Summary
The efficacy of the D1/5 agonist SKF38393 (100 nM-60 µM) to increase long-term potentiation (LTP) in the CA1 region was investigated in the rat hippocampal slice preparation. The receptor specificity of this enhancing effect was confirmed using the D1/5 antagonist SKF83566 (2 µM). Although the ability of D1/5 receptors to increase both the persistence and the early magnitude of LTP has previously been linked to activation of the cAMP/PKA pathway, the subsequent molecular events leading to the enhancement of LTP have not been characterized. In experiments using SKF38393 (20 µM), a requirement for the activation of both protein kinase A (PKA) and Src family tyrosine kinase pathways was demonstrated, as pretreatment with either H89 (10 µM) or PP2 (10 µM) kinase inhibitors prevented the D1/5-mediated enhancement of LTP. In addition, NMDA receptors containing the NR2B subunit were identified as a potential downstream target for this signaling pathway, as pretreatment with the selective antagonist Ro 25–6981 (1 µM) also prevented the D1/5-mediated enhancement of LTP. The results identify a crucial role for NR2B-containing NMDA receptors in the modulation of LTP by D1/5-receptors in the CA1, suggesting that endogenously released dopamine may act through this mechanism as a modulator of hippocampal-dependent learning and memory tasks.
Keywords: hippocampus, synaptic plasticity, neuromodulator, SKF38393, Ro25-6981
1. Introduction
Dopamine is recognized as an important modulator of synaptic plasticity in the CA1 region of the hippocampus (Frey et al., 1990; Lisman and Grace, 2005) and dopamine-dependent alterations in synaptic plasticity in this region are involved in hippocampal-dependent learning (Lemon and Manahan-Vaughan, 2006). Although a role for the D1/5 subtype of dopamine receptor in enhancing both the duration (Frey et al., 1991) and the initial magnitude (Otmakhova and Lisman, 1996) of long-term potentiation (LTP) has been established, it remains unclear how these effects are mediated. In the case of both the enhanced persistence of LTP (Frey et al., 1993), and the facilitating effects of dopamine agonists on the magnitude of early LTP (Otmakhova and Lisman, 1996), the activation of D1/5 receptors has been linked to an increase in cAMP levels. Subsequent steps in this signaling pathway have not been described.
A major portion of the LTP evoked following activation of the Schaffer collateral input to CA1 is dependent upon the activation of N-methyl-D-aspartate receptors (NMDARs, (Collingridge et al., 1983). The contribution of specific NMDAR subunits to the induction of LTP and long-term depression (LTD) has recently been a topic of great interest and ongoing controversy (MacDonald et al., 2006). It has been suggested that the NR2A subunit plays a significant role in the generation of NMDAR-dependent LTP whereas the NR2B subunit may be of primary importance to the induction of NMDAR-dependent LTD (Liu et al., 2004). In this report, we address the question of how the actions of dopamine can alter functional participation of different NMDAR subtypes in the induction of LTP. Aside from any effects on LTP/LTD, it is clear that the activation of D1/5 dopamine receptors could impact the activity of NMDARs through a variety of mechanisms encompassing both changes in channel function (Cepeda et al., 1993; Chen and Roche, 2007; Lee et al., 2002; Levine et al., 1996; Snyder et al., 1998) and/or channel distribution (Dunah and Standaert, 2001; Hallett et al., 2006; Kopp et al., 2007).
In these experiments we have utilized a compressed train, high-frequency stimulation (HFS) protocol that results in the induction of an NMDAR-dependent LTP which is not dependent on the activity of PKA (Woo et al., 2003). This has allowed us to determine, without altering the initial “baseline” level of potentiation observed in the absence of D1/5 receptor activation, whether a D1/5-mediated increase in PKA activity is involved in the ability of the D1/5-selective agonist SKF38393 to enhance the magnitude of LTP. Our findings indicate that dopaminergic activation of PKA promotes the participation of NR2B-containing NMDARs in the induction of LTP in a manner also dependent upon the activation of Src family kinases (SFKs). These results demonstrate a potential link between the gating function of PKA (Blitzer et al., 1995) and a SFK-dependent enhancement of LTP via NR2B-containing NMDARs that can be initiated by D1/5 receptor activation in the CA1 region of the hippocampus.
2. Methods
2.1. Extracellular electrophysiology
Hippocampal slices were prepared from male Sprague-Dawley rats (40–90 days old) using an experimental protocol performed in compliance with the University of Georgia Animal Care and Use guidelines. All rats were anesthetized with halothane prior to decapitation. The brain was removed and submerged in ice-cold, oxygenated (95% O2/ 5% CO2) dissection artificial cerebrospinal fluid (ACSF) containing (mM): NaCl (120), KCl (3), MgCl2 (4), NaH2PO4 (1), NaHCO3 (26), and glucose (10). Horizontal brain slices were cut at a thickness of 500 µM, the hippocampus dissected, and the CA3 region removed. Slices were then perfused with room-temperature, oxygenated (95% O2/5% CO2) standard ACSF containing (mM): NaCl (120), KCl (3), MgCl2 (1.5), NaH2PO4 (1), CaCl2 (2.5), NaHCO3 (26), and glucose (10) at approximately 1 ml/min. Slices recovered in the recording chamber for one hour at room temperature, and then a second hour at 30°C, the temperature at which recordings were obtained. A bipolar stimulating electrode (Kopf Instruments) was placed on the CA3-side of the CA1 region in the stratum radiatum and a 1.0 MΩ tungsten recording microelectrode (World Precision Instruments) was then positioned in the same layer in CA1.
2.2. Quantification of synaptic plasticity
Data were digitized at 10 kHz, low-pass filtered at 1 kHz, and analyzed with pCLAMP 9.2 software (Axon Instruments). The initial slope of the population fEPSP was measured by fitting a straight line to a 1 msec window immediately following the fiber volley. A stimulus-response curve was obtained at the beginning of each experiment, with stimulus pulses consisting of a single square wave of 270 µs duration delivered at 40–160 µA. The stimulation intensity was adjusted to obtain a field EPSP amplitude of 1.0–1.5mV to begin baseline recording, and fEPSPs were elicited by stimulation of the Schaffer collateral-commissural pathway in stratum radiatum once every 60 s (.0167 Hz) for the duration of the experiment. Synaptic responses were normalized by dividing all slopes by the average of the 5 fEPSP slopes obtained from the 5 min prior to tetanization. The tetanization protocol used to induce LTP in all experiments was a standard HFS protocol consisting of 3 trains of 100 Hz/1s administered at 20 s intertrain intervals. Control LTP values were collected periodically throughout the study. Planned comparisons with control were made using unpaired t-tests. In reporting our results, n-values indicate first the number of slices, and then the number of animals.
2.3. Drugs
None of the drugs applied in these experiments significantly altered the baseline fEPSP response at the indicated doses. For all experiments in which either SKF38393 or SKF81297 was tested, application of the D1/5 agonist began 30 min prior to induction of LTP, and fEPSP responses were monitored for an additional 30 min post HFS in the continued presence of the drug (Fig 1&Fig 5). When antagonists or inhibitors were tested, they were applied for at least 20 min prior to initiating the D1/5 agonist co-application, from which point all drugs continued to be applied for the duration of recording (Fig 2–Fig 7). 8-Bromo-2,3,4,5-tetrahydro-3-methyl-5-phenyl-1H-3-benzazepin-7-ol hydrobromide (SKF83566), 3-(4-chlorophenyl) 1-(1,1-dimethylethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine (PP2), (αR,βS)-α-(4-Hydroxyphenyl)-β-methyl-4-(phenylmethyl)-1 -piperidinepropanol maleate (Ro25–6981), 6-Chloro-2,3,4,5-tetrahydro-1-phenyl-1H-3-benzazepine hydrobromide (SKF81297), and 3-(4-[4-Chlorophenyl]piperazin-1-yl)-methyl-1H-pyrrolo[2,3-b]pyridine trihydrochloride (L745870) were obtained from Tocris. N-[2-(p-Bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide dihydrochloride (H89) was obtained from Sigma. (±)-1-Phenyl-2,3,4,5-tetrahydro-(1H)-3-benzazepine-7,8-diol hydrochloride (SKF38393) was obtained from both Sigma and Tocris over the course of the study. Drugs were applied by addition to the perfusion reservoir; stocks for both PP2 and SKF81297 were initially dissolved in DMSO, with the final concentrations of this solvent being 0.04% and 0.08%, respectively.
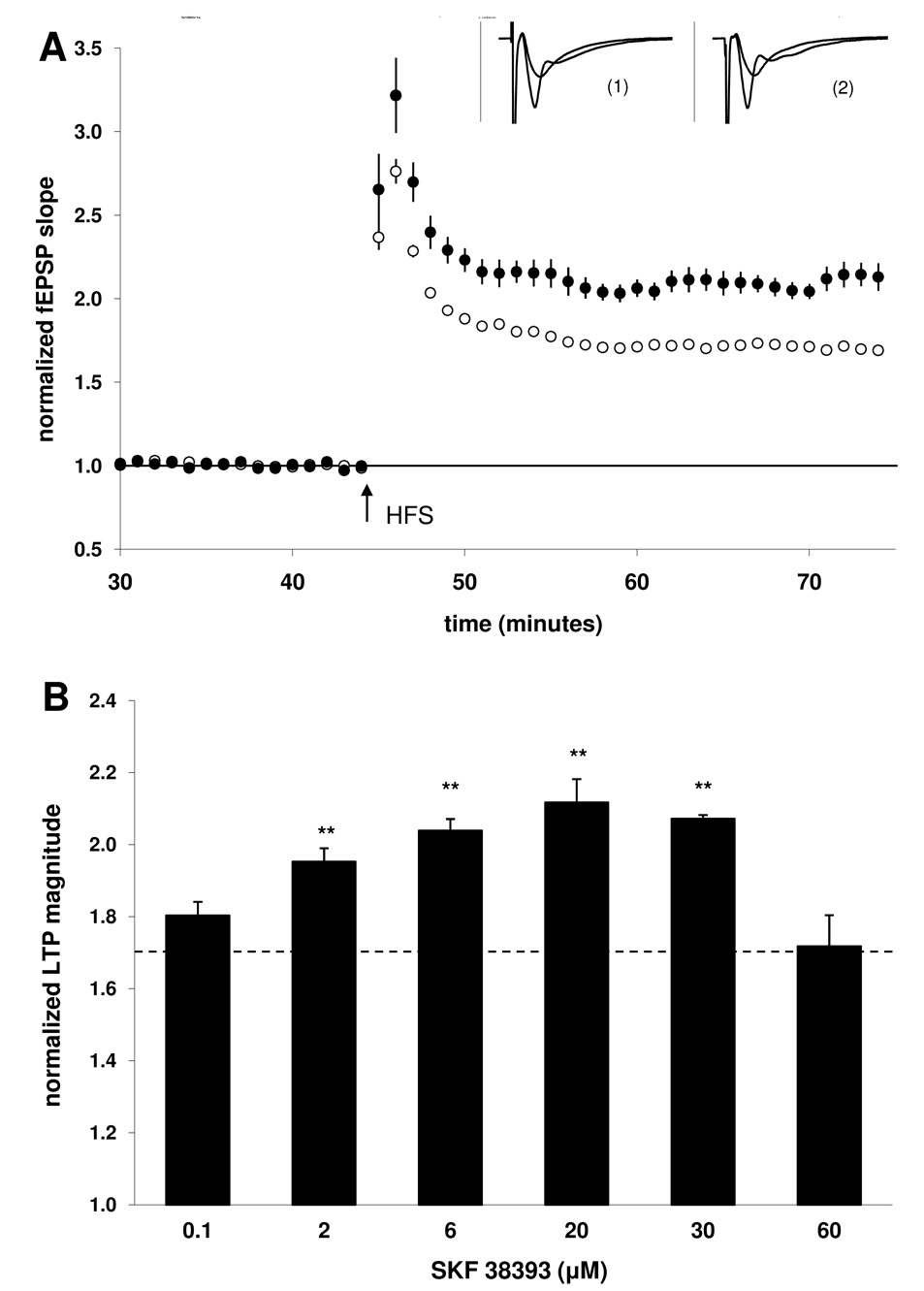
Figure 1. The dopaminergic agonist SKF38393 induced a dose-dependent modulation of LTP.
A) Summary plot of normalized fEPSP slope measurements evoked and recorded in the stratum radiatum layer of the CA1 region. Open circles show responses from control slices; closed circles depict responses from SKF38393-treated slices (20 µM). Insets are 50 ms sweeps averaged from all experiments illustrating the mean fEPSP 1–5 min prior to and 26–30 min post-tetanus (vertical scale bar is 3 mV). The left pair of sweeps (1) is from non-drug, control slices and the right pair (2) is from SKF38393-treated slices. B) Summary quantification of LTP magnitude in the presence of increasing concentrations of SKF38393. Control LTP is indicated by the dashed line. LTP magnitude was significantly enhanced at concentrations of 2 µM, 6 µM, 20 µM, and 30 µM relative to control (** p < 0.01). Error bars show ± SEM.
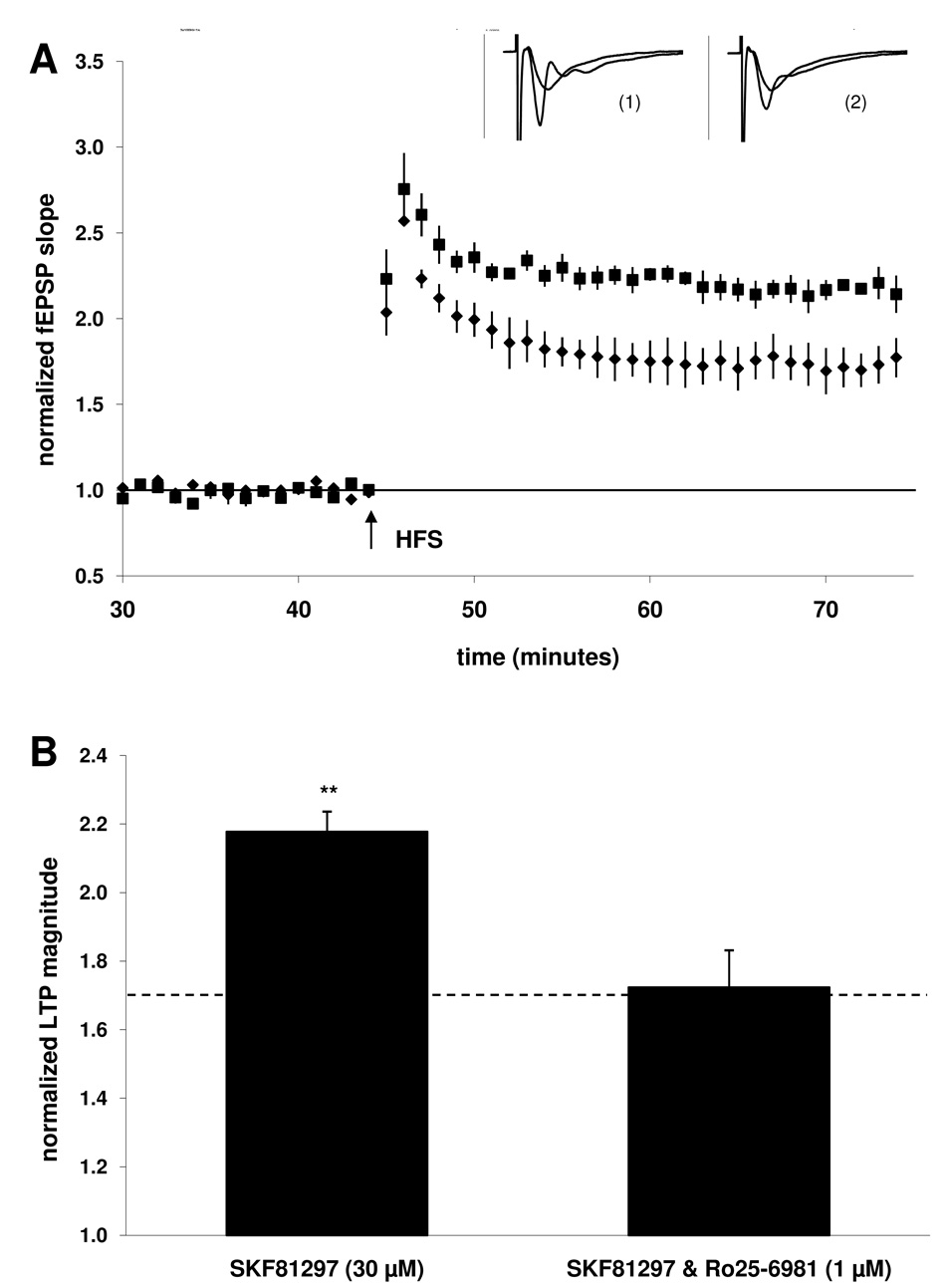
Figure 5. Co-application with NR2B antagonist Ro25–6981 (1 µM) also blocked the ability of D1/5 full agonist SKF81297 to enhance LTP.
A) Summary plot of normalized fEPSP measurements. Closed squares depict slices treated with D1/5 agonist SKF81297 (30 µM). Closed circles depict slices treated with both Ro25–6981 (1 µM) and SKF81297 (30 µM). Insets are 50 ms sweeps averaged from all experiments illustrating the mean fEPSP 1–5 min prior to and 26–30 min post-tetanus (vertical scale bar is 3 mV). The left pair of sweeps (1) is from SKF81297-treated slices and the right pair (2) is from Ro25–6981 & SKF81297-treated slices. B) Summary quantification of LTP magnitude in the presence of SKF81297 alone and when co-applied with Ro25–6981 (significance is relative to the control group indicated by the dashed line, **p < 0.01). Error bars show ± SEM.
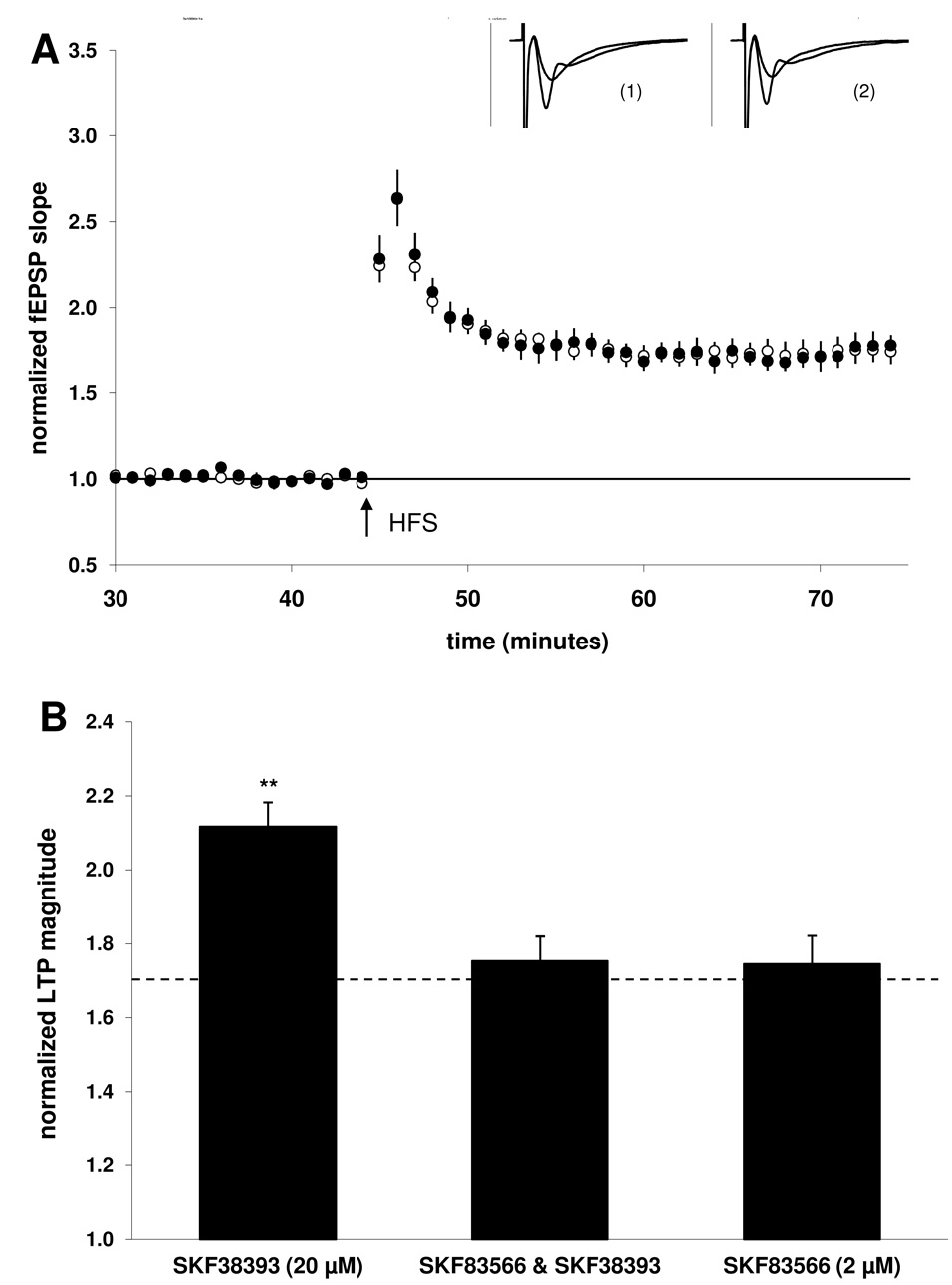
Figure 2. Co-application with the D1/5 antagonist SKF83566 (2 µM) blocked the SKF38393-mediated enhancement of LTP.
A) Summary plot of normalized fEPSP measurements. Open circles show normalized fEPSP slope from slices treated with SKF83566 (2 µM) alone; closed circles depict slices treated with both SKF83566 (2 µM) and SKF38393 (20 µM). Insets are 50 ms sweeps averaged from all experiments illustrating the mean fEPSP 1–5 min prior to and 26–30 min post-tetanus (vertical scale bar is 3 mV). The left pair of sweeps (1) is from SKF83566-treated slices and the right pair (2) is from SKF83566 & SKF38393-treated slices. B) Summary quantification of LTP magnitude in the presence of the agonist SKF38393 alone (taken from Fig. 1, illustrated for comparison), when co-applied with the antagonist SKF83566, or SKF83566 alone. Significance is relative to the control group (indicated by the dashed line, ** p < 0.01). Error bars show ± SEM.
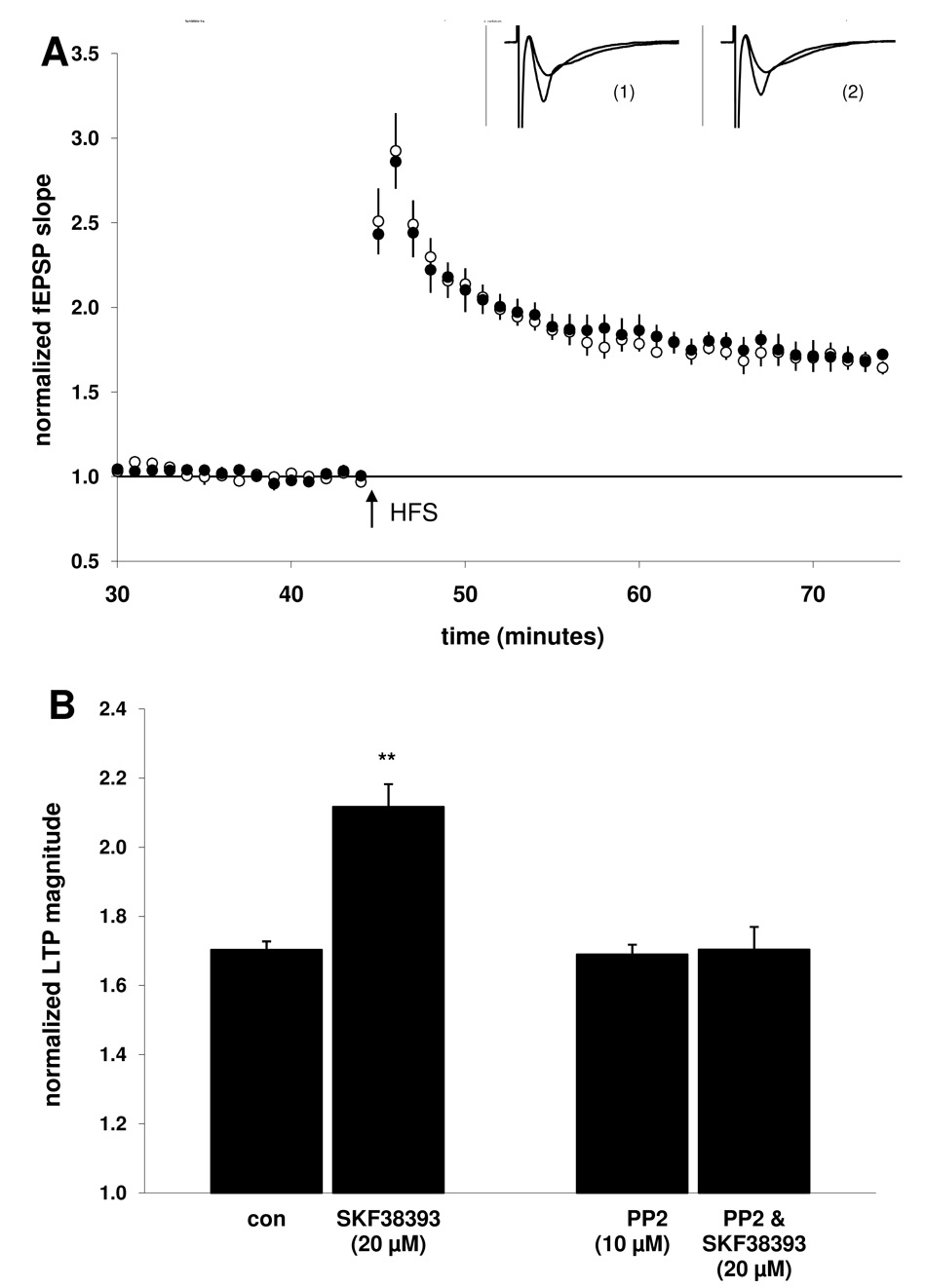
Figure 7. Co-application with the Src-family tyrosine kinase inhibitor PP2 (10 µM) blocked the SKF38393-mediated enhancement of LTP.
A) Summary plot of normalized fEPSP measurements. Open circles show normalized fEPSP slope from slices treated with PP2 (10 µM); closed circles depict slices treated with both PP2 (10 µM) and SKF38393 (20 µM). Insets are 50 ms sweeps averaged from all experiments illustrating the mean fEPSP 1–5 min prior to and 26–30 min post-tetanus (vertical scale bar is 3 mV). The left pair of sweeps (1) is from PP2-treated slices and the right pair (2) is from PP2& SKF38393-treated slices. B) Summary quantification of LTP magnitude in the presence of PP2 alone and when co-applied with SKF38393, as compared to the control and SKF38393 (20 µM) alone groups (taken from Fig. 1, illustrated for comparison, **p < 0.01). Error bars show ± SEM.
3. Results
3.1. The dopaminergic agonist SKF38393 dose-dependently facilitates LTP
Field EPSP (fEPSP) responses were monitored in s. radiatum layer of the CA1 region and LTP was evoked with HFS using 3 × 100 Hz/1 s trains administered at 20 s intertrain intervals. For a cumulative control set of slices collected throughout the study, the fEPSP slope was increased 70 ± 2% (n = 60 slices, 20 animals) 30 min following LTP induction (Fig 1A). In groups of slices treated with the dopamine agonist SKF38393, a dose-dependent modulation of LTP was evident (Fig 1B). At a concentration of 100 nM SKF38393 the magnitude of LTP was 80 ± 4%; n = 9, 3. Higher concentrations of SKF38393 significantly enhanced LTP [2 µM: (95 ± 5%; n = 9, 3; p < 0.01), 6 µM: (104 ± 3%; n = 9, 3; p < 0.01), 20 µM: (112 ± 7%; n = 20, 7; p < 0.01), 30 µM: (107 ± 1%; n = 9, 3; p < 0.01)]. In contrast, application of 60 µM SKF38393 resulted in an LTP magnitude (72 ± 9%; n = 9, 3) which was not significantly different from the control group.
3.2. D1/5 receptor antagonism prevents enhancement of LTP by SKF38393
Previous studies have indicated that activation of D1/5 receptors in the CA1 can enhance the magnitude of LTP measured 30 min post-HFS in both slices (Otmakhova and Lisman, 1996) and in vivo (Lemon and Manahan-Vaughan, 2006). Consistent with these reports, when the D1/5 receptor selective antagonist SKF83566 (2 µM) was applied prior to the agonist SKF38393 (20 µM), LTP was no longer enhanced in the presence of the dopaminergic agonist (75 ± 7; n = 12, 4; Fig 2B). Applied alone, the antagonist did not significantly affect LTP magnitude (74 ± 8%; n = 18, 6) when compared to the control group, suggesting that basal activation of D1/5 receptors by endogenously released dopamine was not required for expression of the early component of LTP induced using our HFS protocol.
3.3. Involvement of cAMP-dependent protein kinase
As it has been reported that cAMP and protein kinase A activity can affect the magnitude of early LTP (Blitzer et al., 1995; Otmakhova et al., 2000), and since D1/5 receptors are positively coupled to cAMP/PKA, we tested the selective PKA inhibitor H89 in our experiments. When H89 (10 µM) was applied prior to SKF38393 (20 µM), LTP was no longer enhanced in the presence of the dopaminergic agonist (71 ± 5%; n = 9, 3; Fig 3B). Applied alone, the PKA inhibitor did not significantly affect LTP magnitude (69 ± 6%; n = 9, 3) when compared to the control group. This result is consistent with the expectations outlined by Woo et al. (2003) for a temporally “compressed” HFS pattern, which was the type of conditioning protocol utilized in our experiments. Taken together, these findings suggest that although the early LTP evoked under our induction conditions was not dependent upon PKA activation, the D1/5-mediated enhancement was PKA-dependent.
Figure 3. Co-application with the PKA inhibitor H89 (10 µM) blocked the SKF38393-mediated enhancement of LTP.
A) Summary plot of normalized fEPSP measurements. Open circles show normalized fEPSP slope from slices treated with H89 (10 µM); closed circles depict slices treated with both H89 (10 µM) and SKF38393 (20 µM). Insets are 50 ms sweeps averaged from all experiments illustrating the mean fEPSP 1–5 min prior to and 26–30 min post-tetanus (vertical scale bar is 3 mV). The left pair of sweeps (1) is from H89-treated slices and the right pair (2) is from H89 & SKF38393-treated slices. B) Summary quantification of LTP magnitude in the presence of H89 alone and when co-applied with SKF38393, as compared to both the control and SKF38393 (20 µM) alone groups taken from Fig. 1 ( **p < 0.01). Error bars show ± SEM.
3.4. NR2B NMDARs are required for the D1/5 enhancement of LTP
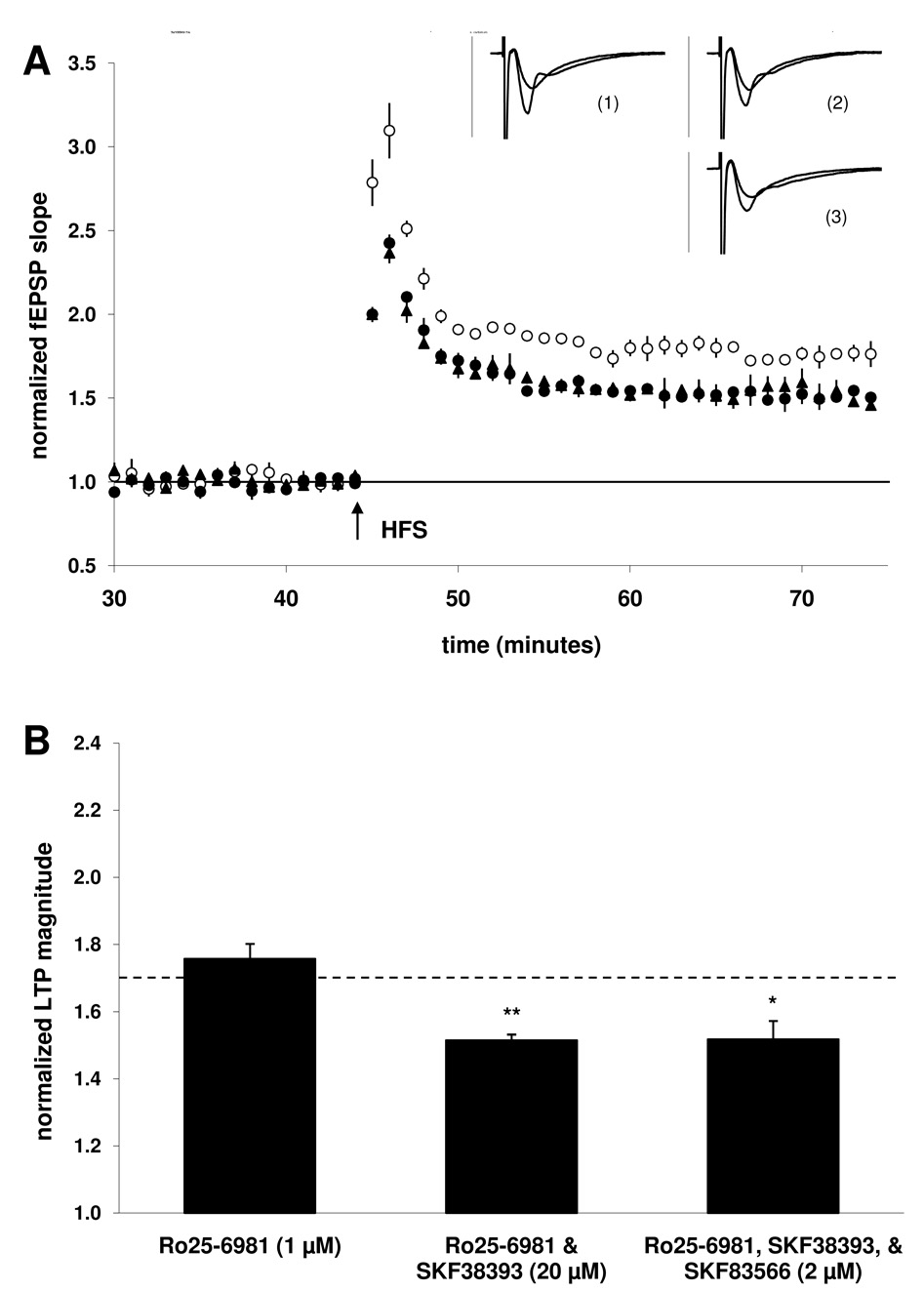
One mechanism by which increased PKA activity might be postulated to enhance LTP is via enhanced NMDA receptor activity. Because we were able to prevent D1/5-mediated increases in early LTP without affecting the “baseline” magnitude of potentiation, we tested the possibility that NR2B-containing NMDARs were selectively participating in the D1/5-mediated enhancement of early LTP, as it has been suggested that these receptors are not required for LTP (Liu et al., 2004). In the presence of the selective NR2B antagonist Ro25–6981 (1 µM) alone, LTP magnitude was not significantly different from that of the control group (76 ± 5%; n = 9, 3; Fig 4B). Thus the early LTP evoked using our induction protocol was not dependent upon the participation of NR2B NMDARs. However, prior application of Ro25–6981 prevented the enhancement of LTP by SKF38393 (20 µM), and furthermore, early LTP was significantly decreased (51 ± 2%; n = 9, 3; p < 0.01) when compared to the control group. Co-application of the D1/5 antagonist SKF83566 (2 µM) did not mitigate this decrease, as LTP magnitude in the presence of all three compounds was still significantly reduced (52 ± 6%; n = 9, 3; p < 0.02). This result suggests that the SKF38393-mediated decrease in LTP magnitude observed in the presence of Ro25–6981 was not mediated by D1/5 receptors.
Figure 4. Co-application with the NR2B antagonist Ro25–6981 (1 µM) blocked the SKF38393-mediated enhancement of LTP.
A) Summary plot of normalized fEPSP measurements. Open circles show normalized fEPSP slope from slices treated with Ro25–6981 (1 µM); closed circles depict slices treated with both Ro25–6981 (1 µM) and SKF38393 (20 µM); closed triangles depict slices treated with these drugs in addition to D1/5 antagonist SKF83566 (2 µM). Insets are 50 ms sweeps averaged from all experiments illustrating the mean fEPSP 1–5 min prior to and 26–30 min post-tetanus (vertical scale bar is 3 mV). The left pair of sweeps (1) is from Ro25–6981-treated slices, the right upper pair (2) is from Ro25–6981 & SKF38393-treated slices, the right lower pair (3) is from Ro25–6981/SKF83566 & SKF38393-treated slices. B) Summary quantification of LTP magnitude in the presence of Ro25–6981 alone, when Ro25–6981 is co-applied with SKF38393, and when both Ro25–6981 and the antagonist SKF83566 are co-applied with SKF38393 (significance is relative to the control group indicated by the dashed line, *p < 0.05, **p < 0.01). Error bars show ± SEM.
3.5. NR2B NMDARs are required for the D1/5 enhancement of LTP (continued)
In groups of slices treated with a D1/5 full agonist, SKF81297 (30 µM), the magnitude of LTP was significantly enhanced (118 ± 6%; n = 9, 3; p < 0.01; Fig 5B) when compared to the control group. LTP was no longer enhanced when NR2B antagonist Ro25–6981 (1 µM) was applied prior the dopaminergic agonist (72 ± 11%; n = 11, 4). In addition, slices exposed to both Ro25–6981 and SKF81297 did not show a decrease in LTP magnitude when compared to the control group, in contrast to the results obtained following co-application of Ro25–6981 and SKF38393 in the previous figure. These findings with SKF81297 further suggest that the decrease in LTP observed in the presence of co-applied Ro25–6981 and SKF38393 (Fig 4) is not attributable to D1/5 receptor activation, and is instead an effect of SKF38393 which is not shared by SKF81297.
3.6. Activation of D4 receptors contributes to the descending portion of the SKF38393 dose-response curve
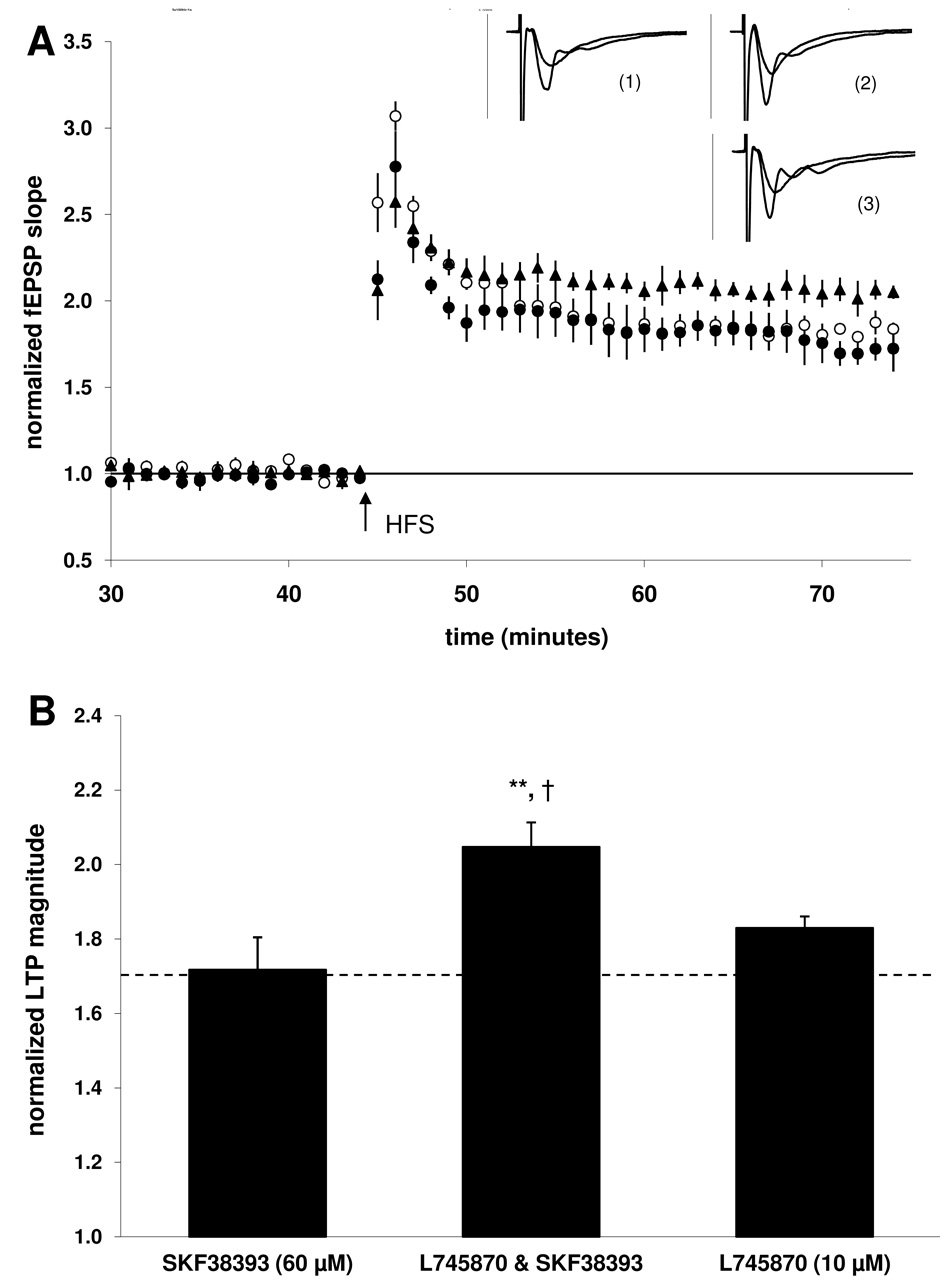
As previously noted (see 3.1 above), application of 60 µM SKF38393 resulted in an LTP magnitude which was not significantly different from the control group. However, when the D4 receptor selective antagonist L745870 (10 µM) was applied prior to the D1/5 agonist SKF38393 (60 µM), LTP was significantly enhanced (105 ± 7; n = 9, 3; p < 0.01; Fig 6B). The D4 antagonist alone did not significantly affect LTP magnitude (83 ± 3%; n = 9, 3) when compared to the control group, suggesting that basal activation of D4 receptors by endogenously released dopamine does not play a significant role in expression of the early component of LTP induced under our conditions.
Figure 6. Co-application with the D4 antagonist L745870 (10 µM) enabled 60 µM SKF38393 to enhance LTP.
A) Summary plot of normalized fEPSP measurements. Open circles show normalized fEPSP slope from slices treated with L745870 (10 µM); closed circles depict slices treated with SKF38393 (60 µM); closed triangles depict slices treated with both L745870 (10 µM) and SKF38393 (60 µM). Insets are 50 ms sweeps averaged from all experiments illustrating the mean fEPSP 1–5 min prior to and 26–30 min post-tetanus (vertical scale bar is 3 mV). The left pair of sweeps (1) is from SKF38393-treated slices, the right upper pair (2) is from L745870 & SKF38393-treated slices, the right lower pair (3) is from L745870-treated slices. B) Summary quantification of LTP magnitude in the presence of SKF38393 alone, when L745870 is co-applied with SKF38393, and when L745870 is applied alone (significance relative to the control group indicated by the dashed line, **p < 0.01; significance relative to the L745870 group is also indicated, †p < 0.05). Error bars show ± SEM.
3.7. Src-family tyrosine kinase activity is required for the D1/5 enhancement of LTP
One example of a functional connection between the cAMP/PKA pathway and the regulation of NR2B-containing NMDARs is via the activation of Src-family tyrosine kinase (Yaka et al., 2003). When the SFK inhibitor PP2 (10 µM) was applied prior to SKF38393 (20 µM), LTP was no longer enhanced in the presence of the dopaminergic agonist (70 ± 7%; n = 15, 5; Fig 5B). Applied alone, the SFK inhibitor did not significantly affect LTP magnitude (69 ± 3%; n = 12, 4). These results suggest that although baseline LTP evoked under our conditions did not require SFK activation, the D1/5-mediated enhancement of LTP was SFK-dependent.
4. Discussion
In this study, we have determined that the dopaminergic agonist SKF38393 is capable of facilitating LTP via activation of D1/5 dopamine receptors in a dose-dependent manner that involves NMDA receptors containing the NR2B subunit. We have also provided evidence that this mechanism is mediated by both protein kinase A and Src-family tyrosine kinase activities.
The dopaminergic agonist SKF38393 has been widely used in characterizing the role of D1/5 receptors in LTP (Huang and Kandel, 1995; Mockett et al., 2004; Navakkode et al., 2007; Swanson-Park et al., 1999), and we have tested a range of SKF38393 concentrations under our recording conditions. The results demonstrate that relatively low concentrations were capable of significantly elevating LTP above control levels, and this enhancement reached a maximum at a concentration of 20 µM. Unexpectedly, LTP magnitude returned toward the control level when slices were exposed to a higher concentration of this drug, an effect that was possibly due to a nonspecific (i.e. non D1/5-mediated) action of SKF38393. In order to confirm that the enhancement of potentiation was in fact mediated via D1/5 receptors, we tested SKF38393 in the presence of the D1/5 antagonist SKF83566. As this antagonist was able to block the ability of SKF38393 to enhance LTP beyond the control level seen in drug naïve slices, a D1/5-specific drug effect was confirmed. The nature of the nonspecific activity of SKF38393 to inhibit LTP was not the focus of these studies, but we note that this compound exhibits binding affinity in the micromolar range for several other receptors (Toll et al., 1998; Van Tol et al., 1991). Some possible contributors to this nonspecific activity could include D4, 5HT1A, or 5HT2A receptors, as activation of any of these would be expected to inhibit LTP in the CA1 region (Kotecha et al., 2002; Mori et al., 2001; Wang and Arvanov, 1998). Accordingly, our results with D4 antagonist L745870 indicate that the negative consequences for LTP following D4 receptor activation (Kotecha et al., 2002) significantly contributes to the summed effects of 60 µM SKF38393 on LTP magnitude. When the suppressive influence of D4 receptor activation was blocked, the enhancement of LTP via D1/5 receptor activity was restored.
Utilizing the selective PKA inhibitor H89, we determined that the magnitude of early LTP expressed under our conditions was not reliant upon PKA activity. This is consistent with a previous report that specifically tested “compressed” (20 s intertrain interval) vs. “spaced” (5 min intertrain interval) induction protocols and found that early LTP induced by compressed protocols was independent of PKA activity (Woo et al., 2003). Our ability to induce a baseline amount of LTP without the involvement of PKA activity afforded us the opportunity to examine the possible PKA-dependence of the D1/5 receptor-mediated enhancement of LTP. As D1/5 receptors are positively coupled to adenylate cyclase, we hypothesized that exposing slices to H89 prior to and during application of SKF38393 would impede the drug’s ability to enhance LTP. Indeed, under such conditions LTP magnitude was not altered from control levels, supporting the postulate that stimulation of cAMP/PKA is an initial step in the resultant signaling cascade following D1/5 receptor activation.
It is clear that there are a number of routes by which G-protein coupled receptors (GPCRs) are capable of modifying NMDAR activity at synapses in the hippocampal CA1 region, and evidence for a positive regulation of NR2B NMDAR activity by pituitary adenylate cyclase activating peptide has been reported (Yaka et al., 2003). Our findings provide additional evidence that GPCR activation (exemplified here by D1/5 receptors) has the ability to recruit signaling mechanisms that target NR2B receptor activity. Using the selective antagonist Ro25–6981, we determined that NR2B-containing NMDA receptor activation does not play a significant role in control LTP under our conditions. This was not unexpected, as it has been previously suggested that the dominant form of NMDAR NR2 subunit involved with LTP is NR2A, while NR2B has been suggested to be more critical in the expression of LTD (Liu et al., 2004). Applying Ro25–6981 to slices prior to and during application of either SKF38393 or the SKF81297 rendered these D1/5 agonists incapable of enhancing LTP. In sum, these findings demonstrate that NR2B-containing NMDARs are a key component in the D1/5 receptor-mediated neuromodulation of LTP in the hippocampal CA1 region.
It has previously been shown that Src-family kinase activation is involved in the induction of LTP in the CA1 region (Lu et al., 1998). Under our experimental conditions, this appears not to be the case as the SFK inhibitor PP2 had no effect on LTP. However, in order to examine the role of Src-family tyrosine kinase activity in the D1/5-mediated enhancement of LTP, we exposed slices to PP2 prior to and during application of SKF38393. In the presence of this inhibitor, D1/5 agonist SKF38393 was unable to significantly increase LTP beyond a control level. This provides evidence for the involvement of SFK activity in mediating the dopaminergic enhancement of LTP. Our findings with PKA and SFK are consistent with a model for Gs-coupled phosphorylation of the NR2B subunit in which both kinase activities participate in GPCR-mediated enhancement of NMDAR activity (Yaka et al., 2003). The model describes a trimolecular signaling complex which regulates the phosphorylation of NR2B-containing NMDARs in CA1 pyramidal cells. In this complex, the receptor for activated C-kinase 1 (RACK1) associates with both the NR2B subunit and SFKs, obstructing their ability to phosphorylate tyrosine residues on the cytoplasmic tail of the NR2B subunit. PKA-mediated phoshorylation of RACK1 causes a dissociation of the protein from the NR2B subunit, thereby allowing SFK activity to potentiate NMDAR currents. D1/5 receptor activation would be expected to initiate this regulatory mechanism via activation of either Gs- or Gq-coupled cascades (Salter and Kalia, 2004).
Although these current investigations are focused on the ability of D1/5 activation to enhance the magnitude of early LTP, a number of studies have investigated dopaminergic involvement in the late, protein-synthesis dependent stage of LTP (Frey et al., 1991; Frey et al., 1990; Huang and Kandel, 1995; Matthies et al., 1997; O'Carroll and Morris, 2004; Swanson-Park et al., 1999). In particular, early work revealed that activation of both D2-like and D1-like dopamine receptors during the tetanus event is necessary for the persistence of LTP, and D1-deficient mice were found to be incapable of expressing late LTP (Frey et al., 1989; Frey et al., 1991; Frey et al., 1990; Matthies et al., 1997). Furthermore, some reports have presented evidence that activation of D1/5 receptors in the absence of a tetanus event is sufficient to induce a potentiation that is similar to late LTP (Huang and Kandel, 1995; Navakkode et al., 2007), although this has not been a consistent observation (Mockett et al., 2004; Otmakhova and Lisman, 1996). Similar to the latter reports, we found no significant effects on baseline synaptic responses with any of the dopaminergic agonists applied during the 30 minute application that preceded the induction of LTP in our experiments. As previously noted (Mockett et al., 2004; Navakkode et al., 2007), there are several methodological considerations that may underlie this discrepancy.
Finally, it is interesting to consider the potential role that endogenously released dopamine may have on the modulation of synaptic plasticity. Recent work from our laboratory has explored the mechanism by which cocaine and the dopamine transporter blocker GBR12935 enhance LTP (Thompson et al., 2005), both of which exert their effect via D3-receptor activation and the inhibition of inhibitory synaptic input on CA1 pyramidal neurons (Hammad and Wagner, 2006; Swant and Wagner, 2006). Now in this report, we have confirmed the enhancement of LTP via D1/5-receptor activation and described a mechanism which directly involves the facilitation of excitatory input on CA1 pyramidal neurons. As the net effects of both of these mechanisms are excitatory, they might be additive or even synergistic, since reducing IPSCs on dendritic shafts would potentially allow increased depolarization and activation of extrasynaptic NR2B-containing NMDARs. Alternatively, D3 receptor activation could be permissive for the actions of endogenously released dopamine in the CA1 since this receptor subtype has the highest affinity for dopamine, and disinhibition could be a requirement for extrasynaptic NR2B-containing NMDARs to become activated under physiological conditions. Regardless, together our results point toward an increase in dopaminergic activity in the CA1 region of the hippocampus to be working to enhance the synaptic plasticity processes thought to be involved with learning and memory via participation of NR2B-containing NMDARs in synaptic signaling.
Acknowledgements
J.J.W. was funded by National Institute on Drug Abuse (DA 16302).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blitzer R, Wong T, Nouranifar R, Iyengar R, Landau E. Postsynaptic cAMP pathway gates early LTP in hippocampal CA1 region. Neuron. 1995;15:1403–1414. doi: 10.1016/0896-6273(95)90018-7. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Buchwald N, Levine M. Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid receptor subtypes activated. Proc. Natl. Acad. Sci. U. S. A. 1993;90:9576–9580. doi: 10.1073/pnas.90.20.9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Roche K. Regulation of NMDA receptors by phosphorylation. Neuropharmacology. 2007;53:362–368. doi: 10.1016/j.neuropharm.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge C, Kehl S, McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J. Phyiol. 1983;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunah A, Standaert D. Dopamine D1 receptor-dependent trafficking of striatal NMDA glutamate receptors to the postsynaptic membrane. J. Neurosci. 2001;21:5546–5558. doi: 10.1523/JNEUROSCI.21-15-05546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Hartmann S, Matthies H. Domperidone, an inhibitor of the D2-receptor, blocks a late phase of an electrically induced long-term potentiation in the CA1-region in rats. Biomed. Biochim. Acta. 1989;48:473–476. [PubMed] [Google Scholar]
- Frey U, Huang Y, Kandel E. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- Frey U, Matthies H, Reymann K, Matthies H. The effect of dopaminergic D1 receptor blockade during tetanization on the expression of long-term potentiation in the rat CA1 region in vitro. Neurosci. Lett. 1991;129:111–114. doi: 10.1016/0304-3940(91)90732-9. [DOI] [PubMed] [Google Scholar]
- Frey U, Schroeder H, Matthies H. Dopaminergic antagonists prevent long-term maintenance of posttetanic LTP in the CA1 region of rat hippocampal slices. Brain Res. 1990;522:69–75. doi: 10.1016/0006-8993(90)91578-5. [DOI] [PubMed] [Google Scholar]
- Hallett P, Spoelgen R, Hyman B, Standaert D, Dunah A. Dopamine D1 activation potentiates striatal NMDA receptors by tyrosine phosphorylation-dependent subunit trafficking. J. Neurosci. 2006;26:4690–4700. doi: 10.1523/JNEUROSCI.0792-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad H, Wagner J. Dopamine-mediated disinhibition in the CA1 region of rat hippocampus via D3 receptor activation. J. Pharmacol. Exp. Ther. 2006;316:113–120. doi: 10.1124/jpet.105.091579. [DOI] [PubMed] [Google Scholar]
- Huang Y, Kandel E. D1/D5 receptor agonists induce a protein synthesis-dependent late potentiation in the CA1 region of the hippocampus. Proc. Natl. Acad. Sci. U. S. A. 1995;92:2446–2450. doi: 10.1073/pnas.92.7.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp C, Longordo F, Lüthi A. Experience-dependent changes in NMDA receptor composition at mature central synapses. Neuropharmacology. 2007;53:1–9. doi: 10.1016/j.neuropharm.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Kotecha S, Oak J, Jackson M, Perez Y, Orser B, Van_Tol H, MacDonald J. A D2 class dopamine receptor transactivates a receptor tyrosine kinase to inhibit NMDA receptor transmission. Neuron. 2002;35:1111–1122. doi: 10.1016/s0896-6273(02)00859-0. [DOI] [PubMed] [Google Scholar]
- Lee F, Xue S, Pei L, Vukusic B, Chery N, Wang Y, Wang Y, Niznik H, Yu X, Liu F. Dual regulation of NMDA receptor functions by direct protein-protein interactions with the dopamine D1 receptor. Cell. 2002;111:219–230. doi: 10.1016/s0092-8674(02)00962-5. [DOI] [PubMed] [Google Scholar]
- Lemon N, Manahan-Vaughan D. Dopamine D1/D5 receptors gate the acquisition of novel information through hippocampal long-term potentiation and long-term depression. J. Neurosci. 2006;26:7723–7729. doi: 10.1523/JNEUROSCI.1454-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M, KL A, Cepeda C, Cromwell H, Crawford C, Ariano M, Drago J, Sibley D, Westphal H. Modulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid receptor subtypes activated. J. Neurosci. 1996;16:5870–5882. doi: 10.1523/JNEUROSCI.16-18-05870.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Grace A. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Liu L, Wong T, Pozza M, Lingenhoehl K, Wang Y, Sheng M, Auberson Y, Wang Y. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Lu Y, Roder J, Davidow J, Salter M. Src activation in the induction of long-term potentiation in CA1 hippocampal neurons. Science. 1998;279:1363–1367. doi: 10.1126/science.279.5355.1363. [DOI] [PubMed] [Google Scholar]
- MacDonald J, Jackson M, Beazely M. Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors. Crit. Rev. Neurobiol. 2006;18:71–84. doi: 10.1615/critrevneurobiol.v18.i1-2.80. [DOI] [PubMed] [Google Scholar]
- Matthies H, Becker A, Schröeder H, Kraus J, Höllt V, Krug M. Dopamine D1-deficient mutant mice do not express the late phase of hippocampal long-term potentiation. Neuroreport. 1997;8:3533–3535. doi: 10.1097/00001756-199711100-00023. [DOI] [PubMed] [Google Scholar]
- Mockett B, Brooks W, Tate W, Abraham W. Dopamine D1/D5 receptor activation fails to initiate an activity-independent late-phase LTP in rat hippocampus. Brain Res. 2004;1021:92–100. doi: 10.1016/j.brainres.2004.06.039. [DOI] [PubMed] [Google Scholar]
- Mori K, Togashi H, Kojima T, Matsumoto M, Ohashi S, Ueno K, Yoshioka M. Different effects of anxiolytic agents, diazepam and 5-HT(1A) agonist tandospirone, on hippocampal long-term potentiation in vivo. Pharmacol. Biochem. Behav. 2001;69:367–372. doi: 10.1016/s0091-3057(01)00546-9. [DOI] [PubMed] [Google Scholar]
- Navakkode S, Sajikumar S, Frey JU. Synergistic requirements for the induction of dopaminergic D1/D5-receptor-mediated LTP in hippocampal slices of rat CA1 in vitro. Neuropharmacology. 2007;52:1547–1554. doi: 10.1016/j.neuropharm.2007.02.010. [DOI] [PubMed] [Google Scholar]
- O'Carroll CM, Morris RGM. Heterosynaptic co-activation of glutamatergic and dopaminergic afferents is required to induce persistent long-term potentiation. Neuropharmacology. 2004;47:324–332. doi: 10.1016/j.neuropharm.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Otmakhova N, Lisman J. D1/D5 Dopamine Receptor Activation Increases the Magnitude of Early Long-Term Potentiation at CA1 Hippocampal Synapses. J. Neurosci. 1996;16:7478–7486. doi: 10.1523/JNEUROSCI.16-23-07478.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhova N, Otmakhov N, Mortenson L, Lisman J. Inhibition of the cAMP Pathway Decreases Early Long-Term Potentiation at CA1 Hippocampal Synapses. J. Neurosci. 2000;20:4446–4451. doi: 10.1523/JNEUROSCI.20-12-04446.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nature Reviews Neuroscience. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- Snyder G, Fienberg A, Huganir R, Greengard P. A dopamine/D1 receptor/protein kinase A/dopamine- and cAMP-regulated phosphoprotein (Mr 32 kDa)/protein phosphatase-1 pathway regulates dephosphorylation of the NMDA receptor. J. Neurosci. 1998;18:10297–10303. doi: 10.1523/JNEUROSCI.18-24-10297.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson-Park J, Coussens C, Mason-Parker S, Raymond C, Hargreaves E, Draqunow M, Cohen A, Abraham W. A double dissociation within the hippocampus of dopamine D1/D5 receptor and beta-adrenergic receptor contributions to the persistence of long-term potentiation. Neuroscience. 1999;92:485–497. doi: 10.1016/s0306-4522(99)00010-x. [DOI] [PubMed] [Google Scholar]
- Swant J, Wagner J. Dopamine transporter blockade increases LTP in the CA1 region of the rat hippocampus via activation of the D3 dopamine receptor. Learn. Mem. 2006;13:161–167. doi: 10.1101/lm.63806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A, Swant J, Wagner J. Cocaine-induced modulation of long-term potentiation in the CA1 region of rat hippocampus. Neuropharmacology. 2005;49:185–194. doi: 10.1016/j.neuropharm.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Toll L, Berzetei-Gurske I, Polgar W, Brandt S, Adapa I, Rodriguez L, Schwartz R, Haggart D, O'Brien A, White A, Kennedy J, Craymer K, Farrington L, Auh J. Standard binding and functional assays related to medications development division testing for potential cocaine and opiate narcotic treatment medications. NIDA Res. Monogr. 1998;178:440–466. [PubMed] [Google Scholar]
- Van_Tol H, Bunzow J, Guan H, Sunahara R, Seeman P, Niznik H, Civelli O. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature. 1991;350:610–614. doi: 10.1038/350610a0. [DOI] [PubMed] [Google Scholar]
- Wang R, Arvanov V. M100907, a highly selective 5-HT2A receptor antagonist and a potential atypical antipsychotic drug, facilitates induction of long-term potentiation in area CA1 of the rat hippocampal slice. Brain Res. 1998;779:309–313. doi: 10.1016/s0006-8993(97)01174-8. [DOI] [PubMed] [Google Scholar]
- Woo N, Duffy S, Abel T, Nguyen P. Temporal spacing of synaptic stimulation critically modulates the dependence of LTP on cyclic AMP-dependent protein kinase. Hippocampus. 2003;13:293–300. doi: 10.1002/hipo.10086. [DOI] [PubMed] [Google Scholar]
- Yaka R, He D, Phamluong K, Ron D. Pituitary adenylate cyclase-activating polypeptide (PACAP(1–38)) enhances N-methyl-D-aspartate receptor function and brain-derived neurotrophic factor expression via RACK1. J. Biol. Chem. 2003;278:9630–9638. doi: 10.1074/jbc.M209141200. [DOI] [PubMed] [Google Scholar]