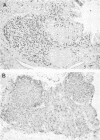
Abstract
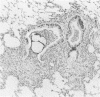
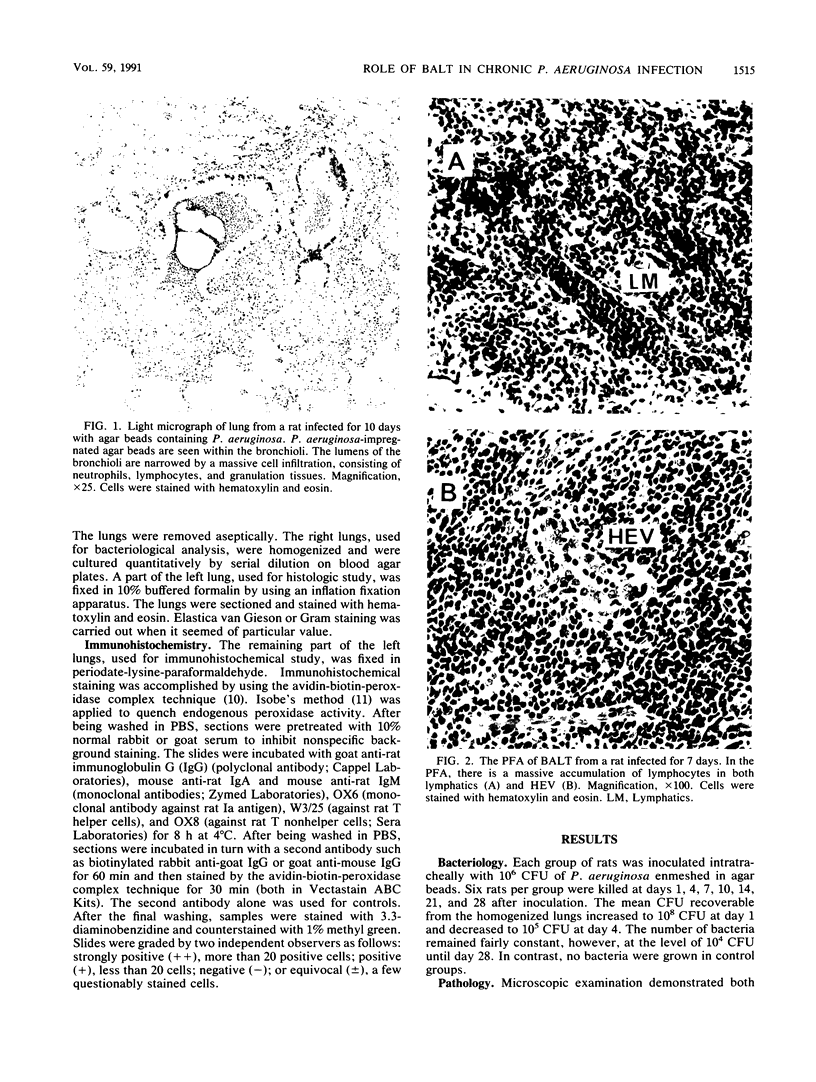
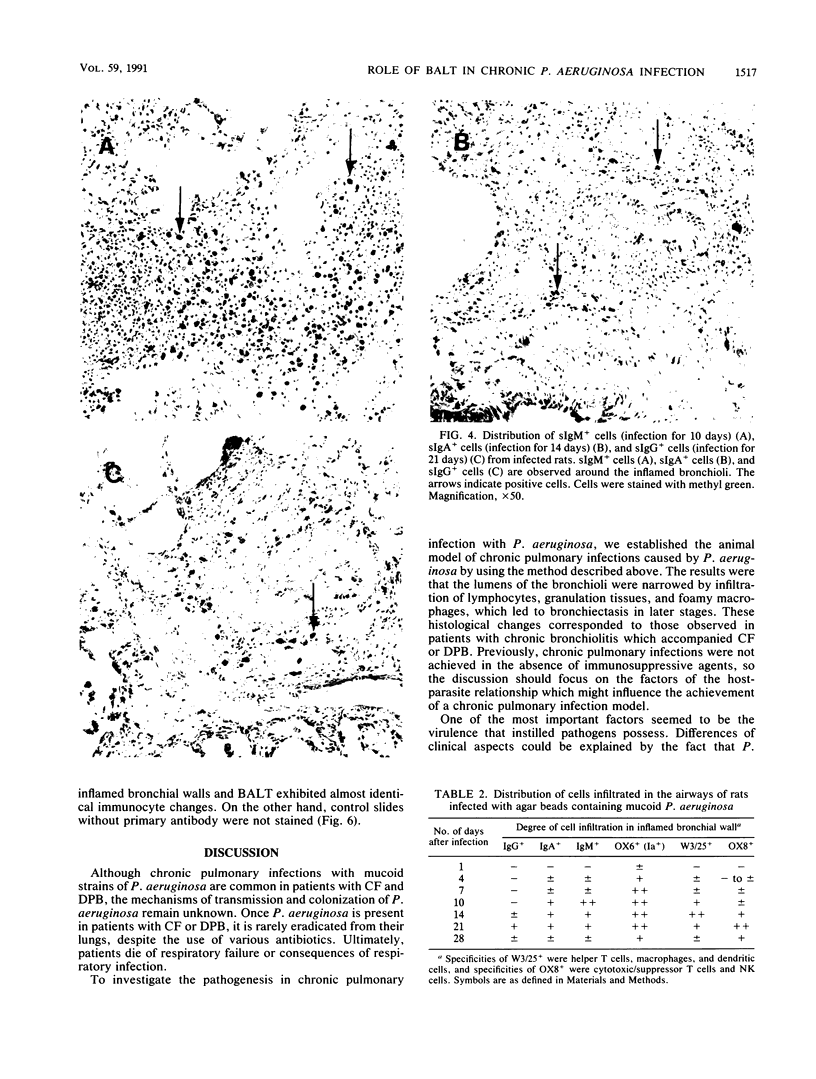
Pseudomonas aeruginosa is one of the most frequently encountered bacterial pathogens in patients with chronic pulmonary infections, including cystic fibrosis and diffuse panbronchiolitis. Bronchus-associated lymphoid tissue (BALT), noted frequently in patients with cystic fibrosis and diffuse panbronchiolitis, is considered to play an important role in the local immunologic defense mechanisms in the respiratory tract. To investigate the role of BALT in chronic pulmonary infections, we developed an animal model for chronic pulmonary infection and studied the morphological and immunohistochemical characteristics of BALT. Experimental pneumonia was produced in rats by intratracheal inoculation of P. aeruginosa enmeshed in agar beads. The histological changes corresponded to those occurring in chronic bronchiolitis. Immunohistochemically, surface immunoglobulin M-positive (sIgM+) cells and sIgA+ cells were recognized in the inflamed bronchial walls from day 4, and sIgG+ cells were recognized from day 14, W3/25+ cells exceeded OX8+ cells in number until day 14. In the BALT, there was a massive accumulation of lymphocytes in the lymphatics and high endothelial venules. The development of germinal centers was accompanied by increased numbers of sIgM+ and sIgA+ cells. W3/25+ cells exceeded OX8+ cells in number in the BALT until day 14. On the other hand, OX8+ cells were predominant in comparison with W3/25+ cells at day 21, and then both sIgM+ and sIgA+ cells and inflammatory changes in the lung decreased at day 28. These findings suggest that BALT regulates the local immune responses against chronic pulmonary infection due to P. aeruginosa.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bienenstock J., Johnston N., Perey D. Y. Bronchial lymphoid tissue. I. Morphologic characteristics. Lab Invest. 1973 Jun;28(6):686–692. [PubMed] [Google Scholar]
- Bienenstock J., Johnston N., Perey D. Y. Bronchial lymphoid tissue. II. Functional characterisitics. Lab Invest. 1973 Jun;28(6):693–698. [PubMed] [Google Scholar]
- Bockman D. E., Stevens W. Gut-associated lymphoepithelial tissue: bidirectional transport of tracer by specialized epithelial cells associated with lymphoid follicles. J Reticuloendothel Soc. 1977 Apr;21(4):245–254. [PubMed] [Google Scholar]
- Cabral D. A., Loh B. A., Speert D. P. Mucoid Pseudomonas aeruginosa resists nonopsonic phagocytosis by human neutrophils and macrophages. Pediatr Res. 1987 Oct;22(4):429–431. doi: 10.1203/00006450-198710000-00013. [DOI] [PubMed] [Google Scholar]
- Cash H. A., Woods D. E., McCullough B., Johanson W. G., Jr, Bass J. A. A rat model of chronic respiratory infection with Pseudomonas aeruginosa. Am Rev Respir Dis. 1979 Mar;119(3):453–459. doi: 10.1164/arrd.1979.119.3.453. [DOI] [PubMed] [Google Scholar]
- Fournier M., Vai F., Derenne J. P., Pariente R. Bronchial lymphoepithelial nodules in the rat: morphologic features and uptake and transport of exogenous proteins. Am Rev Respir Dis. 1977 Oct;116(4):685–694. doi: 10.1164/arrd.1977.116.4.685. [DOI] [PubMed] [Google Scholar]
- Homma H., Yamanaka A., Tanimoto S., Tamura M., Chijimatsu Y., Kira S., Izumi T. Diffuse panbronchiolitis. A disease of the transitional zone of the lung. Chest. 1983 Jan;83(1):63–69. doi: 10.1378/chest.83.1.63. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Høiby N., Döring G., Schiøtz P. O. Pathogenic mechanisms of chronic Pseudomonas aeruginosa infections in cystic fibrosis patients. Antibiot Chemother (1971) 1987;39:60–76. [PubMed] [Google Scholar]
- Jericho K. W., Austwick P. K., Hodges R. T., Dixon J. B. Intrapulmonary lymphoid tissue of pigs exposed to aerosols of carbon particles, of Salmonella oranienburg, of Mycoplasma granularum, and to an oral inoculum of larvae of Metastrongylus apri. J Comp Pathol. 1971 Jan;81(1):13–21. doi: 10.1016/0021-9975(71)90050-8. [DOI] [PubMed] [Google Scholar]
- Jericho K. W., Derbyshire J. B., Jones J. E. Intrapulmonary lymphoid tissue of pigs exposed to aerosols of haemolytic streptococcus group L and porcine adenovirus. J Comp Pathol. 1971 Jan;81(1):1–11. doi: 10.1016/0021-9975(71)90049-1. [DOI] [PubMed] [Google Scholar]
- Kiyono H., McGhee J. R., Mosteller L. M., Eldridge J. H., Koopman W. J., Kearney J. F., Michalek S. M. Murine Peyer's patch T cell clones. Characterization of antigen-specific helper T cells for immunoglobulin A responses. J Exp Med. 1982 Oct 1;156(4):1115–1130. doi: 10.1084/jem.156.4.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraal G., Twisk A. The interaction of high endothelial venules with T and B cells in peripheral lymph nodes after antigenic stimulation. Eur J Immunol. 1984 Jun;14(6):586–588. doi: 10.1002/eji.1830140617. [DOI] [PubMed] [Google Scholar]
- Meuwissen H. J., Hussain M. Bronchus-associated lymphoid tissue in human lung: correlation of hyperplasia with chronic pulmonary disease. Clin Immunol Immunopathol. 1982 May;23(2):548–561. doi: 10.1016/0090-1229(82)90139-8. [DOI] [PubMed] [Google Scholar]
- Pier G. B. Pulmonary disease associated with Pseudomonas aeruginosa in cystic fibrosis: current status of the host-bacterium interaction. J Infect Dis. 1985 Apr;151(4):575–580. doi: 10.1093/infdis/151.4.575. [DOI] [PubMed] [Google Scholar]
- Ramphal R., Pier G. B. Role of Pseudomonas aeruginosa mucoid exopolysaccharide in adherence to tracheal cells. Infect Immun. 1985 Jan;47(1):1–4. doi: 10.1128/iai.47.1.1-4.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rácz P., Tenner-Rácz K., Myrvik Q. N., Fainter L. K. Functional architecture of bronchial associated lymphoid tissue and lymphoepithelium in pulmonary cell-mediated reactions in the rabbit. J Reticuloendothel Soc. 1977 Jul;22(1):59–83. [PubMed] [Google Scholar]
- Rácz P., Tenner-Rácz K., Myrvik Q. N., Shannon B. T., Love S. H. Sinus reactions in the hilar lymph node complex of rabbits undergoing a pulmonary cell-mediated immune response: sinus macrophage clumping reaction, sinus lymphocytosis and immature sinus histiocytosis. J Reticuloendothel Soc. 1978 Nov;24(5):499–525. [PubMed] [Google Scholar]
- Stamper H. B., Jr, Woodruff J. J. An in vitro model of lymphocyte homing. I. Characterization of the interaction between thoracic duct lymphocytes and specialized high-endothelial venules of lymph nodes. J Immunol. 1977 Aug;119(2):772–780. [PubMed] [Google Scholar]
- Starke J. R., Edwards M. S., Langston C., Baker C. J. A mouse model of chronic pulmonary infection with Pseudomonas aeruginosa and Pseudomonas cepacia. Pediatr Res. 1987 Dec;22(6):698–702. doi: 10.1203/00006450-198712000-00017. [DOI] [PubMed] [Google Scholar]
- Tenner-Rácz K., Rácz P., Myrvik Q. N., Ockers J. R., Geister R. Uptake and transport of horseradish peroxidase by lymphoepithelium of the bronchus-associated lymphoid tissue in normal and bacillus Calmette-Guérin-immunized and challenged rabbits. Lab Invest. 1979 Aug;41(2):106–115. [PubMed] [Google Scholar]