Abstract
Dysfunction of mitochondrial complex I leads to degeneration of dopaminergic neurons of the substantia nigra pars compacta, as seen in Parkinson’s disease, through activation of mitochondria-dependent programmed cell death pathways. In this scenario, complex I blockade increases the soluble pool of cytochrome c in the mitochondrial intermembrane space through oxidative mechanisms, whereas activation of pro-cell death protein Bax triggers neuronal death by permeabilizing the outer mitochondrial membrane and releasing cytochrome c into the cytosol. Targeting either Bax transcriptional or post-translational activation results in a marked attenuation of dopaminergic cell death caused by complex I inhibition.
Keywords: Apoptosis, mitochondria, complex I, oxidative stress, Bax, BH3-only, p53
Introduction
Programmed cell death (PCD) is a physiological process that occurs naturally during development and morphogenesis in which intrinsic molecular programs are activated to cause cell death [1]. PCD is highly regulated by proteins of the Bcl-2 family, comprising members that have either anti-PCD (such as Bcl-2 and Bcl-xL) or pro-PCD (such as Bax and Bak) effects [2]. Structurally, all these proteins share some degree of similarity and can have up to four Bcl-2-homology domains (BH1–BH4). In addition to the Bcl-2 members that contain multiple BH domains, such as Bcl-2 and Bax, there are molecules that share sequence homology only with the BH3 domain (so-called “BH3-only” proteins), which induce cell death by playing a crucial role in activating Bax, either by directly facilitating Bax oligomerization and translocation to the mitochondria or by inactivating anti-PCD proteins such as Bcl-2 [3,4]. At least 10 different BH3-only molecules have been described so far in mammals, including Puma (p53-upregulated modulator of apoptosis), Noxa (the Greek word for ‘‘damage’’) or Bim (Bcl-2-interacting mediator of cell death).
Mitochondria play a central role in the regulation of PCD. Multidomain Bcl-2 members can preserve or disrupt mitochondrial integrity by regulating the release of mitochondrial apoptogenic factors such as cytochrome c, Smac/Diablo, Omi/HTRA2, endonuclease G or apoptosis-inducing factor (AIF). Once outside the mitochondria, these factors may initiate cell death in a caspase-dependent or a caspase-independent manner [5].
PCD and Parkinson’s disease
Mounting evidence indicates that inappropriate re-activation of PCD in adulthood may contribute to substantia nigra pars compacta (SNpc) dopaminergic (DAergic) neurodegeneration linked to complex I deficiency in Parkinson’s disease (PD). In particular, studies in rodents and human post-mortem PD samples have shown that PD-related DAergic neurodegeneration occurs, at least in part, through activation of mitochondria-dependent PCD molecular pathways [6–8]. After complex I blockade with MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) in mice, there is a time-dependent and region-specific mitochondrial release of cytochrome c that occurs in association with activation of caspase-9 and -3 [7]. All of these molecular alterations, including SNpc DAergic cell death, appear to be regulated by the cell death agonist Bax, because they coincide with Bax upregulation and translocation to the mitochondria and are prevented by genetic ablation of Bax [7,9]. The involvement of the mitochondrial-dependent PCD pathway in PD-related neurodegeneration is further supported by the neuroprotective effect obtained when targeting other molecules of this pathway such as caspase-9 or Apaf-1 in MPTP-intoxicated mice [10,11]. In addition, activation of Bax, caspase-9 and caspase-3 has been detected in SNpc DAergic neurons of human post-mortem PD brains [10,12–14].
It was widely assumed that complex I inhibition and the subsequent impairment of mitochondrial respiration occurring in PD could directly trigger the release of cytochrome c from the affected mitochondria and induce cell death [15–18]. Contrary to this view, we observed that complex I inhibition in experimental models of PD does not directly induce cytochrome c release from isolated brain mitochondria but, instead, it increases the “releasable” soluble pool of cytochrome c in the mitochondrial intermembrane space that can be subsequently released to the cytosol by cell death agonists, such as Bax [7]. This phenomenon is mediated by mitochondrial oxidative damage secondary to complex I inhibition, such as peroxidation of the inner mitochondrial lipid cardiolipin, which affects the binding of cytochrome c to the mitochondrial inner membrane, leading to an increased soluble pool of cytochrome c in the mitochondrial intermembrane space [7]. Membrane-permeant antioxidants block the neuronal-death-promoting interaction between mitochondrial oxidative damage and Bax-induced permeabilization of the outer mitochondrial membrane, suggesting that these compounds, of which a few are near approval for human use, may prove effective in mitigating neurodegeneration in complex I cytopathies, such as PD [7].
We have also identified the molecular mechanisms responsible for Bax transcriptional and post-translational activation in experimental PD, which involve the cooperation of the transcriptional factor p53 with the BH3-only protein Bim [8]. In particular, by using gene-targeted mice, we have shown that while p53 mediates Bax transcriptional induction following complex I blockade with MPTP, it does not participate in Bax mitochondrial translocation in this model, neither by a transcription-independent mechanism nor through induction of the BH3-only proteins Puma or Noxa [8]. In this context, post-translational activation of Bax relied on JNK-dependent activation of the BH3-only protein Bim [8]. Targeting either Bax transcriptional induction or Bax post-translational activation resulted in a marked attenuation of mitochondria-dependent apoptotic SNpc DAergic cell death caused by complex I inhibition with MPTP [8].
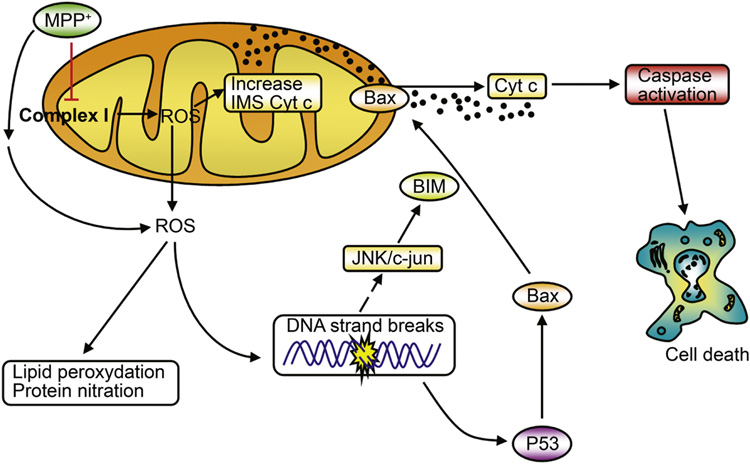
Proposed mechanism of PCD-dependent dopaminergic cell death
Based on our results, we proposed a pathogenic scenario in which neuronal death caused by complex I deficiency results from a self-amplifying cascade of deleterious events starting at the mitochondria by the alteration of the oxidative phosphorylation and finishing also at the mitochondria by the activation of the PCD machinery [7,8]. In this scenario (Figure 1), MPTP impairs mitochondrial respiration in SNpc DAergic neurons by inhibiting complex I of the electron transport chain. Inhibition of complex I blocks the flow of electrons along the mitochondrial electron transport chain, which results in an increased production of reactive oxygen species (ROS). Mitochondrial ROS then increases the soluble pool of cytochrome c in the mitochondrial intermembrane space by a mechanism involving peroxidation of cardiolipin, while ROS outside the mitochondria, probably emanating also from sources other than complex I inhibition [19], damage different cellular elements, such as lipids, proteins and DNA. DNA damage activates both p53 and JNK. p53 induces transcriptional upregulation of Bax, while JNK participates in Bax mitochondrial translocation through transcriptional activation of the BH3-only protein Bim. Once localized to the mitochondrial outer membrane, Bax induces the release of cytochrome c into the cytosol and the ensuing caspase activation and cell death. Supporting the relevance of this scenario to SNpc DAergic neurodegeneration occurring in PD, several elements of this molecular cascade have been demonstrated in post-mortem human brain samples from PD patients, including complex I deficiency [20–22], ROS production [23], oxidative damage to lipids [24,25], proteins [26,27] and DNA [28,29], JNK activation [30–32], Bax activation [13,14] and activation of caspase-9 [10] and caspase-3 [12,14].
Figure 1.
Proposed pathogenic mechanism induced by complex I deficiency with MPTP. In this scenario, SNpc DAergic neuronal death results from a self-amplifying cascade of deleterious events that start at the mitochondria with the alteration of oxidative phosphorylation and finish at the mitochondria with the activation of the programmed cell death machinery. See text for details. Figure adapted from ref. [8].
Of note, while the mechanism by which Bax induces cytochrome c release is still a matter of intense debate, our results suggested that, in the context of complex I deficiency, this phenomenon seems to be independent of the mitochondrial permeability transition pore, since Bax-induced cytochrome c release in MPP+ (1-methyl-4-phenylpyridinium ion)-treated brain mitochondria was not responsive to the mitochondrial pore blocker cyclosporin A and mice deficient for cyclophilin D, a critical component of the mitochondrial pore, did not exhibit a reduced susceptibility to MPTP [8]. It is also worth mentioning that although other pro-apoptotic molecules such as Bid and Bak are known for cooperating with Bax to initiate mitochondria-dependent apoptosis in response to the ligation of cell-surface death receptors, they seem to be dispensable in complex I deficiency-mediated neuronal death [10,33].
Conclusion
Activation of mitochondria-dependent PCD pathways appear to be instrumental in the death of SNpc DAergic neurons in experimental models of PD. Our studies support a pathogenic scenario in which complex I deficiency linked to PD does not autonomously kill cells but rather sensitizes neurons to the action of death agonists such as Bax, through mitochondrial oxidative damage. The complexity of this molecular cascade guarantees an exquisite control over neuronal cell death and provides several targets of potential therapeutic significance. Approaches aimed at targeting different key elements of this cascade, especially those acting upstream of cytochrome c release such as Bax activation, all result in a significant attenuation of MPTP-induced SNpc DAergic neurodegeneration [6–9]. In contrast, interfering at a more downstream level, such as caspase activation, seems to produce more variable results [6]. This is of therapeutic relevance because, once the caspase executioner program is in place, its inhibition might only be able to delay, but not prevent, cell death. However, pharmacological targeting of Bax has been precluded so far by the lack of specific molecular tools. The recent introduction of new molecules presumably capable of inhibiting Bax post-translational activation [34–36] may provide new insights on the potential beneficial effect of therapeutic strategies aimed at preventing Bax-dependent cell death in many incurable human pathologies, including complex I deficiencies such as PD.
Acknowledgments
This work was supported by the Marie Curie Excellence Grant action (EU), the Marie Curie International Reintegration Grant action (EU), Fundació la Caixa (Spain), the National Institutes of Health/National Institute on Aging (USA) and the US Department of Defense (USA).
This article is based on a presentation given at the LIMPE Seminars 2007 “Experimental Models in Parkinson’s Disease” held in September 2007 at the “Porto Conte Ricerche” Congress Center in Alghero, Sardinia, Italy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors have declared no conflicts of interest.
References
- 1.Vaux DL, Strasser A. The molecular biology of apoptosis. Proc Natl Acad Sci USA. 1996;93(6):2239–2244. doi: 10.1073/pnas.93.6.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13(15):1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 3.Letai A, Bassik M, Walensky L, Sorcinelli M, Weiler S, Korsmeyer S. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2(3):183. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 4.Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315(5813):856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 5.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116(2):205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 6.Vila M, Przedborski S. Neurological diseases: Targeting programmed cell death in neurodegenerative diseases. Nat Rev Neurosci. 2003;4(5):365–375. doi: 10.1038/nrn1100. [DOI] [PubMed] [Google Scholar]
- 7.Perier C, Tieu K, Guegan C, Caspersen C, Jackson-Lewis V, Carelli V. Complex I deficiency primes Bax-dependent neuronal apoptosis through mitochondrial oxidative damage. Proc Natl Acad Sci U S A. 2005;102(52):19126–19131. doi: 10.1073/pnas.0508215102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perier C, Bove J, Wu DC, Dehay B, Choi DK, Jackson-Lewis V, et al. Two molecular pathways initiate mitochondria-dependent dopaminergic neurodegeneration in experimental Parkinson's disease. Proc Natl Acad Sci U S A. 2007;104(19):8161–8166. doi: 10.1073/pnas.0609874104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vila M, Jackson-Lewis V, Vukosavic S, Djaldetti R, Liberatore G, Offen D, et al. Bax ablation prevents dopaminergic neurodegeneration in the 1-methyl- 4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. Proc Natl Acad Sci USA. 2001;98(5):2837–2842. doi: 10.1073/pnas.051633998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viswanath V, Wu Y, Boonplueang R, Chen S, Stevenson FF, Yantiri F, et al. Caspase-9 activation results in downstream caspase-8 activation and bid cleavage in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson's disease. J Neurosci. 2001;21(24):9519–9528. doi: 10.1523/JNEUROSCI.21-24-09519.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mochizuki H, Hayakawa H, Migita M, Shibata M, Tanaka R, Suzuki A, et al. An AAV-derived Apaf-1 dominant negative inhibitor prevents MPTP toxicity as antiapoptotic gene therapy for Parkinson's disease. Proc Natl Acad Sci USA. 2001;98(19):10918–10923. doi: 10.1073/pnas.191107398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartmann A, Hunot S, Michel PP, Muriel MP, Vyas S, Faucheux BA, et al. Caspase-3: A vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson's disease. Proc Natl Acad Sci USA. 2000;97(6):2875–2880. doi: 10.1073/pnas.040556597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartmann A, Michel PP, Troadec JD, Mouatt-Prigent A, Faucheux BA, Ruberg M, et al. Is Bax a mitochondrial mediator in apoptotic death of dopaminergic neurons in Parkinson's disease? J Neurochem. 2001;76(6):1785–1793. doi: 10.1046/j.1471-4159.2001.00160.x. [DOI] [PubMed] [Google Scholar]
- 14.Tatton NA. Increased Caspase 3 and Bax Immunoreactivity Accompany Nuclear GAPDH Translocation and Neuronal Apoptosis in Parkinson's Disease. Exp Neurol. 2000;166(1):29–43. doi: 10.1006/exnr.2000.7489. [DOI] [PubMed] [Google Scholar]
- 15.Clayton R, Clark JB, Sharpe M. Cytochrome c release from rat brain mitochondria is proportional to the mitochondrial functional deficit: implications for apoptosis and neurodegenerative disease. J Neurochem. 2005;92(4):840–849. doi: 10.1111/j.1471-4159.2004.02918.x. [DOI] [PubMed] [Google Scholar]
- 16.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305(5684):626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 17.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 18.Cassarino DS, Parks JK, Parker WD, Jr, Bennett JP., Jr The parkinsonian neurotoxin MPP+ opens the mitochondrial permeability transition pore and releases cytochrome c in isolated mitochondria via an oxidative mechanism. Biochim Biophys Acta. 1999;1453(1):49–62. doi: 10.1016/s0925-4439(98)00083-0. [DOI] [PubMed] [Google Scholar]
- 19.Lotharius J, O'Malley KL. The parkinsonism-inducing drug 1-methyl-4-phenylpyridinium triggers intracellular dopamine oxidation. A novel mechanism of toxicity. J Biol Chem. 2000;275(49):38581–38588. doi: 10.1074/jbc.M005385200. [DOI] [PubMed] [Google Scholar]
- 20.Parker WD, Jr, Boyson SJ, Parks JK. Abnormalities of the electron transport chain in idiopathic Parkinson's disease. Ann Neurol. 1989;26:719–723. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- 21.Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson's disease. J Neurochem. 1990;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- 22.Keeney PM, Xie J, Capaldi RA, Bennett JP., Jr Parkinson's disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J Neurosci. 2006;26(19):5256–5264. doi: 10.1523/JNEUROSCI.0984-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu DC, Teismann P, Tieu K, Vila M, Jackson-Lewis V, Ischiropoulos H, et al. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine model of Parkinson's disease. Proc Natl Acad Sci USA. 2003;100:6145–6150. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dexter DT, Carter CJ, Wells FR, Javoy-Agid F, Agid Y, Lees A, et al. Basal lipid peroxidation in substantia nigra is increased in Parkinson's disease. J Neurochem. 1989;52:381–389. doi: 10.1111/j.1471-4159.1989.tb09133.x. [DOI] [PubMed] [Google Scholar]
- 25.Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc Natl Acad Sci USA. 1996;93(7):2696–2701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alam ZI, Daniel SE, Lees AJ, Marsden DC, Jenner P, Halliwell B. A generalised increase in protein carbonyls in the brain in Parkinson's but not incidental Lewy body disease. J Neurochem. 1997;69(3):1326–1329. doi: 10.1046/j.1471-4159.1997.69031326.x. [DOI] [PubMed] [Google Scholar]
- 27.Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, et al. Oxidative damage linked to neurodegeneration by selective alpha- synuclein nitration in synucleinopathy lesions. Science. 2000;290(5493):985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 28.Alam ZI, Jenner A, Daniel SE, Lees AJ, Cairns N, Marsden CD, et al. Oxidative DNA damage in the parkinsonian brain: An apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J Neurochem. 1997;69(3):1196–1203. doi: 10.1046/j.1471-4159.1997.69031196.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Perry G, Smith MA, Robertson D, Olson SJ, Graham DG, et al. Parkinson's disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons. Am J Pathol. 1999;154(5):1423–1429. doi: 10.1016/S0002-9440(10)65396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia XG, Harding T, Weller M, Bieneman A, Uney JB, Schulz JB. Gene transfer of the JNK interacting protein-1 protects dopaminergic neurons in the MPTP model of Parkinson's disease. Proc Natl Acad Sci USA. 2001;98(18):10433–10438. doi: 10.1073/pnas.181182298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teismann P, Tieu K, Choi DK, Wu DC, Naini A, Hunot S, et al. Cyclooxygenase-2 is instrumental in Parkinson's disease neurodegeneration. Proc Natl Acad Sci USA. 2003;100:5473–5478. doi: 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunot S, Vila M, Teismann P, Davis RJ, Hirsch EC, Przedborski S, et al. JNK-mediated induction of cyclooxygenase 2 is required for neurodegeneration in a mouse model of Parkinson's disease. Proc Natl Acad Sci USA. 2004;101(2):665–670. doi: 10.1073/pnas.0307453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fannjiang Y, Kim CH, Huganir RL, Zou SF, Lindsten T, Thompson CB, et al. BAK alters neuronal excitability and can switch from anti- to pro-death function during postnatal development. Developmental Cell. 2003;4(4):575–585. doi: 10.1016/s1534-5807(03)00091-1. [DOI] [PubMed] [Google Scholar]
- 34.Sawada M, Hayes P, Matsuyama S. Cytoprotective membrane-permeable peptides designed from the Bax-binding domain of Ku70. Nat Cell Biol. 2003;5(4):352–357. doi: 10.1038/ncb955. [DOI] [PubMed] [Google Scholar]
- 35.Guo B, Zhai D, Cabezas E, Welsh K, Nouraini S, Satterthwait AC, et al. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature. 2003;423(6938):456–461. doi: 10.1038/nature01627. [DOI] [PubMed] [Google Scholar]
- 36.Hetz C, Vitte PA, Bombrun A, Rostovtseva TK, Montessuit S, Hiver A, et al. Bax channel inhibitors prevent mitochondrion-mediated apoptosis and protect neurons in a model of global brain ischemia. J Biol Chem. 2005;280(52):42960–42970. doi: 10.1074/jbc.M505843200. [DOI] [PubMed] [Google Scholar]