all inhibitors and new intermediates, and a Lineweaver–Burke analysis of 53 are provided (see www.pnas.org).
Abbreviation
- FAAH
fatty acid amide hydrolase
References
- 1.Boger D L, Henriksen S J, Cravatt B F. Curr Pharm Des. 1998;4:303–314. [PubMed] [Google Scholar]
- 2.Cravatt B F, Prospero-Garcia O, Siuzdak G, Gilula N B, Henriksen S J, Boger D L, Lerner R A. Science. 1995;268:1506–1509. doi: 10.1126/science.7770779. [DOI] [PubMed] [Google Scholar]
- 3.Cravatt B F, Lerner R A, Boger D L. J Am Chem Soc. 1996;118:580–590. [Google Scholar]
- 4.Lerner R A, Siuzdak G, Prospero-Garcia O, Henriksen S J, Boger D L, Cravatt B F. Proc Natl Acad Sci USA. 1994;91:9505–9508. doi: 10.1073/pnas.91.20.9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huidobro-Toro J P, Harris R A. Proc Natl Acad Sci USA. 1996;93:8078–8082. doi: 10.1073/pnas.93.15.8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas E A, Carson M J, Neal M J, Sutcliffe J G. Proc Natl Acad Sci USA. 1997;94:14115–14119. doi: 10.1073/pnas.94.25.14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boger D L, Patterson J E, Jin Q. Proc Natl Acad Sci USA. 1998;95:4102–4107. doi: 10.1073/pnas.95.8.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas E A, Cravatt B F, Sutcliffe J G. J Neurochem. 1999;72:2370–2378. doi: 10.1046/j.1471-4159.1999.0722370.x. [DOI] [PubMed] [Google Scholar]
- 9.Cheer J F, Cadogan A-K, Marsden C A, Fone K C F, Kendall D A. Neuropharmacology. 1999;38:533–541. doi: 10.1016/s0028-3908(98)00208-1. [DOI] [PubMed] [Google Scholar]
- 10.Yost C S, Hampson A J, Leonoudakis D, Koblin D D, Bornheim L M, Gray A T. Anesth Analg. 1998;86:1294–1299. doi: 10.1097/00000539-199806000-00031. [DOI] [PubMed] [Google Scholar]
- 11.Guan X, Cravatt B F, Ehring G R, Hall J E, Boger D L, Lerner R A, Gilula N B. J Cell Biol. 1997;139:1785–1792. doi: 10.1083/jcb.139.7.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boger D L, Patterson J E, Guan X, Cravatt B F, Lerner R A, Gilula N B. Proc Natl Acad Sci USA. 1998;95:4810–4815. doi: 10.1073/pnas.95.9.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mechoulam R, Fride E, Hanus L, Sheskin T, Bisogno T, Di Marzo V, Bayewitch M, Vogel Z. Nature (London) 1997;389:25–26. doi: 10.1038/37891. [DOI] [PubMed] [Google Scholar]
- 14.Basile A S, Hanus L, Mendelsen W B. NeuroReport. 1999;10:947–951. doi: 10.1097/00001756-199904060-00010. [DOI] [PubMed] [Google Scholar]
- 15.Hanus L O, Fales H M, Spande T F, Basile A S. Anal Biochem. 1999;270:159–166. doi: 10.1006/abio.1999.4083. [DOI] [PubMed] [Google Scholar]
- 16.Devane W A, Hanus L, Breuer A, Pertwee R G, Stevenson L A, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 17.Di Marzo V, De Petrocellis L, Bisogno T, Melck D. Lipids. 1999;34:S319–S325. doi: 10.1007/BF02562332. [DOI] [PubMed] [Google Scholar]
- 18.Di Marzo V. Biochim Biophys Acta. 1998;1392:153–175. doi: 10.1016/s0005-2760(98)00042-3. [DOI] [PubMed] [Google Scholar]
- 19.Axelrod J, Felder C C. Neurochem Res. 1998;23:575–581. doi: 10.1023/a:1022418217479. [DOI] [PubMed] [Google Scholar]
- 20.Mechoulam R, Fride E, Di Marzo V. Eur J Pharmacol. 1998;359:1–18. doi: 10.1016/s0014-2999(98)00649-9. [DOI] [PubMed] [Google Scholar]
- 21.Boger D L, Sato H, Lerner A E, Guan X, Gilula N B. Bioorg Med Chem Lett. 1999;9:1151–1154. doi: 10.1016/s0960-894x(99)00148-1. [DOI] [PubMed] [Google Scholar]
- 22.Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz J-C, Piomelli D. Nature (London) 1994;372:686–689. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- 23.Fan P. J Neurophysiol. 1995;73:907–910. doi: 10.1152/jn.1995.73.2.907. [DOI] [PubMed] [Google Scholar]
- 24.Kimura T, Ohta T, Watanabe K, Yoshimura H, Yamamoto I. Biol Pharm Bull. 1998;21:224–226. doi: 10.1248/bpb.21.224. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez E M, Alavez M S, Navarro L, Gonzalez D M, Colin R D, Prospero-Garcia O. Brain Res. 1998;812:270–274. doi: 10.1016/s0006-8993(98)00969-x. [DOI] [PubMed] [Google Scholar]
- 26.De Petrocellis L, Melck D, Polmisano A, Bisogno T, Laezza C, Bifulco M, Di Marzo V. Proc Natl Acad Sci USA. 1998;95:8375–8380. doi: 10.1073/pnas.95.14.8375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calignano A, La Rana G, Giuffrida A, Piomelli D. Nature (London) 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- 28.Walker J M, Huang S M, Strangman N M, Tsou K, Sanudo-Pena M C. Proc Natl Acad Sci USA. 1999;96:12198–12203. doi: 10.1073/pnas.96.21.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zygmunt P M, Petersson J, Andersson D A, Chuang H, Sorgard M, DiMarzo V, Julius D, Hogestatt E D. Nature (London) 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 30.Arafat E S, Trimble J W, Andersen R N, Dass C, Desiderio D M. Life Sci. 1989;45:1679–1687. doi: 10.1016/0024-3205(89)90278-6. [DOI] [PubMed] [Google Scholar]
- 31.Schmid H H O, Schmid P C, Natarajan V. Prog Lipid Res. 1990;29:1–43. doi: 10.1016/0163-7827(90)90004-5. [DOI] [PubMed] [Google Scholar]
- 32.Wakamatsu K, Masaki T, Itoh F, Kondo K, Sudo K. Biochem Biophys Res Commun. 1990;168:423–429. doi: 10.1016/0006-291x(90)92338-z. [DOI] [PubMed] [Google Scholar]
- 33.Barg J, Fride E, Hanus L, Levy R, Matus-Leibovitch N, Heldman E, Bayewitch M, Mechoulam R, Vogel Z. Eur J Pharmacol. 1995;287:145–152. doi: 10.1016/0014-2999(95)00487-4. [DOI] [PubMed] [Google Scholar]
- 34.Skaper S D, Buriani A, Dal Toso R, Petrelli L, Romanello S, Facci L, Leon A. Proc Natl Acad Sci USA. 1996;93:3984–3989. doi: 10.1073/pnas.93.9.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cravatt B F, Giang D K, Mayfield S P, Boger D L, Lerner R A, Gilula N B. Nature (London) 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 36.Giang D K, Cravatt B F. Proc Natl Acad Sci USA. 1997;94:2238–2242. doi: 10.1073/pnas.94.6.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patricelli M P, Lashuel H A, Giang D K, Kelly J W, Cravatt B F. Biochemistry. 1998;37:15177–15187. doi: 10.1021/bi981733n. [DOI] [PubMed] [Google Scholar]
- 38.Deutsch D G, Chin S A. Biochem Pharmacol. 1993;46:791–796. doi: 10.1016/0006-2952(93)90486-g. [DOI] [PubMed] [Google Scholar]
- 39.Desarnaud F, Cadas H, Piomelli D. J Biol Chem. 1995;270:6030–6035. doi: 10.1074/jbc.270.11.6030. [DOI] [PubMed] [Google Scholar]
- 40.Hillard C J, Wilkison D M, Edgemond W S, Campbell W B. Biochim Biophys Acta. 1995;1257:249–256. doi: 10.1016/0005-2760(95)00087-s. [DOI] [PubMed] [Google Scholar]
- 41.Ueda N, Kurahashi Y, Yamamoto S, Tokunaga T. J Biol Chem. 1995;270:23823–23827. doi: 10.1074/jbc.270.40.23823. [DOI] [PubMed] [Google Scholar]
- 42.Omeir R L, Chin S, Hong Y, Ahern D G, Deutsch D G. Life Sci. 1995;56:1999–2005. doi: 10.1016/0024-3205(95)00181-5. [DOI] [PubMed] [Google Scholar]
- 43.Maurelli S, Bisogno T, De Petrocellis L, Di Luccia A, Marino G, Di Marzo V. FEBS Lett. 1995;377:82–86. doi: 10.1016/0014-5793(95)01311-3. [DOI] [PubMed] [Google Scholar]
- 44.Maccarrone M, van der Stelt M, Rossi A, Veldink G A, Vllegenthart J F G, Agro A F. J Biol Chem. 1998;273:32332–32339. doi: 10.1074/jbc.273.48.32332. [DOI] [PubMed] [Google Scholar]
- 45.Thomas E A, Cravatt B F, Danielson P E, Gilula N B, Sutcliffe J G. J Neurosci Res. 1997;50:1047–1052. doi: 10.1002/(SICI)1097-4547(19971215)50:6<1047::AID-JNR16>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 46.Lang W, Qin C, Lin S, Khanolkar A D, Goutspoulos A, Fan P, Abouzid K, Meng Z, Biegel D, Makriyannis A. J Med Chem. 1999;42:896–902. doi: 10.1021/jm980461j. [DOI] [PubMed] [Google Scholar]
- 47.Goparaju S K, Ueda N, Yamaguchi H, Yamamoto S. FEBS Lett. 1998;442:69–73. doi: 10.1016/s0014-5793(97)01603-7. [DOI] [PubMed] [Google Scholar]
- 48.Kurahashi Y, Ueda N, Suzuki H, Suzuki M, Yamamoto S. Biochem Biophys Res Commun. 1997;237:512–515. doi: 10.1006/bbrc.1997.7180. [DOI] [PubMed] [Google Scholar]
- 49.Bisogno T, Sepe N, Melck D, Maurelli S, De Petrocellis L, Di Marzo V. Biochem J. 1997;322:671. doi: 10.1042/bj3220671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Di Marzo V, Bisogno T, Sugiura T, Melck D, De Petrocellis L. Biochem J. 1998;331:15–19. doi: 10.1042/bj3310015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patricelli M P, Patterson J E, Boger D L, Cravatt B F. Bioorg Med Chem Lett. 1998;8:613–618. doi: 10.1016/s0960-894x(98)00073-0. [DOI] [PubMed] [Google Scholar]
- 52.Torii S, Mitsumori K, Inubushi S, Yanagisawa I. Psychopharmacologia. 1973;29:65–75. doi: 10.1007/BF00421212. [DOI] [PubMed] [Google Scholar]
- 53.Koutek B, Prestwich G D, Howlett A C, Chin S A, Salehani D, Akhavan N, Deutsch D G. J Biol Chem. 1994;269:22937–22940. [PubMed] [Google Scholar]
- 54.Patterson J E, Ollmann I R, Cravatt B F, Boger D L, Wong C-H, Lerner R A. J Am Chem Soc. 1996;118:5938–5945. [Google Scholar]
- 55.Boger D L, Sato H, Lerner A E, Austin B J, Patterson J E, Patricelli M P, Cravatt B F. Bioorg Med Chem Lett. 1999;9:167–172. doi: 10.1016/s0960-894x(98)00734-3. [DOI] [PubMed] [Google Scholar]
- 56.Deutsch D G, Omeir R, Arreaza G, Salehani D, Prestwich G D, Huang Z, Howlett A. Biochem Pharmacol. 1997;53:255–260. doi: 10.1016/s0006-2952(96)00830-1. [DOI] [PubMed] [Google Scholar]
- 57.Deutsch D G, Lin S, Hill W A G, Morse K L, Salehani D, Arreaza G, Omeir R L, Makriyannis A. Biochem Biophys Res Commun. 1997;231:217–221. doi: 10.1006/bbrc.1997.6072. [DOI] [PubMed] [Google Scholar]
- 58.De Petrocellis L, Melck D, Ueda N, Maurelli S, Kurahashi Y, Yamamoto S, Marino G, Di Marzo V. Biochem Biophys Res Commun. 1997;231:82–88. doi: 10.1006/bbrc.1997.6000. [DOI] [PubMed] [Google Scholar]
- 59.De Petrocellis L, Melck D, Ueda N, Kurahashi Y, Bisogno T, Yamamoto S, Di Marzo V. Recent Advances in Prostaglandin, Thromboxane, and Leukotriene Research. New York: Plenum; 1998. pp. 259–263. [Google Scholar]
- 60.Edwards P D, Meyer E F, Jr, Vijayalakshmi J, Tuthill P A, Andisik D A, Gomes B, Strimpler A. J Am Chem Soc. 1992;114:1854–1863. [Google Scholar]
- 61.Edwards P D, Wolanin D J, Andisik D W, Davis M W. J Med Chem. 1995;38:76–85. doi: 10.1021/jm00001a013. [DOI] [PubMed] [Google Scholar]
- 62.Edwards P D, Zottola M A, Davis M, Williams J, Tuthill P A. J Med Chem. 1995;38:3972–3982. doi: 10.1021/jm00020a011. [DOI] [PubMed] [Google Scholar]
- 63.Tsutsumi S, Okonogi T, Shibahara S, Patchett A A, Christensen B G. Bioorg Med Chem Lett. 1994;4:831–834. [Google Scholar]
- 64.Tsutsumi S, Okonogi T, Shibahara S, Ohuchi S, Hatsushiba E, Patchett A A, Christensen B G. J Med Chem. 1994;37:3492–3502. doi: 10.1021/jm00047a007. [DOI] [PubMed] [Google Scholar]
- 65.Costanzo M J, Maryanoff B E, Hecker L R, Schott M R, Yabut S C, Zhang H-C, Andrade-Gordon P, Kauffman J A, Lewis J M, Krishnan R, et al. J Med Chem. 1996;39:3039–3043. doi: 10.1021/jm9603274. [DOI] [PubMed] [Google Scholar]
- 66.Akiyama Y, Tsutsumi S, Hatsushiba E, Ohuchi S, Okonogi T. Bioorg Med Chem Lett. 1997;7:533–538. [Google Scholar]
- 67.Tamura S Y, Shamblin B M, Brunck T K, Ripka W C. Bioorg Med Chem Lett. 1997;7:1359–1364. [Google Scholar]
- 68.Ogilvie W, Bailey M, Poupart M A, Abraham A, Bhavsar A, Bonneau P, Bordeleau J, Bousquet Y, Chabot C, Duceppe J S, et al. J Med Chem. 1997;40:4113–4135. doi: 10.1021/jm970104t. [DOI] [PubMed] [Google Scholar]
- 69.Boatman P D, Ogbu C O, Eguchi M, Kim H-O, Nakanishi H, Cao B, Shea J P, Kahn M. J Med Chem. 1999;42:1367–1375. doi: 10.1021/jm980354p. [DOI] [PubMed] [Google Scholar]
- 70.Cregge R J, Durham S L, Farr R A, Gallion S L, Hare C M, Hoffman R V, Janusz M J, Kim H-O, Koehl J R, Mehdi S, et al. J Med Chem. 1998;41:2461–2480. doi: 10.1021/jm970812e. [DOI] [PubMed] [Google Scholar]
- 71.Conde-Frieboes K, Reynolds L J, Lio Y-C, Hale M R, Wasserman H H, Dennis E A. J Am Chem Soc. 1996;118:5519–5525. [Google Scholar]
- 72.Patricelli M P, Cravatt B F. Biochemistry. 1999;38:14125–14130. doi: 10.1021/bi991876p. [DOI] [PubMed] [Google Scholar]
- 73.Patricelli M P, Lovato M A, Cravatt B F. Biochemistry. 1999;38:9804–9812. doi: 10.1021/bi990637z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.