Abstract
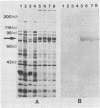
The antigen-coding region of a 4.2-kb PstI fragment of Chlamydia pneumoniae (pLC3), which encodes a 75-kDa immunoreactive protein recognized during human C. pneumoniae infection, was localized to a 2.0-kb EcoRI fragment. This subclone expressed an immunoreactive fusion protein of ca. 82 kDa. Nucleotide sequence analysis of the C. pneumoniae gene revealed that it consisted of a 1,980-base open reading frame with an inferred 71,550-Da protein of 660 amino acids. Putative Escherichia coli-like promoters and a ribosomal binding site were located in the 5' upstream region, and an 11-base dyad forming a stable stem-loop structure following two in-frame stop codons was identified. The C. pneumoniae 75-kDa protein is a member of the hsp70 family of heat shock proteins and has 87% amino acid similarity with the Chlamydia trachomatis protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birkelund S., Lundemose A. G., Christiansen G. Characterization of native and recombinant 75-kilodalton immunogens from Chlamydia trachomatis serovar L2. Infect Immun. 1989 Sep;57(9):2683–2690. doi: 10.1128/iai.57.9.2683-2690.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkelund S., Lundemose A. G., Christiansen G. The 75-kilodalton cytoplasmic Chlamydia trachomatis L2 polypeptide is a DnaK-like protein. Infect Immun. 1990 Jul;58(7):2098–2104. doi: 10.1128/iai.58.7.2098-2104.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunham R. C., Peeling R., Maclean I., McDowell J., Persson K., Osser S. Postabortal Chlamydia trachomatis salpingitis: correlating risk with antigen-specific serological responses and with neutralization. J Infect Dis. 1987 Apr;155(4):749–755. doi: 10.1093/infdis/155.4.749. [DOI] [PubMed] [Google Scholar]
- Campbell L. A., Kuo C. C., Grayston J. T. Characterization of the new Chlamydia agent, TWAR, as a unique organism by restriction endonuclease analysis and DNA-DNA hybridization. J Clin Microbiol. 1987 Oct;25(10):1911–1916. doi: 10.1128/jcm.25.10.1911-1916.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L. A., Kuo C. C., Thissen R. W., Grayston J. T. Isolation of a gene encoding a Chlamydia sp. strain TWAR protein that is recognized during infection of humans. Infect Immun. 1989 Jan;57(1):71–75. doi: 10.1128/iai.57.1.71-75.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilition S. L., Maclean I. W., Peeling R., Winston S., Brunham R. C. The 75-kilodalton protein of Chlamydia trachomatis: a member of the heat shock protein 70 family? Infect Immun. 1990 Jan;58(1):189–196. doi: 10.1128/iai.58.1.189-196.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayston J. T., Campbell L. A., Kuo C. C., Mordhorst C. H., Saikku P., Thom D. H., Wang S. P. A new respiratory tract pathogen: Chlamydia pneumoniae strain TWAR. J Infect Dis. 1990 Apr;161(4):618–625. doi: 10.1093/infdis/161.4.618. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleid D. G., Yansura D., Small B., Dowbenko D., Moore D. M., Grubman M. J., McKercher P. D., Morgan D. O., Robertson B. H., Bachrach H. L. Cloned viral protein vaccine for foot-and-mouth disease: responses in cattle and swine. Science. 1981 Dec 4;214(4525):1125–1129. doi: 10.1126/science.6272395. [DOI] [PubMed] [Google Scholar]
- Kuo C. C., Chen H. H., Wang S. P., Grayston J. T. Identification of a new group of Chlamydia psittaci strains called TWAR. J Clin Microbiol. 1986 Dec;24(6):1034–1037. doi: 10.1128/jcm.24.6.1034-1037.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Maclean I. W., Peeling R. W., Brunham R. C. Characterization of Chlamydia trachomatis antigens with monoclonal and polyclonal antibodies. Can J Microbiol. 1988 Feb;34(2):141–147. doi: 10.1139/m88-028. [DOI] [PubMed] [Google Scholar]
- Morrison R. P., Belland R. J., Lyng K., Caldwell H. D. Chlamydial disease pathogenesis. The 57-kD chlamydial hypersensitivity antigen is a stress response protein. J Exp Med. 1989 Oct 1;170(4):1271–1283. doi: 10.1084/jem.170.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison R. P., Lyng K., Caldwell H. D. Chlamydial disease pathogenesis. Ocular hypersensitivity elicited by a genus-specific 57-kD protein. J Exp Med. 1989 Mar 1;169(3):663–675. doi: 10.1084/jem.169.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardinia L. M., Engel J. N., Ganem D. Chlamydial gene encoding a 70-kilodalton antigen in Escherichia coli: analysis of expression signals and identification of the gene product. J Bacteriol. 1989 Jan;171(1):335–341. doi: 10.1128/jb.171.1.335-341.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler K. R., Rosser D. S., Berk A. J. Analysis of adenovirus transforming proteins from early regions 1A and 1B with antisera to inducible fusion antigens produced in Escherichia coli. J Virol. 1984 Jan;49(1):132–141. doi: 10.1128/jvi.49.1.132-141.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Mullenbach G., Sanchez-Pescador R., Agabian N. Sequence analysis of the major outer membrane protein gene from Chlamydia trachomatis serovar L2. J Bacteriol. 1986 Dec;168(3):1277–1282. doi: 10.1128/jb.168.3.1277-1282.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Young R. A., Elliott T. J. Stress proteins, infection, and immune surveillance. Cell. 1989 Oct 6;59(1):5–8. doi: 10.1016/0092-8674(89)90861-1. [DOI] [PubMed] [Google Scholar]