Abstract
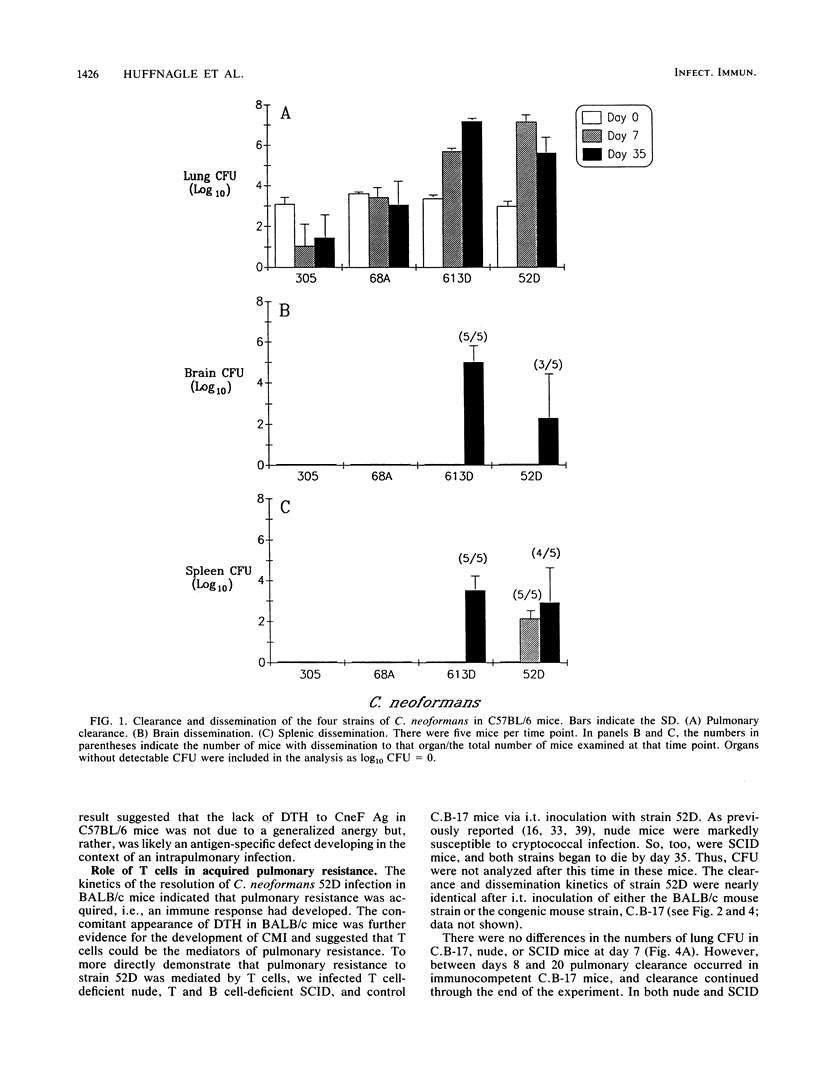
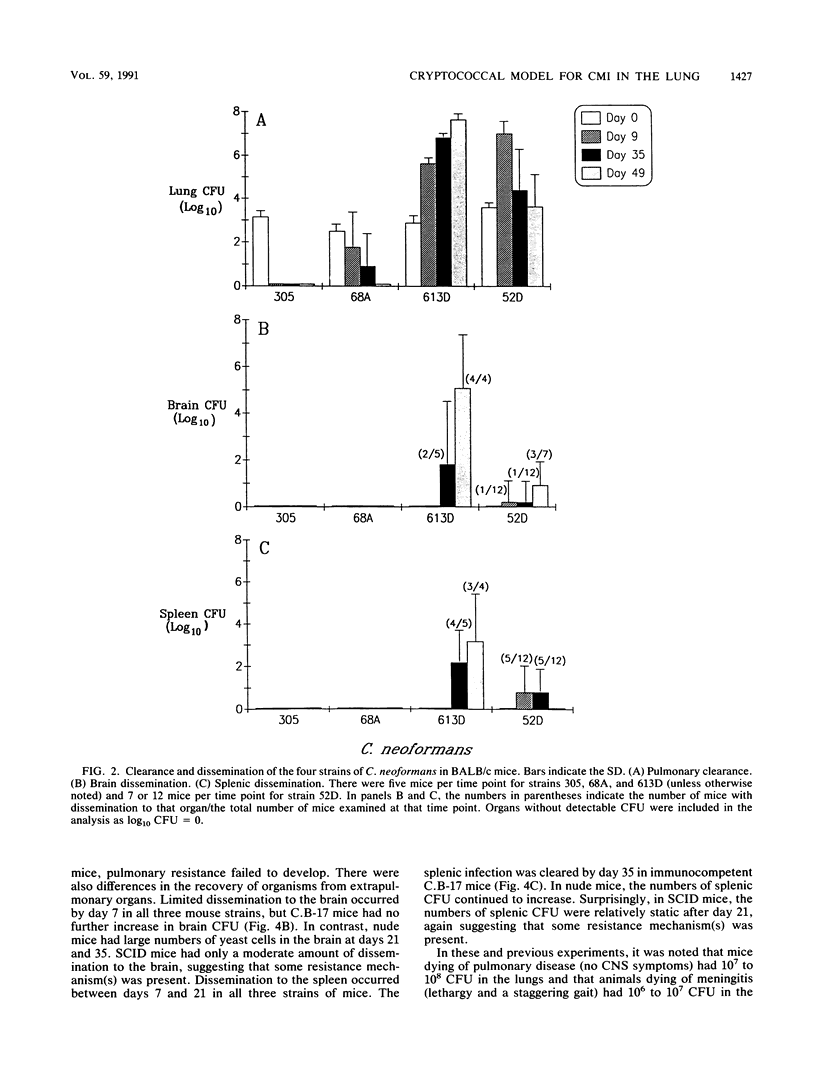
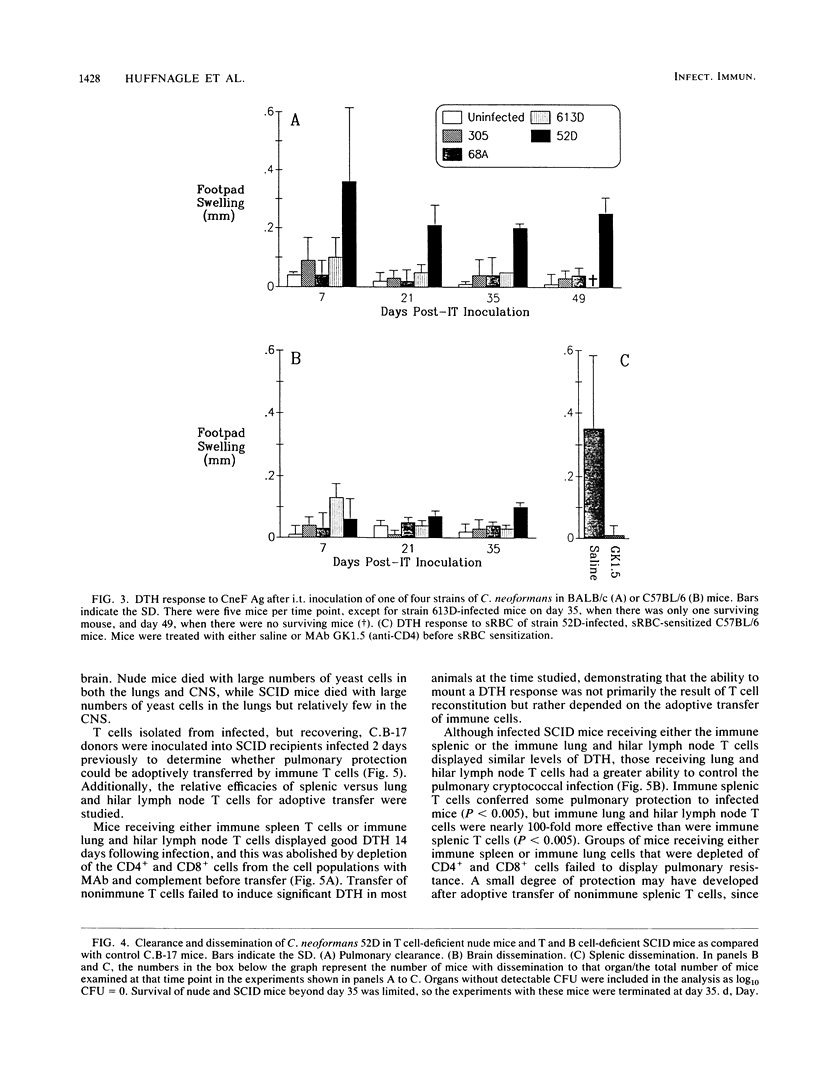
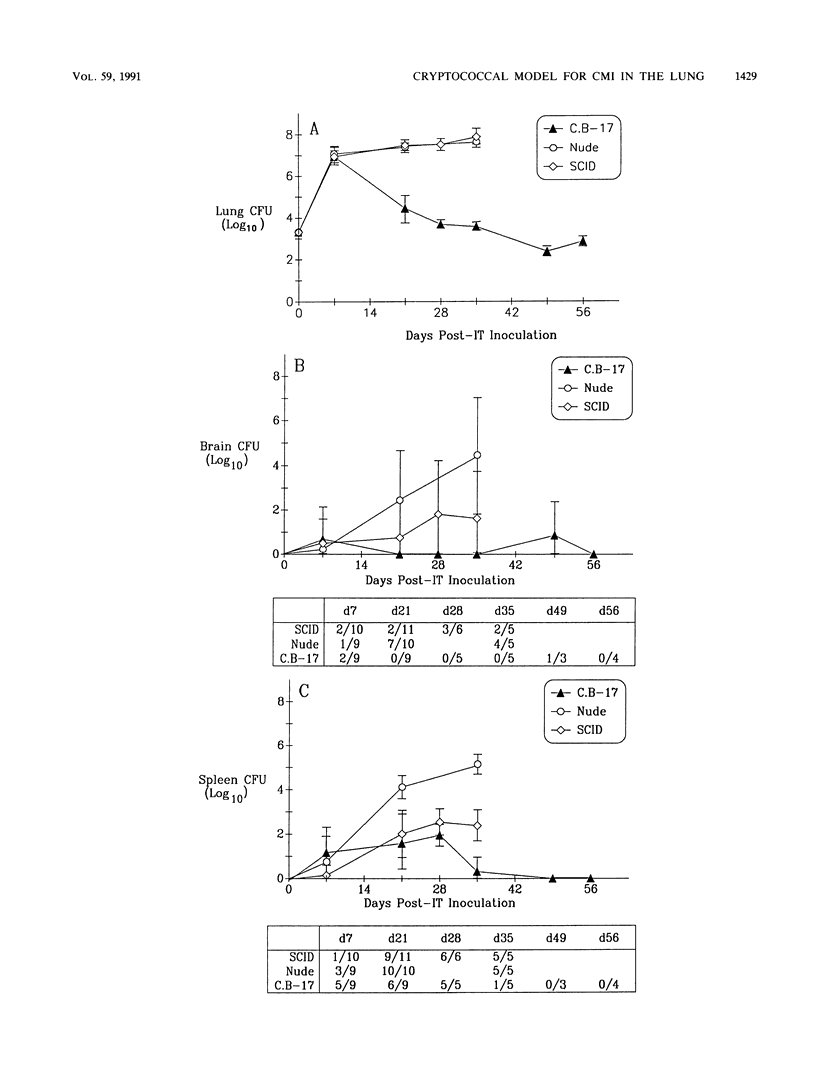
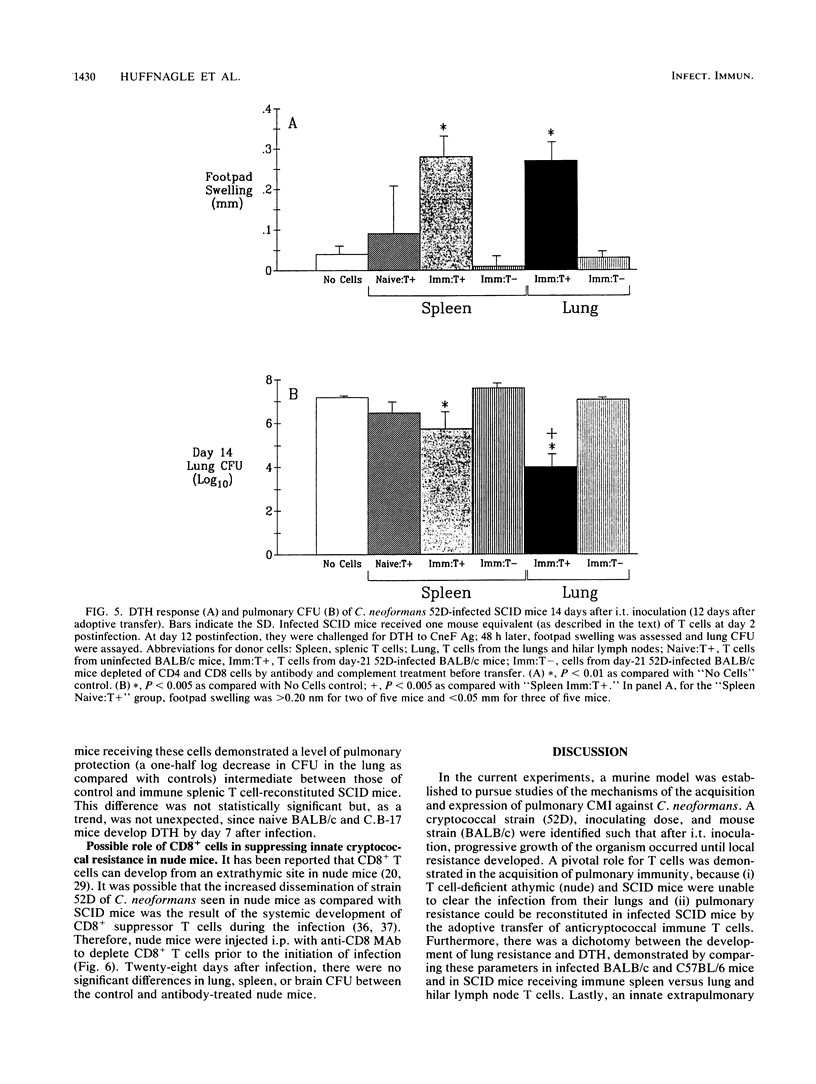
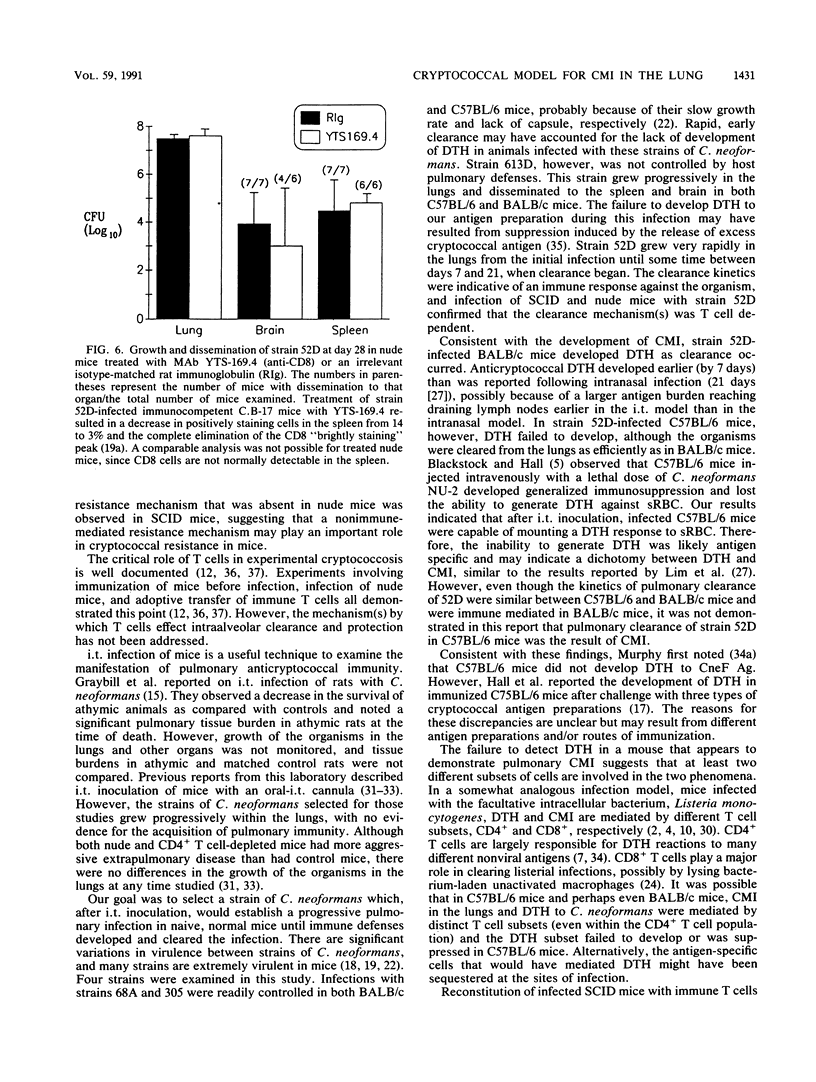
T cells are important in systemic anticryptococcal defenses, but a role in controlling an initial pulmonary infection has not been demonstrated. A murine model with intratracheal inoculation was developed to study the acquisition and expression of pulmonary T cell-mediated immunity against Cryptococcus neoformans. Infections with four strains of C. neoformans (305, 68A, 613D, and 52D) in two strains of mice (BALB/c and C57BL/6) were examined. Unencapsulated strain 305 and slowly growing strain 68A were readily controlled apparently by nonimmune pulmonary defenses, and no extrapulmonary dissemination was detected. Strain 613D grew progressively in the lungs and disseminated to the brain and spleen. Strain 52D initially grew rapidly in the lungs and disseminated to the spleen, but a clearance mechanism developed in the lungs after day 7 postinfection and in the spleen after day 28. SCID and athymic nude mice were unable to clear a strain 52D pulmonary infection, and a lethal disseminated infection occurred. Pulmonary clearance could be adoptively transferred into SCID mice infected with strain 52D by use of immune T cells from the spleen and lungs and hilar lymph nodes of infected immunocompetent donors. Furthermore, pulmonary clearance was almost 100-fold better in SCID mice that received immune T cells from the lungs and hilar lymph nodes than in those that received immune T cells from the spleen, even though equivalent levels of delayed-type hypersensitivity were transferred by both cell populations. These adoptive transfer studies suggested that the lung and hilar lymph node T cells from immune animals either are enriched in such a way as to mediate protective immunity or home to the lungs better than do splenic T cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. A., Sagha H. M. Persistence of infection in mice inoculated intranasally with Cryptococcus neoformans. Mycopathologia. 1988 Dec;104(3):163–169. doi: 10.1007/BF00437432. [DOI] [PubMed] [Google Scholar]
- Baldridge J. R., Barry R. A., Hinrichs D. J. Expression of systemic protection and delayed-type hypersensitivity to Listeria monocytogenes is mediated by different T-cell subsets. Infect Immun. 1990 Mar;58(3):654–658. doi: 10.1128/iai.58.3.654-658.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berche P., Decreusefond C., Theodorou I., Stiffel C. Impact of genetically regulated T cell proliferation on acquired resistance to Listeria monocytogenes. J Immunol. 1989 Feb 1;142(3):932–939. [PubMed] [Google Scholar]
- Blackstock R., Hall N. K. Non-specific immunosuppression by Cryptococcus neoformans infection. Mycopathologia. 1984 Apr 30;86(1):35–43. doi: 10.1007/BF00437227. [DOI] [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Bottomly K. A functional dichotomy in CD4+ T lymphocytes. Immunol Today. 1988 Sep;9(9):268–274. doi: 10.1016/0167-5699(88)91308-4. [DOI] [PubMed] [Google Scholar]
- Cobbold S. P., Jayasuriya A., Nash A., Prospero T. D., Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984 Dec 6;312(5994):548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- Czuprynski C. J., Brown J. F., Young K. M., Cooley A. J. Administration of purified anti-L3T4 monoclonal antibody impairs the resistance of mice to Listeria monocytogenes infection. Infect Immun. 1989 Jan;57(1):100–109. doi: 10.1128/iai.57.1.100-109.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Dorshkind K., Keller G. M., Phillips R. A., Miller R. G., Bosma G. C., O'Toole M., Bosma M. J. Functional status of cells from lymphoid and myeloid tissues in mice with severe combined immunodeficiency disease. J Immunol. 1984 Apr;132(4):1804–1808. [PubMed] [Google Scholar]
- Godfrey K. Statistics in practice. Comparing the means of several groups. N Engl J Med. 1985 Dec 5;313(23):1450–1456. doi: 10.1056/NEJM198512053132305. [DOI] [PubMed] [Google Scholar]
- Graybill J. R., Ahrens J., Nealon T., Paque R. Pulmonary cryptococcosis in the rat. Am Rev Respir Dis. 1983 May;127(5):636–640. doi: 10.1164/arrd.1983.127.5.636. [DOI] [PubMed] [Google Scholar]
- Graybill J. R., Mitchell L., Drutz D. J. Host defense in cryptococcosis. III. Protection of nude mice by thymus transplantation. J Infect Dis. 1979 Oct;140(4):546–552. doi: 10.1093/infdis/140.4.546. [DOI] [PubMed] [Google Scholar]
- HASENCLEVER H. F., EMMONS C. W. THE PREVALENCE AND MOUSE VIRULENCE OF CRYPTOCOCCUS NEOFORMANS STRAINS ISOLATED FROM URBAN AREAS. Am J Hyg. 1963 Sep;78:227–231. doi: 10.1093/oxfordjournals.aje.a120340. [DOI] [PubMed] [Google Scholar]
- HASENCLEVER H. F., MITCHELL W. O. Virulence and growth rates of Cryptococcus neoformans in mice. Ann N Y Acad Sci. 1960 Aug 27;89:156–162. doi: 10.1111/j.1749-6632.1960.tb20138.x. [DOI] [PubMed] [Google Scholar]
- Hall N. K., Maluf K. C., Blackstock R. Functional testing and chemical composition of cryptococcal extracts. Sabouraudia. 1984;22(5):439–442. doi: 10.1080/00362178485380701. [DOI] [PubMed] [Google Scholar]
- Jacobson E. S., Ayers D. J., Harrell A. C., Nicholas C. C. Genetic and phenotypic characterization of capsule mutants of Cryptococcus neoformans. J Bacteriol. 1982 Jun;150(3):1292–1296. doi: 10.1128/jb.150.3.1292-1296.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagaya K., Yamada T., Miyakawa Y., Fukazawa Y., Saito S. Characterization of pathogenic constituents of Cryptococcus neoformans strains. Microbiol Immunol. 1985;29(6):517–532. doi: 10.1111/j.1348-0421.1985.tb00854.x. [DOI] [PubMed] [Google Scholar]
- Karaoui R. M., Hall N. K., Larsh H. W. Role of macrophages in immunity and pathogenesis of experimental cryptococcosis induced by the airborne route--Part II: Phagocytosis and intracellular fate of Cryptococcus neoformans. Mykosen. 1977 Nov;20(11):409–412. [PubMed] [Google Scholar]
- Kaufmann S. H. CD8+ T lymphocytes in intracellular microbial infections. Immunol Today. 1988 Jun;9(6):168–174. doi: 10.1016/0167-5699(88)91292-3. [DOI] [PubMed] [Google Scholar]
- Kovacs J. A., Kovacs A. A., Polis M., Wright W. C., Gill V. J., Tuazon C. U., Gelmann E. P., Lane H. C., Longfield R., Overturf G. Cryptococcosis in the acquired immunodeficiency syndrome. Ann Intern Med. 1985 Oct;103(4):533–538. doi: 10.7326/0003-4819-103-4-533. [DOI] [PubMed] [Google Scholar]
- Kozel T. R., Cazin J. Nonencapsulated Variant of Cryptococcus neoformans I. Virulence Studies and Characterization of Soluble Polysaccharide. Infect Immun. 1971 Feb;3(2):287–294. doi: 10.1128/iai.3.2.287-294.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lim T. S., Murphy J. W., Cauley L. K. Host-etiological agent interactions in intranasally and intraperitoneally induced Cryptococcosis in mice. Infect Immun. 1980 Aug;29(2):633–641. doi: 10.1128/iai.29.2.633-641.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscomb M. F. Lung defenses against opportunistic infections. Chest. 1989 Dec;96(6):1393–1399. doi: 10.1378/chest.96.6.1393. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Blanc C., Lees R. K., Sordat B. Abnormal distribution of T cell subsets in athymic mice. J Immunol. 1986 Jun 15;136(12):4337–4339. [PubMed] [Google Scholar]
- Mielke M. E., Ehlers S., Hahn H. T-cell subsets in delayed-type hypersensitivity, protection, and granuloma formation in primary and secondary Listeria infection in mice: superior role of Lyt-2+ cells in acquired immunity. Infect Immun. 1988 Aug;56(8):1920–1925. doi: 10.1128/iai.56.8.1920-1925.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody C. H., Lipscomb M. F., Street N. E., Toews G. B. Depletion of CD4+ (L3T4+) lymphocytes in vivo impairs murine host defense to Cryptococcus neoformans. J Immunol. 1990 Feb 15;144(4):1472–1477. [PubMed] [Google Scholar]
- Mody C. H., Toews G. B., Lipscomb M. F. Cyclosporin A inhibits the growth of Cryptococcus neoformans in a murine model. Infect Immun. 1988 Jan;56(1):7–12. doi: 10.1128/iai.56.1.7-12.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody C. H., Toews G. B., Lipscomb M. F. Treatment of murine cryptococcosis with cyclosporin-A in normal and athymic mice. Am Rev Respir Dis. 1989 Jan;139(1):8–13. doi: 10.1164/ajrccm/139.1.8. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Murphy J. W. Clearance of Cryptococcus neoformans from immunologically suppressed mice. Infect Immun. 1989 Jul;57(7):1946–1952. doi: 10.1128/iai.57.7.1946-1952.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W. Immunoregulation in cryptococcosis. Immunol Ser. 1989;47:319–345. [PubMed] [Google Scholar]
- Murphy J. W., Mosley R. L., Cherniak R., Reyes G. H., Kozel T. R., Reiss E. Serological, electrophoretic, and biological properties of Cryptococcus neoformans antigens. Infect Immun. 1988 Feb;56(2):424–431. doi: 10.1128/iai.56.2.424-431.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., Pahlavan N. Cryptococcal culture filtrate antigen for detection of delayed-type hypersensitivity in cryptococcosis. Infect Immun. 1979 Jul;25(1):284–292. doi: 10.1128/iai.25.1.284-292.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RITTER R. C., LARSH H. W. THE INFECTION OF WHITE MICE FOLLOWING AN INTRANASAL INSTILLATION OF CRYPTOCOCCUS NEOFORMANS. Am J Hyg. 1963 Sep;78:241–246. doi: 10.1093/oxfordjournals.aje.a120342. [DOI] [PubMed] [Google Scholar]
- SMITH C. D., RITTER R., LARSH H. W., FURCOLOW M. L. INFECTION OF WHITE SWISS MICE WITH AIRBORNE CRYPTOCOCCUS NEOFORMANS. J Bacteriol. 1964 Jun;87:1364–1368. doi: 10.1128/jb.87.6.1364-1368.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler W., Weiler I. J., Schuler A., Phillips R. A., Rosenberg N., Mak T. W., Kearney J. F., Perry R. P., Bosma M. J. Rearrangement of antigen receptor genes is defective in mice with severe combined immune deficiency. Cell. 1986 Sep 26;46(7):963–972. doi: 10.1016/0092-8674(86)90695-1. [DOI] [PubMed] [Google Scholar]
- Waldorf A. R. Pulmonary defense mechanisms against opportunistic fungal pathogens. Immunol Ser. 1989;47:243–271. [PubMed] [Google Scholar]


