Abstract
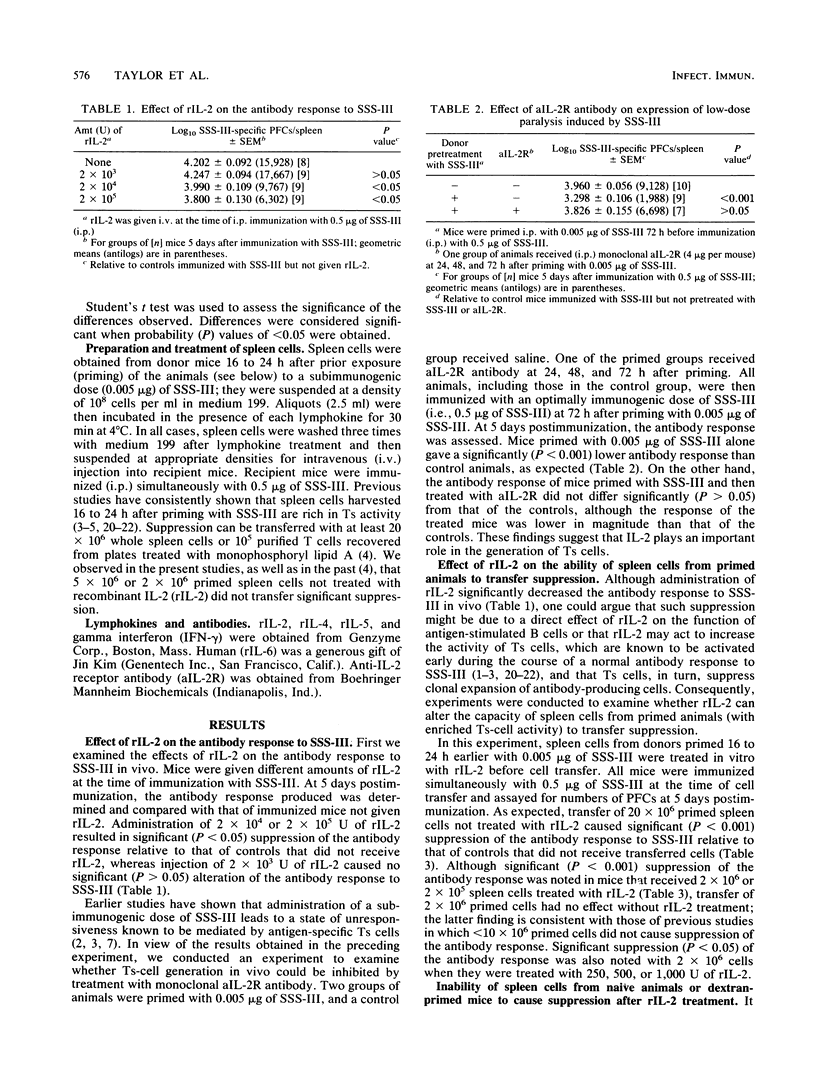
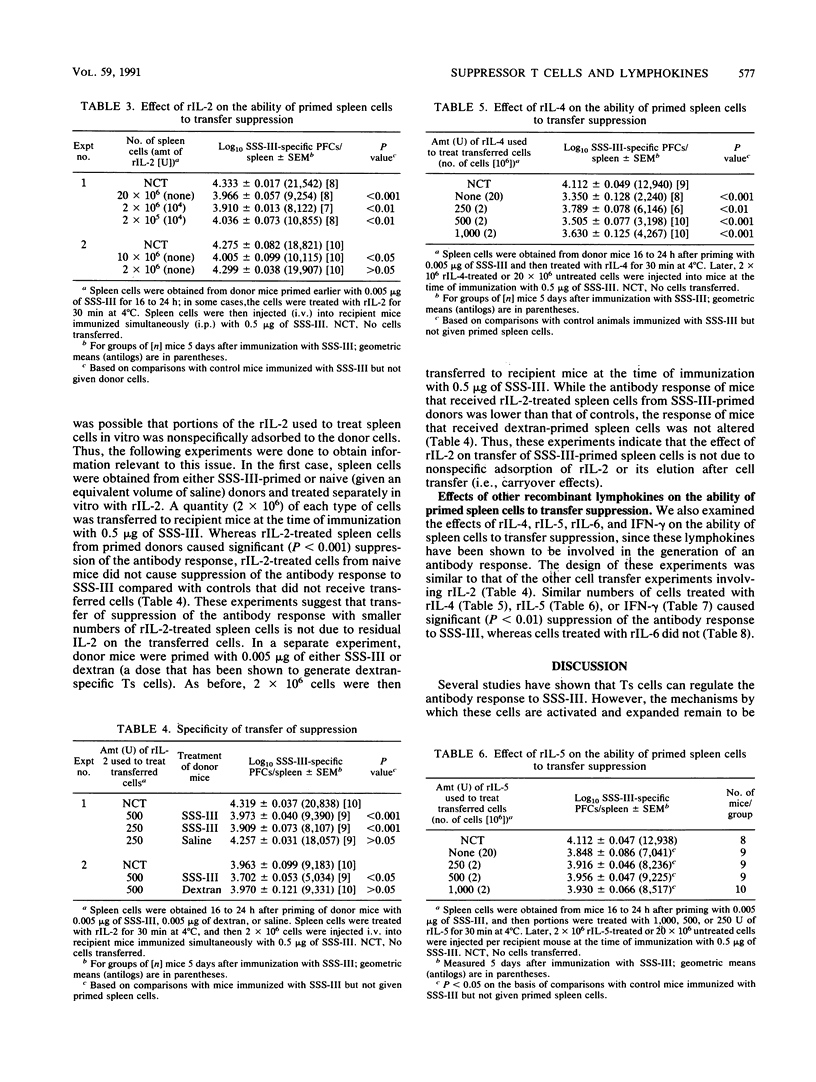
Previous studies have shown that transfer of whole spleen cell populations obtained from primed donors or transfer of purified T cells enriched for suppressor activity (Ts) to recipient mice decreased the antibody response to pneumococcal polysaccharide type III (SSS-III) when the animals were simultaneously immunized with SSS-III. In the present studies, such suppression of the antibody response was transferred with 10- to 100-fold fewer primed spleen cells when the cells were treated in vitro with recombinant interleukin-2 (rIL-2) before transfer; spleen cells from naive mice or mice primed with an unrelated antigen (dextran) and then treated with rIL-2 did not cause suppression of the antibody response to SSS-III, thereby eliminating the possibility of nonspecific carryover effects induced by rIL-2. In vivo administration of rIL-2 at the time of immunization with an optimally immunogenic dose of SSS-III resulted in significant (P less than 0.05) suppression of the antibody response relative to that of control animals, suggesting that IL-2 augments the clonal expansion of Ts cells in vivo. Further, the ability of passively administered anti-IL-2 receptor antibody to inhibit generation of Ts cells in vivo is consistent with such a view. Spleen cells from primed animals treated with rIL-4, rIL-5, or gamma interferon--but not those from primed animals treated with rIL-6--likewise were able to transfer suppression of the antibody response with fewer cells than those required when primed cells not treated with lymphokines were used. Thus, these studies indicate that Ts cell activity is greatly influenced by lymphokines produced by helper T cells. The studies also suggest that these lymphokines are required during activation and/or clonal expansion of Ts cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. H., Stashak P. W. Quantitative and qualitative studies on the primary antibody response to pneumococcal polysaccharides at ehe cellular level. J Immunol. 1969 Dec;103(6):1342–1348. [PubMed] [Google Scholar]
- Baker P. J., Amsbaugh D. F., Stashak P. W., Caldes G., Prescott B. Direct evidence for the involvement of T suppressor cells in the expression of low-dose paralysis to type III pneumococcal polysaccharide. J Immunol. 1982 Mar;128(3):1059–1062. [PubMed] [Google Scholar]
- Baker P. J., Amsbaugh D. F., Stashak P. W., Caldes G., Prescott B. Regulation of the antibody response to pneumococcal polysaccharide by thymus-derived cells. Rev Infect Dis. 1981 Mar-Apr;3(2):332–341. doi: 10.1093/clinids/3.2.332. [DOI] [PubMed] [Google Scholar]
- Baker P. J., Haslov K. R., Fauntleroy M. B., Stashak P. W., Myers K., Ulrich J. T. Enrichment of suppressor T cells by means of binding to monophosphoryl lipid A. Infect Immun. 1990 Mar;58(3):726–731. doi: 10.1128/iai.58.3.726-731.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Hiernaux J. R., Fauntleroy M. B., Prescott B., Cantrell J. L., Rudbach J. A. Inactivation of suppressor T-cell activity by nontoxic monophosphoryl lipid A. Infect Immun. 1988 May;56(5):1076–1083. doi: 10.1128/iai.56.5.1076-1083.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Prescott B., Stashak P. W., Amsbaugh D. F. Characterization of the antibody response to type 3 pneumococcal polysaccharide at the cellular level. 3. Studies on the average avidity of the antibody produced by specific plaque-forming cells. J Immunol. 1971 Sep;107(3):719–724. [PubMed] [Google Scholar]
- Baker P. J., Reed N. D., Stashak P. W., Amsbaugh D. F., Prescott B. Regulation of the antibody response to type 3 pneumococcal polysaccharide. I. Nature of regulatory cells. J Exp Med. 1973 Jun 1;137(6):1431–1441. doi: 10.1084/jem.137.6.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J. Regulation of magnitude of antibody response to bacterial polysaccharide antigens by thymus-derived lymphocytes. Infect Immun. 1990 Nov;58(11):3465–3468. doi: 10.1128/iai.58.11.3465-3468.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B. Characterization of the antibody response to type 3 pneumococcal polysaccharide at the cellular level. I. Dose-response studies and the effect of prior immunization on the magnitude of the antibody response. Immunology. 1971 Apr;20(4):469–480. [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B. Characterization of the antibody response to type 3 pneumococcal polysaccharide at the cellular level. II. Studies on the relative rate of antibody synthesis and release by antibody-producing cells. Immunology. 1971 Apr;20(4):481–492. [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Prescott B. Use of erythrocytes sensitized with purified pneumococcal polysaccharides for the assay of antibody and antibody-producing cells. Appl Microbiol. 1969 Mar;17(3):422–426. doi: 10.1128/am.17.3.422-426.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Taylor C., Fauntleroy M. B., Stashak P. W., Prescott B. The role of antigen in the activation of regulatory T cells by immune B cells. Cell Immunol. 1985 Dec;96(2):376–385. doi: 10.1016/0008-8749(85)90368-5. [DOI] [PubMed] [Google Scholar]
- Elkins K. L., Stashak P. W., Baker P. J. Metabolic activity is necessary for activation of T suppressor cells by B cells. J Immunol. 1990 Apr 15;144(8):2859–2864. [PubMed] [Google Scholar]
- Elkins K. L., Stashak P. W., Baker P. J. Transferred B cells from autoimmune NZB/N mice fail to activate T suppressor cells. Cell Immunol. 1987 Nov;110(1):14–27. doi: 10.1016/0008-8749(87)90097-9. [DOI] [PubMed] [Google Scholar]
- Gottlieb C. F. Application of transformations to normalize the distribution of plaque-forming cells. J Immunol. 1974 Jul;113(1):51–57. [PubMed] [Google Scholar]
- Hamby B. A., Huggins E. M., Jr, Lachman L. B., Dinarello C. A., Sigel M. M. Fish lymphocytes respond to human IL-1. Lymphokine Res. 1986 Spring;5(2):157–162. [PubMed] [Google Scholar]
- Holda J. H., Maier T., Claman H. N. Natural suppressor activity in graft-vs-host spleen and normal bone marrow is augmented by IL 2 and interferon-gamma. J Immunol. 1986 Dec 1;137(11):3538–3543. [PubMed] [Google Scholar]
- Jones J. M., Amsbaugh D. F., Stashak P. W., Prescott B., Baker P. J., Alling D. W. Kinetics of the antibody response to type III pneumococcal polysaccharide. I. Evidence that suppressor cells function by inhibiting the recruitment and proliferation of antibody-producing cells. J Immunol. 1976 Mar;116(3):647–656. [PubMed] [Google Scholar]
- Mitchell M. S., Kempf R. A., Harel W., Shau H., Boswell W. D., Lind S., Bradley E. C. Effectiveness and tolerability of low-dose cyclophosphamide and low-dose intravenous interleukin-2 in disseminated melanoma [corrected]. J Clin Oncol. 1988 Mar;6(3):409–424. doi: 10.1200/JCO.1988.6.3.409. [DOI] [PubMed] [Google Scholar]
- Oh-Ishi T., Goldman C. K., Misiti J., Waldmann T. A. The interaction of interleukin 2 with its receptor in the generation of suppressor T cells in antigen-specific and antigen-nonspecific systems in vitro. Clin Immunol Immunopathol. 1989 Sep;52(3):447–459. doi: 10.1016/0090-1229(89)90159-1. [DOI] [PubMed] [Google Scholar]
- Stashak P. W., Taylor C. E., Caldes G., Prescott B., Baker P. J. Cyclic expression of low-dose paralysis. Cell Immunol. 1983 Apr 1;77(1):143–149. doi: 10.1016/0008-8749(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Taylor C. E., Amsbaugh D. F., Stashak P. W., Caldes G., Prescott B., Baker P. J. Cell surface antigens and other characteristics of T cells regulating the antibody response to type III pneumococcal polysaccharide. J Immunol. 1983 Jan;130(1):19–23. [PubMed] [Google Scholar]
- Taylor C. E., Stashak P. W., Caldes G., Prescott B., Chused T. E., Brooks A., Baker P. J. Activation of antigen-specific suppressor T cells by B cells from mice immunized with type III pneumococcal polysaccharide. J Exp Med. 1983 Sep 1;158(3):703–717. doi: 10.1084/jem.158.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. E., Stashak P. W., Caldes G., Prescott B., Fowlkes B. J., Baker P. J. Lectin-induced modulation of the antibody response to type III pneumococcal polysaccharide. Cell Immunol. 1984 Jan;83(1):26–33. doi: 10.1016/0008-8749(84)90221-1. [DOI] [PubMed] [Google Scholar]
- Thoman M. L., Weigle W. O. Deficiency in suppressor T cell activity in aged animals. Reconstitution of this activity by interleukin 2. J Exp Med. 1983 Jun 1;157(6):2184–2189. doi: 10.1084/jem.157.6.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting C. C., Yang S. S., Hargrove M. E. Induction of suppressor T cells by interleukin 2. J Immunol. 1984 Jul;133(1):261–266. [PubMed] [Google Scholar]
- Volk H. D., Diamantstein T. IL-2 normalizes defective suppressor T cell function of patients with systemic lupus erythematosus in vitro. Clin Exp Immunol. 1986 Dec;66(3):525–531. [PMC free article] [PubMed] [Google Scholar]


