Abstract
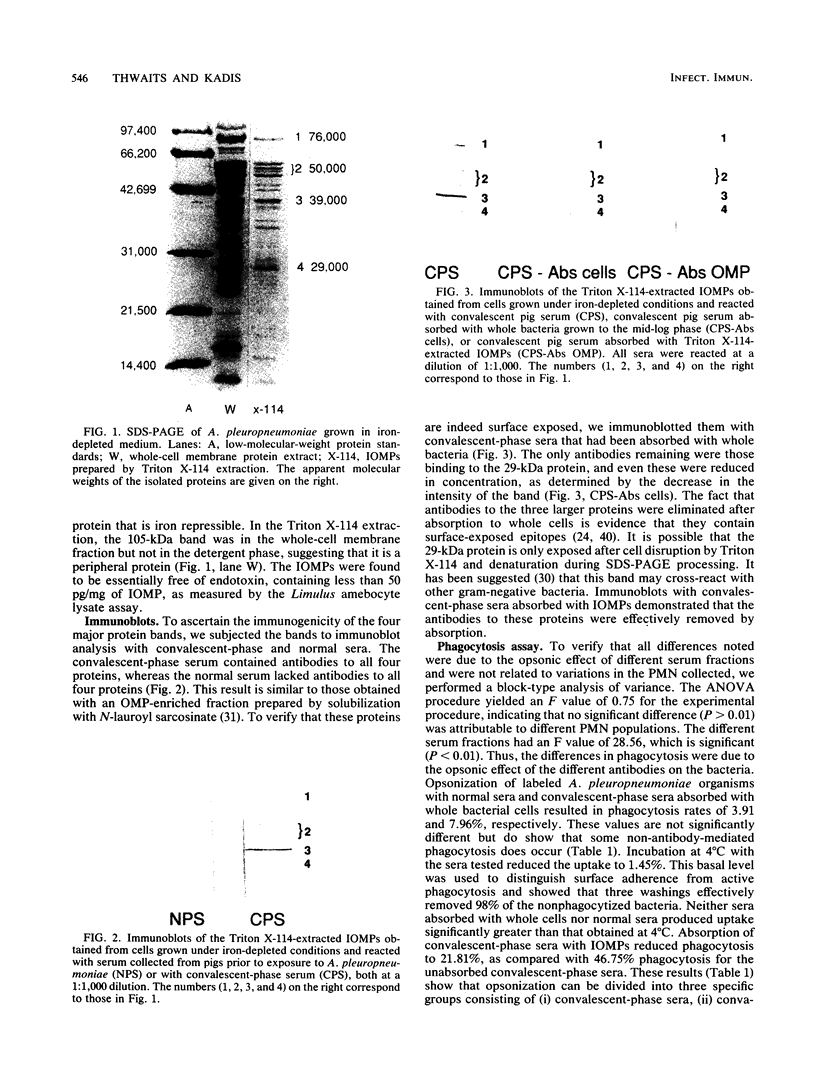
To determine the opsonic effect of antibodies to Actinobacillus (Haemophilus) pleuropneumoniae outer membrane proteins on phagocytosis by porcine polymorphonuclear leukocytes (PMN), we separated the integral outer membrane proteins (IOMPs) by Triton X-114 extraction. Four major IOMPs with molecular masses of 76, 50, 39, and 29 kDa were detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. These IOMPs were found to be essentially free of endotoxin in the Limulus amebocyte lysate assay. The 76-kDa protein exhibited a more intensely stained electrophoresis band when isolated from iron-restricted cultures, and a new band at 105 kDa was present in the whole-membrane fraction but not in the integral fraction, indicating that the 105-kDa iron-repressible protein is a peripheral membrane protein. The 76-, 50-, and 39-kDa proteins were shown to be surface exposed, since antibodies to these IOMPs could be absorbed out of convalescent-phase sera by whole cells. Percentages of phagocytosis by porcine PMN of A. pleuropneumoniae opsonized with convalescent-phase sera, convalescent-phase sera absorbed with IOMPs, or convalescent-phase sera absorbed with whole cells were 46.75, 21.81, and 7.96%, respectively. These results demonstrate that antibodies to IOMPs of A. pleuropneumoniae serve as important opsonins in phagocytosis by porcine PMN.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. M., Roberts I., Boulnois G. J., Saunders J. R., Hart C. A. Contribution of capsular polysaccharide and surface properties to virulence of Escherichia coli K1. Infect Immun. 1987 Nov;55(11):2662–2668. doi: 10.1128/iai.55.11.2662-2668.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bricker B. J., Tabatabai L. B., Deyoe B. L., Mayfield J. E. Conservation of antigenicity in a 31-kDa Brucella protein. Vet Microbiol. 1988 Dec;18(3-4):313–325. doi: 10.1016/0378-1135(88)90096-x. [DOI] [PubMed] [Google Scholar]
- Byrd W., Kadis S. Structures and sugar compositions of lipopolysaccharides isolated from seven Actinobacillus pleuropneumoniae serotypes. Infect Immun. 1989 Dec;57(12):3901–3906. doi: 10.1128/iai.57.12.3901-3906.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. F., Young R., Struck D. K. Cloning and characterization of a hemolysin gene from Actinobacillus (Haemophilus) pleuropneumoniae. DNA. 1989 Nov;8(9):635–647. doi: 10.1089/dna.1.1989.8.635. [DOI] [PubMed] [Google Scholar]
- Deneer H. G., Potter A. A. Effect of iron restriction on the outer membrane proteins of Actinobacillus (Haemophilus) pleuropneumoniae. Infect Immun. 1989 Mar;57(3):798–804. doi: 10.1128/iai.57.3.798-804.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneer H. G., Potter A. A. Identification of a maltose-inducible major outer membrane protein in Actinobacillus (Haemophilus) pleuropneumoniae. Microb Pathog. 1989 Jun;6(6):425–432. doi: 10.1016/0882-4010(89)90084-3. [DOI] [PubMed] [Google Scholar]
- Deneer H. G., Potter A. A. Iron-repressible outer-membrane proteins of Pasteurella haemolytica. J Gen Microbiol. 1989 Feb;135(Pt 2):435–443. doi: 10.1099/00221287-135-2-435. [DOI] [PubMed] [Google Scholar]
- Devenish J., Rosendal S., Johnson R., Hubler S. Immunoserological comparison of 104-kilodalton proteins associated with hemolysis and cytolysis in Actinobacillus pleuropneumoniae, Actinobacillus suis, Pasteurella haemolytica, and Escherichia coli. Infect Immun. 1989 Oct;57(10):3210–3213. doi: 10.1128/iai.57.10.3210-3213.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorka-Cray P. J., Huether M. J., Stine D. L., Anderson G. A. Efficacy of a cell extract from Actinobacillus (Haemophilus) pleuropneumoniae serotype 1 against disease in swine. Infect Immun. 1990 Feb;58(2):358–365. doi: 10.1128/iai.58.2.358-365.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick B. W., Osburn B. I. Immune responses to the lipopolysaccharides and capsular polysaccharides of Haemophilus pleuropneumoniae in convalescent and immunized pigs. Infect Immun. 1986 Nov;54(2):575–582. doi: 10.1128/iai.54.2.575-582.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick B. W., Osburn B. I. Vaccine potential of Haemophilus pleuropneumoniae oligosaccharide-tetanus toxoid conjugates. Infect Immun. 1986 Nov;54(2):583–586. doi: 10.1128/iai.54.2.583-586.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T. R., Williams M. R., Bunch K. J. An improved method for the isolation of a neutrophil-rich fraction from porcine blood. Br Vet J. 1985 Jul-Aug;141(4):355–361. doi: 10.1016/0007-1935(85)90084-3. [DOI] [PubMed] [Google Scholar]
- Higgins R., Larivière S., Mittal K. R., Martineau G. P., Rousseau P., Cameron J. Evaluation of a Killed Vaccine Against Porcine Pleuropneumonia Due to Haemophilus pleuropneumoniae. Can Vet J. 1985 Feb;26(2):86–89. [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J., Mathison B. Serotype specificity and immunogenicity of the capsular polymer of Haemophilus pleuropneumoniae serotype 5. Infect Immun. 1987 Jul;55(7):1580–1587. doi: 10.1128/iai.55.7.1580-1587.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J. Purification and partial characterization of the capsular polymer of Haemophilus pleuropneumoniae serotype 5. Infect Immun. 1987 Jul;55(7):1573–1579. doi: 10.1128/iai.55.7.1573-1579.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J., Tosi M. F., Kaplan S. L., Anderson D. C., Mason E. O., Jr, Williams R. P. Effect of Haemophilus influenzae type b lipopolysaccharide on complement activation and polymorphonuclear leukocyte function. Pediatr Res. 1987 Dec;22(6):659–666. doi: 10.1203/00006450-198712000-00010. [DOI] [PubMed] [Google Scholar]
- Jonson G., Svennerholm A. M., Holmgren J. Vibrio cholerae expresses cell surface antigens during intestinal infection which are not expressed during in vitro culture. Infect Immun. 1989 Jun;57(6):1809–1815. doi: 10.1128/iai.57.6.1809-1815.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp E. M., van Leengoed L. A. Serotype-related differences in production and type of heat-labile hemolysin and heat-labile cytotoxin of Actinobacillus (Haemophilus) pleuropneumoniae. J Clin Microbiol. 1989 Jun;27(6):1187–1191. doi: 10.1128/jcm.27.6.1187-1191.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper D. L. Bacterial capsule--old dogmas and new tricks. J Infect Dis. 1986 Mar;153(3):407–415. doi: 10.1093/infdis/153.3.407. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loeb M. R. Immunoblot method for identifying surface components, determining their cross-reactivity, and investigating cell topology: results with Haemophilus influenzae type b. Anal Biochem. 1984 Nov 15;143(1):196–204. doi: 10.1016/0003-2697(84)90576-1. [DOI] [PubMed] [Google Scholar]
- MacInnes J. I., Rosendal S. Analysis of major antigens of Haemophilus (Actinobacillus) pleuropneumoniae and related organisms. Infect Immun. 1987 Jul;55(7):1626–1634. doi: 10.1128/iai.55.7.1626-1634.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton D. J., Williams P. Characterization of the outer-membrane proteins of Haemophilus parainfluenzae expressed under iron-sufficient and iron-restricted conditions. J Gen Microbiol. 1989 Feb;135(Pt 2):445–451. doi: 10.1099/00221287-135-2-445. [DOI] [PubMed] [Google Scholar]
- Moxon E. R., Deich R. A., Connelly C. Cloning of chromosomal DNA from Haemophilus influenzae. Its use for studying the expression of type b capsule and virulence. J Clin Invest. 1984 Feb;73(2):298–306. doi: 10.1172/JCI111214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niven D. F., Donga J., Archibald F. S. Responses of Haemophilus pleuropneumoniae to iron restriction: changes in the outer membrane protein profile and the removal of iron from porcine transferrin. Mol Microbiol. 1989 Aug;3(8):1083–1089. doi: 10.1111/j.1365-2958.1989.tb00258.x. [DOI] [PubMed] [Google Scholar]
- Rapp V. J., Munson R. S., Jr, Ross R. F. Outer membrane protein profiles of Haemophilus pleuropneumoniae. Infect Immun. 1986 May;52(2):414–420. doi: 10.1128/iai.52.2.414-420.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp V. J., Ross R. F. Antibody response of swine to outer membrane components of Haemophilus pleuropneumoniae during infection. Infect Immun. 1986 Dec;54(3):751–760. doi: 10.1128/iai.54.3.751-760.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. B. Vaccines for the prevention of encapsulated bacterial diseases: current status, problems and prospects for the future. Immunochemistry. 1978 Nov;15(10-11):839–854. doi: 10.1016/0161-5890(78)90117-7. [DOI] [PubMed] [Google Scholar]
- Rosendal S., Carpenter D. S., Mitchell W. R., Wilson M. R. Vaccination against pleuropneumonia of pigs caused by Haemophilus pleuropneumoniae. Can Vet J. 1981 Feb;22(2):34–35. [PMC free article] [PubMed] [Google Scholar]
- SHOPE R. E. PORCINE CONTAGIOUS PLEUROPNEUMONIA. I. EXPERIMENTAL TRANSMISSION, ETIOLOGY, AND PATHOLOGY. J Exp Med. 1964 Mar 1;119:357–368. doi: 10.1084/jem.119.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford S. E., Josephson G. K. Porcine Haemophilus pleuropneumonia epizootic in southwestern Ontario: clinical, microbiological, pathological and some epidemiological findings. Can J Comp Med. 1981 Jan;45(1):2–7. [PMC free article] [PubMed] [Google Scholar]
- Schultz R. A., Young T. F., Ross R. F., Jeske D. R. Prevalence of antibodies to Haemophilus pleuropneumoniae in Iowa swine. Am J Vet Res. 1982 Oct;43(10):1848–1851. [PubMed] [Google Scholar]
- Sebunya T. N., Saunders J. R. Haemophilus pleuropneumoniae infection in swine: a review. J Am Vet Med Assoc. 1983 Jun 15;182(12):1331–1337. [PubMed] [Google Scholar]
- Simms H. H., Frank M. M., Quinn T. C., Holland S., Gaither T. A. Studies on phagocytosis in patients with acute bacterial infections. J Clin Invest. 1989 Jan;83(1):252–260. doi: 10.1172/JCI113867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stull T. L., Mack K., Haas J. E., Smit J., Smith A. L. A comparison of techniques for isolation of the outer membrane proteins of Haemophilus influenzae type b. Anal Biochem. 1985 Nov 1;150(2):471–480. doi: 10.1016/0003-2697(85)90537-8. [DOI] [PubMed] [Google Scholar]
- Swanson J., Barrera O. Immunological characteristics of gonococcal outer membrane protein II assessed by immunoprecipitation, immunoblotting, and coagglutination. J Exp Med. 1983 May 1;157(5):1405–1420. doi: 10.1084/jem.157.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis K. N., Boulnois G. J., Bitter-Suermann D., Cabello F. C. Surface components of Escherichia coli that mediate resistance to the bactericidal activities of serum and phagocytes. Curr Top Microbiol Immunol. 1985;118:197–218. doi: 10.1007/978-3-642-70586-1_11. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truscott W. M., Hirsh D. C. Demonstration of an outer membrane protein with antiphagocytic activity from Pasteurella multocida of avian origin. Infect Immun. 1988 Jun;56(6):1538–1544. doi: 10.1128/iai.56.6.1538-1544.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang V. C., Peralta J. M., Simons A. R. Enzyme-linked immunoelectrotransfer blot techniques (EITB) for studying the specificities of antigens and antibodies separated by gel electrophoresis. Methods Enzymol. 1983;92:377–391. doi: 10.1016/0076-6879(83)92032-3. [DOI] [PubMed] [Google Scholar]
- Udeze F. A., Latimer K. S., Kadis S. Role of haemophilus pleuropneumoniae lipopolysaccharide endotoxin in the pathogenesis of porcine Haemophilus pleuropneumonia. Am J Vet Res. 1987 May;48(5):768–773. [PubMed] [Google Scholar]
- Van Leengoed L. A., Kamp E. M., Pol J. M. Toxicity of Haemophilus pleuropneumoniae to porcine lung macrophages. Vet Microbiol. 1989 Apr;19(4):337–349. doi: 10.1016/0378-1135(89)90099-0. [DOI] [PubMed] [Google Scholar]
- van Leengoed L. A., Kamp E. M. Endobronchial inoculation of various doses of Haemophilus (Actinobacillus) pleuropneumoniae in pigs. Am J Vet Res. 1989 Dec;50(12):2054–2059. [PubMed] [Google Scholar]





