Abstract
There is evidence that nitric oxide (NO) formation in adult cardiomyocytes stimulated with lipopolysaccharide (LPS) does not commensurate with iNOS levels. Tetrahydrobiopterin (BH4) is a key factor in the stabilization and NO production by iNOS homodimer. Thus we hypothesized that BH4 is a limiting factor for NO production in adult cardiomyocytes in response to LPS and cytokines (TNF-α, IL-1, IFN-γ alone or mixed). It was verified that LPS and cytokines induced iNOS expression which did not translate into increased nitrite or 14C-citrulline production. This response coincided with defective BH4 synthesis and low GTP cyclohydrolase activity. Furthermore, supplementation with BH4 and ascorbate failed to increase iNOS activity. This effect was related to preferential accumulation of BH2 rather than BH4 in these cells. Uncoupled iNOS activity in stimulated cells was examined using mitochondrial aconitase activity as an endogenous marker of superoxide anion radical (O2•−) formation, and found not to be significantly inhibited. 2-Hydroxyethidium also showed not to be significantly increased. We conclude that adult cardiomyocytes are an unlikely source of NO and O2•− in inflammatory conditions. This finding adds a new and unexpected layer of complexity to our understanding of the responses of the adult heart to inflammation.
Keywords: Tetrahydrobiopterin, aconitase, nitric oxide, cytokines, lipopolysaccharide
Introduction
Increased expression of inducible nitric oxide synthase (iNOS) has been demonstrated in cardiac failure arising from a variety of inflammatory conditions, including human and experimental animal models of acute cardiac allograft rejection and sepsis. In these conditions, cytokines (TNF-α, IFN-γ and others) and lipopolysaccharide (LPS) increase iNOS protein levels in the heart, liver and other tissues [1–3]. Moreover, the specific iNOS mRNA expression in cardiomyocytes was shown in cells isolated from the hearts of rats receiving LPS [4]. This data suggested that cardiomyocyte-derived nitric oxide (NO) is important in promoting organ damage. Additional studies linked iNOS expression to oxidative stress and apoptosis in cardiomyocytes [5,6].
It is generally accepted that NO production from iNOS is commensurate with iNOS protein levels. However, the actual NO bioactivity as a consequence of iNOS upregulation can be controlled by additional mechanisms. Studies characterizing iNOS properties showed that the stabilization of the homodimer state of the protein is an absolute requirement for optimal levels of NO-forming activity. It has been established that iNOS homodimerization depends on insertion of the heme group [7], while stabilization of the homodimer active form of the enzyme is dependent on L-arginine and tetrahydrobiopterin (BH4). In the absence of BH4, however, the iNOS homodimer easily dissociates into inactive monomers [8].
There is limited information regarding the regulation of BH4 levels within adult cardiomyocytes. The available studies profiling the coordinated regulation of BH4 and iNOS expression with that of concomitant NO production in cardiomyocytes are derived exclusively from neonate rat cardiomyocyte preparations. In a recent study, Kalivendi et al [9] showed that biopterin levels, an index of BH4, were extremely low in adult rat cardiomyocytes even after stimulation with the same LPS concentration that upregulates iNOS expression. This response raises questions about the role of iNOS in the mechanisms of heart dysfunction under inflammatory stimuli. Therefore, it is possible that an upregulation of iNOS without BH4 may render an inactive iNOS-monomer which results in a lack of NO production.
There are indications that not only is an excess of NO detrimental to cardiac allograft rejection, but an increase of reactive oxygen species may also be involved [10,11]. It is possible that upregulation of iNOS in a BH4-deficient environment favors an uncoupled iNOS activity with or without an increase in reactive oxygen species formation. Clearly, this shift to inhibit iNOS enzyme activity may have serious implications in defining the role of iNOS in cardiac injury associated with inflammatory conditions. Therefore, we characterized iNOS expression and coordinated responses of BH4 and NO bioactivity in adult cardiomyocytes in order to fully understand their role in cardiomyocyte dysfunction.
Methods
The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Isolation of adult rat cardiomyocytes
Adult rat ventricular cardiomyocytes were isolated from male Sprague–Dawley adult rats (160–180 g). Animals were given heparin (150 U) intraperitoneally and anesthetized with sodium pentobarbital (50 mg/kg), i.p. The heart was excised and mounted on an aortic cannula and perfused with a buffer (pH 7.4) containing (in mM): NaCl, 110; HEPES, 25; glucose, 11; creatine, 5; taurine, 20; KCl, 2.6; MgSO4, 1.2; and KH2PO4, 1.2. The perfusion buffer was saturated with 100% oxygen and maintained at 37 °C. After 8 min of perfusion with Ca2+-free buffer, the perfusion was continued by recirculation of 60 ml of buffer supplemented with type II collagenase (250 U/ml) and CaCl2 (50 μM). After 25 min, the ventricular tissue was minced and incubated for 5 min in a recirculating medium with BSA (1%) and deoxyribonuclease (20 mg/ml). Cells were carefully released from chunks of tissue by gentle pipetting and then filtered through a 100 μm nylon cell strainer. Cell suspension was washed twice and resuspended in a CaCl2-containing buffer in which CaCl2 was consecutively increased from 0.2 to 0.5 mM. To isolate cardiomyocytes, the cell suspension was layered over BSA solution (4%) in a buffer containing CaCl2 (1mM). Cardiomyocytes were allowed to settle, and then plated on to 100-mm plates pre-coated with laminin (Invitrogen, Carlsbad, CA). For in vitro culture, Media 199 supplemented with BSA (0.2 %), insulin (10 nM), creatine (5 mM), L-carnitine (2 mM), taurine (5 mM), penicillin (100 units/ml), and streptomycin (100 mg/ml) was used. After 2 hr intact cardiomyocytes adhered to the culture plates and damaged cells were washed away with the medium change. Cardiomyocytes (>90% rod-shape phenotype) were cultured under these conditions for 24–48 hr before stimulation with cytokines.
Cytokine and LPS stimulation
Adult rat ventricular cardiomyocytes were maintained in 95% air-5% CO2 in a humidified incubator at 37°C and treated for 12 and 24 hr with cytokines or LPS at the indicated concentrations. All cytokines were purchased from R&D Systems (Minneapolis, MN) and LPS (serotype O6:B11) was from Sigma Chemical Co. (St. Louis, MO).
Reverse transcriptase-PCR (RT-PCR) analysis
Total cellular RNA was isolated by using 1 ml of TRIzol® reagent (Invitrogen, Carlsbad, CA) per 100-mm cardiomyocyte culture plate according to the manufacturer’s protocol. Complementary DNA was synthesized from total RNA (1 μg) and random hexamers primers by using SuperScript® III First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA) according to the manufacturer’s directions. The iNOS primers used for these analyses were: forward (5′-3′): CACCTTGGAGTTTCACCCAGT and reverse (5′-3′): TGTTTGTAGCGCTGTGTGTCA. PCR conditions include: denaturation (94°C for 1 min); annealing (60°C for 1 min); extension (72°C for 1.5 min); number of cycles (30). PCR reactions were performed in a 25-μl volume containing cDNA (1 μl), sequence-specific primers (25 pmol) and Platinum® PCR SuperMix High Fidelity (25 μl) (Invitrogen, Carlsbad, CA). The PCR product (328 bp) was resolved by 1% TAE-agarose gel electrophoresis and densitometric analysis was performed using Alpha Imager (Alpha Innotech Corp, San Leandro, CA).
Western Blot Analysis
Adult rat ventricular myocytes were lysed in RIPA buffer supplemented with phenylmethanesulphonylfluoride (PMSF 1 mM), sodium orthovanadate (1 mM), sodium fluoride (1 mM), and protease inhibitor cocktail (Roche, Basel, Switzerland). Proteins were applied onto SDS-PAGE system and protein separation was determined with the help of prestained protein molecular weight markers (precision plus protein, dual color, BioRad, Hercules, CA), followed by transfer to nitrocellulose. Membranes were probed with specific antibodies for iNOS (1:1000), eNOS (1:1000), and B-actin (1:500) obtained from Santa Cruz Biotechnology (Santa Cruz, CA); or GAPDH (1:1000, Bioscience Research Reagents) and GTPCH (1:200) at 4°C overnight. Immunoblots were developed with ECL Plus Western Blotting Detection System (Amersham, Buckinghamshire, United Kingdom) following the manufacturer’s recommendations.
For the analysis of iNOS dimer, cardiomyocytes were lysed in buffer containing protease inhibitors (Complete™, Roche) with and without the addition of L-arginine (2 mM) and BH4 (10 μM). After incubation for 30 min in ice bath, cells were syringe-lysed and spun down to eliminate cell debris. Cell lysate proteins were prepared in Laemmli buffer without boiling and resolved in 7.5% SDS-PAGE run at 4°C.
Total biopterin and tetrahydrobiopterin measurements
Total intracellular biopterin content was measured in cardiomyocyte cell lysates in 0.1 M HCl and oxidized with KI/I2 reagent for 1 hr at room temperature. Quantification was performed by HPLC with fluorescence detection as described previously [9]. In addition, differential quantification of BH4, BH2 and ascorbate was performed by HPLC with electrochemical detection as previously described [12]. Cell pellets were lysed in 150 μl of 50 mM phosphate buffer (pH. 2.6) containing 0.1 mM DTPA and freshly added 1 mM DTE. Samples were centrifuged at 12,500 rpm for 10 min at 4°C, and supernatants were analyzed on an HPLC system ESA Biosciences CoulArray® system, Model 582 and 542 (ESA, Chelmford, MA). Multi-channel coulometric detection was set between 0–600 mV. Intracellular concentrations were calculated using authentic BH4, BH2 and ascorbate (10–100 nM) as standards and normalized to protein content.
2-Hydroxyethidium measurements
Cardiomyocytes in 100 mm diameter culture dishes were washed twice with DPBS and incubated for 20 min with 10 μM HE in culture media. Cells were washed with ice cold DPBS, and syringe lysed in 0.25 ml DPBS containing 0.1% Triton X-100. Cell debris was spun down and an aliquot of supernatant was removed for protein quantification. Another aliquot was incubated with 0.2 M HClO4 in methanol solution for 1 h on ice. After pelleting proteins an aliquot of the supernatant was combined with 1M phosphate buffer pH 2.6 following by centrifugation for 15 min at 14,000 rpm at 4°C. Supernatants were injected onto a Synergi Polar-RP column (Phenomenex, 4μ, 250mm×4.6 mm) and analyzed by electrochemical detection as described [13]. All the reagents, additions and incubations were performed protected from light exposure to avoid unspecific product formation.
Biochemical Assays
iNOS activity
Nitrite was quantified in the culture media collected after treatments with cytokines. The media was collected and filtered through 10 kDa molecular mass cut-off filters (Millipore, Billerica, MA) at 14000 rpm for 30 min. Aliquots (20 μl) were reacted with KI reagent to release NO, and detected with a Nitric Oxide Analyzer (Sievers Instruments, Inc., Boulder, CO). L-citrulline formation was tested in syringe cell lysates in HEPES buffer containing protease inhibitors (Complete™, Roche supplemented with sodium orthovanadate). An aliquot (50 μl) cell lysate was incubated with a reaction mix containing 14C-L-arginine, NADPH (0.1 mM); BSA (0.1 mg/ml) with and without BH4 (10 μM) in HEPES buffer (50 mM, pH 7.4) at 37 ºC. Concentration of L-citrulline was calculated by scintillation counting after separation in a cationic exchange Dowex column.
GTP cyclohydrolase I (GTPCH) Activity
Cells were suspended in Tris-HCl (50 mM) buffer pH 7.4 containing PMSF (1 mM) and protease inhibitors cocktail (Roche). Aliquots were assayed for activity as described previously [9]. Briefly, cell lysates were incubated with 1 mM GTP, 50 μg/ml bovine serum albumin, and 1 mM dithiothreitol in 50 mM Tris-HCl pH 7.4 in a total volume of 200 μl. After incubation (60 min,37°C), the reaction was stopped by cooling down in ice-water bath and by the addition of 5 N HCl. Next, samples were oxidized with 2% KI/1% I2 solution at room temperature. After 60 min the reaction was stopped by addition of ascorbate. Following treatment with 5 U alkaline phosphatase, neopterin content was analyzed by HPLC using a C18 reverse phase column (C18-kromasil, Alltech) eluted with water:0.1% TFA (1:1) at a flow of 1 ml/min. Neopterin was detected by fluorescence with λexc of 350 and λem of 440 nm. Authentic neopterin was used as standard.
Aconitase activity
Cardiomyocytes were permeabilized with a cold phosphate saline solution containing citrate (5 mM), DTPA (0.1 mM) and Triton X-100 (0.2%). The sample was incubated on ice for 10 min followed by centrifugation at 750 g for 15 min at 4°C to remove cell debris. Enzyme activity was measured following the formation of cis-aconitate from isocitrate for 2 min at 240 nm and the concentration calculated using an extinction coefficient of 3.6 mM−1cm−1. The assay was performed at room temperature in Tris-HCl buffer (100 mM, pH 8.0) containing DL-isocitrate (20 mM). Blanks were performed in the presence of the aconitase inhibitor isofluorocitrate (5 mM).
Statistical analysis
Results are expressed as means±S.D. All of the statistics consisted of ANOVA by Kruskal-Wallis with Dunn’s post test for selected comparisons. Significance was set at P<0.05.
Results
Induction of iNOS expression in adult cardiomyocyte
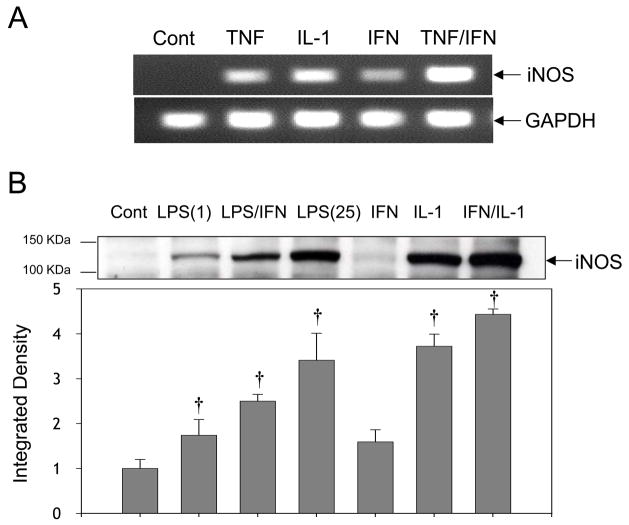
The iNOS gene expression was demonstrated by RT-PCR assay in primary culture adult cardiomyocytes. Cells were stimulated for 12 or 24 hr with cytokines at concentrations that have been previously shown to cause cardiomyocyte dysfunction [14–20]. Both TNF-α (20 ng/ml) or IL-1β (20 ng/ml) alone or in combination for 12 hr potently increased iNOS mRNA levels whereas IFN-γ (50 ng/ml) was less effective (Fig. 1A). These findings confirm that iNOS mRNA induction in adult cardiomyocytes follows a similar trend to that shown previously using neonatal cardiomyocytes [21,22]. A robust increase in iNOS protein expression was also detected after 12 hr incubation with IL-1α (0.125 ng/ml) and IFN-γ (20 ng/ml), with a comparable response induced by IL-1α alone (Fig. 1B). Also high iNOS was induced by LPS (1, 25 and 50 μg/ml) alone or in combination with IFN-γ (20 ng/ml) (Fig. 1B and 1C). This response was abolished by co-treatment with the protein synthesis inhibitor cycloheximide indicating that iNOS is the product of new protein synthesis. The specificity of the antibody for iNOS was demonstrated by comparing the responses with recombinant iNOS protein (Fig. 1C). Additionally, cardiomyocytes proteins do not react with antibodies against eNOS that produce a strong signal in endothelial cells extracts (Fig. 1D). It was also verified that at 24 hr post-stimulation, similar levels of iNOS protein were found (data not shown), indicating that the majority of iNOS expression occurs before 12 hr with little or no changes thereafter.
Figure 1. Induction of iNOS expression in primary culture adult rat cardiomyoctes.
Cells were stimulated with freshly prepared cytokines and/or LPS in M199-supplemented media for 12 h. (A) RT-PCR analysis of iNOS mRNA levels following stimulation with control; TNF-α (20 ng/ml); IL-1β (20 ng/ml); IFN-γ (50 ng/ml); TNF-α (20 ng/ml) plus IFN-γ (50 ng/ml). (B) Western blot analysis of iNOS protein in cardiomyocytes stimulated or not (control) with; LPS (1 μg/ml); LPS (1 μg/ml) plus IFN-γ (20 ng/ml); LPS (25 μg/ml); IFN-γ (20 ng/ml); IL-1 (0.125 ng/ml); IFN-γ (20 ng/ml) plus IL-1 (0.125 ng/ml). (C) Western blot analysis of recombinant iNOS (1.25 μg) and cardiomyocytes stimulated or not (control) with LPS(25 μg/ml); LPS (50 μg/ml); LPS (50 μg/ml) plus IFN-γ (20 ng/ml); LPS (50 μg/ml) plus IL-1 (0.125 ng/ml) and LPS (50 μg/ml) plus cycloheximide (CHX, 2 μM). (D) eNOS detection in cardiomyocytes and human coronary aorta endothelial cells (HAECs). Cardiomyocytes were treated or not (control) with TNF-α (20 ng/ml); IL-1β (20 ng/ml); IFN-γ (50 ng/ml); TNF-α (20 ng/ml) plus IFN-γ (50 ng/ml). The increase in iNOS and eNOS protein levels were estimated by integrated densitometry values taking control values as 1. Results are representative of at least three experiments, and are expressed as Mean±SD. (†) P<0.05.
iNOS activity in stimulated cardiomyocytes
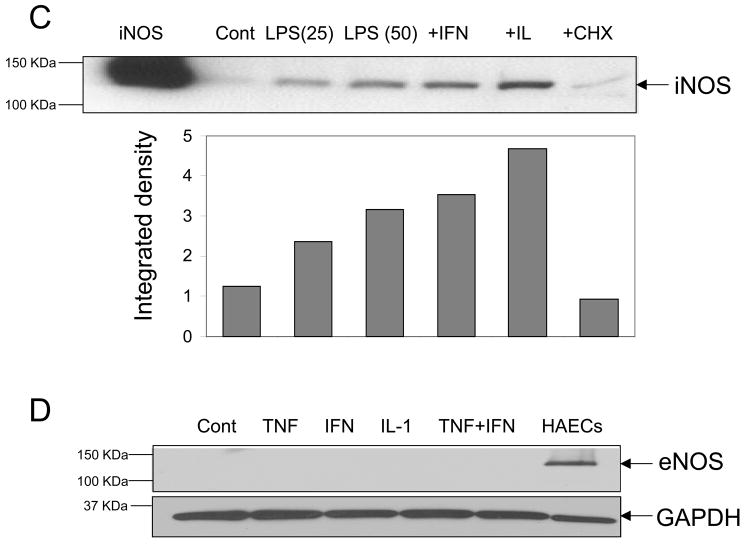
iNOS activity was assessed by measuring the accumulation of nitrite in the culture media after 12 hr incubation of adult cardiomyocytes with LPS or cytokines alone or in combination. As shown in Figure 2, neither LPS nor cytokines were able to significantly increase iNOS activity over control levels despite an upregulation of iNOS protein expression. Since nitrite can be converted to other nitrogen species, we examined the conversion of 14C-L-arginine to 14C-L-citrulline as an additional, but more direct method for assessing iNOS activity. Similarly, increases in citrulline generation were insignificant upon stimulation with either LPS or cytokines as above. Exceedingly high LPS concentrations (25 μg/ml) stimulated activity only ~ 4 fmol citrulline/min/mg protein higher than control non-stimulated cells (Fig. 2). This level of activity is remarkably low considering that purified iNOS protein presents a specific activity of ~300 nmoles/min/mg protein and higher [23]. To illustrate this point further, we found that RAW264.7 cells stimulated with LPS for the same time and dose dramatically increased citrulline production to ~2000 fmol/min/mg protein. Collectively, these results indicate that inflammatory mediators increase iNOS expression in adult rat ventricular myocytes, but they fail to stimulate NO-producing activity to significant levels.
Figure 2. iNOS activity in cytokine and LPS-stimulated adult cardiomyocytes.
Enzyme activity was measured by quantifying (A) nitrite and (B) 14C-L citrulline formation in cardiomyocytes stimulated or not (control) with LPS (1 μg/ml); LPS (1 μg/ml) plus IFN-γ (20 ng/ml); LPS (25 μg/ml); IFN-γ (20 ng/ml); IL-1α (0.125 ng/ml); IFN-γ (20 ng/ml) plus IL-1α (0.125 ng/ml). Results are represented as Mean±SD (n=4 nitrite). Citrulline results represent the average of two experiments.
iNOS dimeric and monomeric distribution
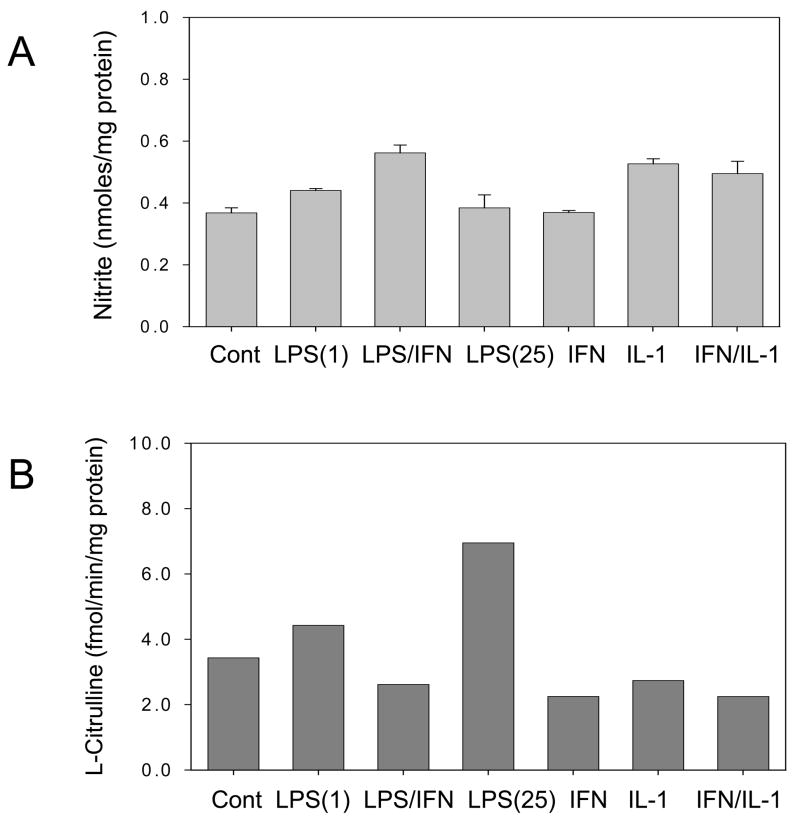
The lack of significant increases in iNOS activity may be explained by the destabilization of the dimeric state of iNOS protein. Thus, iNOS monomer:dimer distribution in cytokine-stimulated cardiomyocytes was examined. To minimize potential changes in dimer-monomer during cellular protein extraction, the lysis buffer was supplemented or not with L-arginine and BH4. All of the conditions tested showed significant levels of iNOS monomers (Fig. 3) which could explain the lack of enzyme activity. Therefore, since dimer formation and stabilization requires optimal amounts of heme, substrate and BH4, limiting the supply of any of these cofactors will likely produce inactive iNOS monomers.
Figure 3. Western blot analysis of iNOS monomer:dimer distribution in cytokine-stimulated adult cardiomyocytes.
Cell lysates were prepared in buffer without and with 10 μM BH4 and 2 mM L-arginine. Cardiomyocytes were stimulated or not (control) with: TNF-α (20 ng/ml); IFN-γ (50 ng/ml); IL-1β (20 ng/ml); TNF-α (20 ng/ml) plus IFN-γ (50 ng/ml).
Changes in total biopterin, tetrahydrobiopterin and ascorbate in LPS and cytokine-stimulated cardiomyocytes
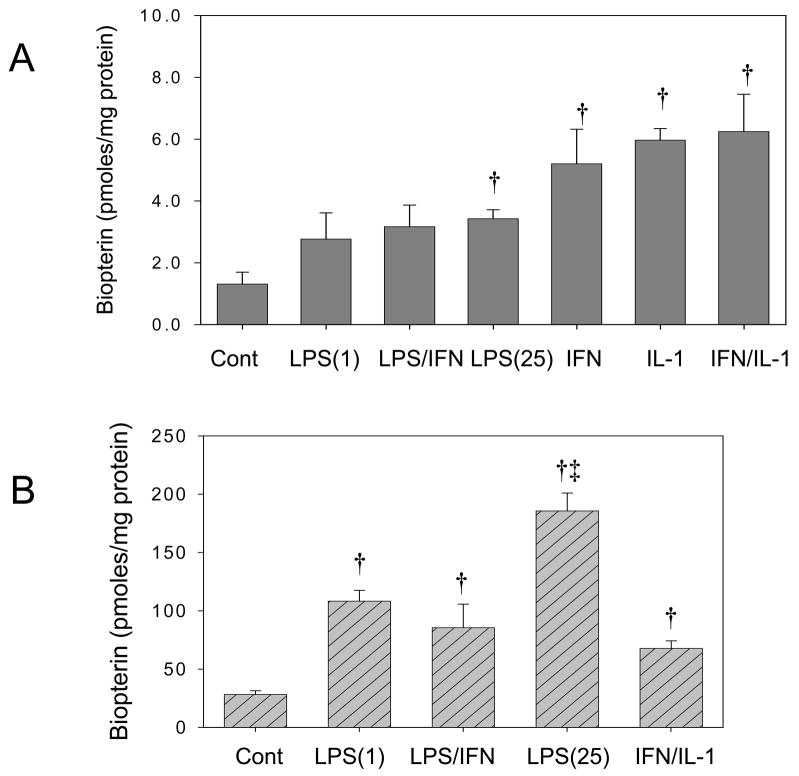
The role of BH4 in the lack of activity and preferential iNOS monomer distribution was examined in adult cardiomyocytes stimulated with LPS and cytokines. Total biopterin measurements represent the sum of the concentrations of BH4 and other redox forms and are taken as a general index for increased synthesis of BH4. The analysis of biopterin by HPLC with fluorescence showed that IFN-γ (20 ng/ml) or IL-1α (0.125 ng/ml) alone or a combination were equally efficient at increasing total biopterin from 1.25±0.3 to ~5.5 pmoles/mg protein (Fig. 4A). Likewise, treatment with LPS alone or in combination with IFN-γ increased total biopterin to ~3 pmoles/mg protein. In RAW264.7 cells, however, the same treatment induced a much stronger increase in biopterin production (Fig. 4B) indicating that the responses in adult cardiomyocytes are marginal. To further examine the effects of cytokine stimulation on BH4 synthesis, we directly quantified BH4 levels using HPLC with electrochemical detection (HPLC-EC) [12]. This analysis showed negligible increases in BH4 levels in cardiomyocytes treated with TNF-α or IFN-γ alone or a combination (Table 1). Collectively, our data indicate that adult cardiomyocytes are BH4-deficient under basal conditions and that cytokines and/or LPS fail to stimulate BH4 production.
Figure 4. Total biopterin analysis in cytokine and LPS-stimulated adult cardiomyocytes.
Quantification of total biopterin (BH4+BH2+biopterin) was performed by HPLC with fluorescent detection after acid oxidation of cell lysates with KI/I2. (A) Cardiomyocytes were stimulated or not (control) with: (LPS 1 μg/ml); LPS (1 μg/ml) plus IFN-γ (20 ng/ml); LPS (25 μg/ml); IFN-γ (20 ng/ml); IL-1α (0.125 ng/ml); IFN-γ (20 ng/ml) plus IL-1α (0.125 ng/ml). (B) RAW264.7 cells were stimulated or not (control) with: LPS (1 μg/ml); LPS (1 μg/ml) plus IFN-γ (20 ng/ml); LPS (25 μg/ml); and IFN-γ (20 ng/ml) plus IL-1α (0.125 ng/ml). Results are represented as Mean±SD (n=4). (†) P<0.05 respect to non-treated controls. ; (‡) P<0.05 with respect to LPS(1).
Table 1.
Direct quantification of BH4, BH2 and ascorbate in adult rat cardiomyocytes by HPLC with electrochemical detection.
| Treatment | BH4 (pmol/mg protein) | BH2 (pmol/mg protein) | Ascorbate (pmol/mg protein) |
|---|---|---|---|
| Control | 0.066±0.058 | 0.0 | 13.80±2.10 |
| TNF-α | 0.039±0.058 | 0.0 | 13.23±0.14 |
| IFN-γ | 0.057±0.035 | 0.0 | 13.83±1.92 |
| IL-1 | 0.077±0.087 | 0.0 | 12.51±1.57 |
| TNF-α + I FN-γ | 0.078±0.093 | 0.0 | 20.68±1.40 |
Adult cardiomyocytes were treated with cytokines 12 hr as follows: TNF-α (20 ng/ml); IFN-γ (50 ng/ml); IL-1 (20 ng/ml). Values represent the mean±SD of at least 3 experiments.
GTPCH activity in adult cardiomyocytes
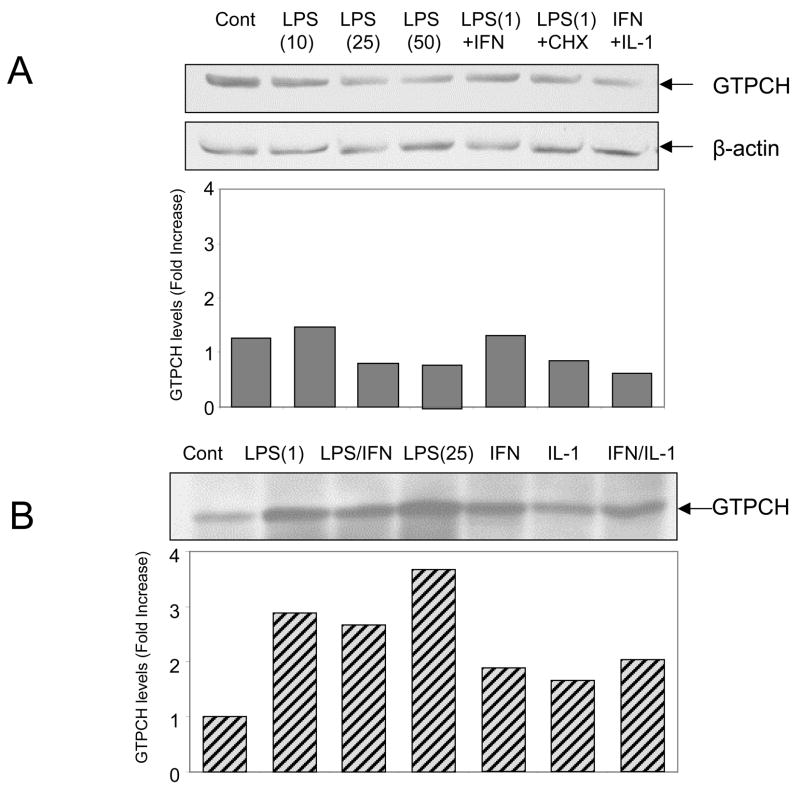
The enzyme activity of GTP cyclohydrolase I (GTPCH) was determined in order to explain the absence of BH4 in adult cardiomyocytes. Cells treated with LPS did not increase GTPCH activity over control values of <0.25 pmol/h/mg protein (not shown). The low GTPCH activity, however, did not correlate with expression levels of GTPCH protein that is found constitutively expressed in the cells. The treatment with LPS or IFN/IL-1 did not stimulate GTPCH expression in adult cardiomyocytes. In contrast, the same treatments increased GTPCH protein expression levels in RAW267.4 (Fig. 5B). Therefore, low BH4 levels in adult cardiomyocytes can not be attributed to deficient expression of GTPCH protein but by the fact this protein is found mostly inactive.
Figure 5. Evaluation of GTP cyclohydrolase expression levels in adult cardiomyocytes and RAW264.7 cells upon stimulation with LPS and cytokines.
(A) Cardiomyocytes were stimulated or not (control) with: LPS (10 μg/ml); LPS (25 μg/ml); LPS (50 μg/ml); LPS (1 μg/ml) plus IFN-γ (20 ng/ml); LPS (1 μg/ml) plus cycloheximide (CHX, 2 μM); IFN-γ (20 ng/ml) plus IL-1α (0.125 ng/ml). Results are represented as mean values from two different cardiomyocytes preparations. (B) RAW264.7 cells were stimulated or not (control) with: LPS (1 μg/ml); LPS (1 μg/ml) plus IFN-γ (20 ng/ml); LPS (25 μg/ml); IFN-γ (20 ng/ml); IL-1α (0.125 ng/ml); IFN-γ (20 ng/ml) plus IL-1α (0.125 ng/ml). Results are representative of two independent experiments.
Supplementation of adult cardiomyocytes with BH4
The possibility of increasing cardiomyocyte BH4 content to stimulate iNOS activity was examined in cardiomyocytes pre-treated with BH4 (10 μM) and ascorbate (75 μM) 6 hr prior to stimulation with cytokines or LPS for 12 hr. The intracellular concentration of BH4, BH2 and ascorbate at the end of the incubation period was determined by HPLC with electrochemical detection (HPLC-EC). This analysis showed a significant but modest increase in BH4 compared to the marked accumulation of the oxidized metabolite, 7,8 dihydrobiopterin (BH2), in all of the treatments (Table 2). Interestingly, iNOS activity was not increased in cells treated with BH4 and ascorbate upon stimulation with LPS or cytokines (not shown). This result may be explained by the disproportional accumulation of BH2 which does not sustain NOS activity.
Table 2.
Electrochemical HPLC detection of BH4, BH2 and ascorbate in adult rat cardiomyocytes supplemented with ascorbate and BH4.
| Treatment | BH4 (pmol/mg protein) | BH2 (pmol/mg protein) | Ascorbate (pmol/mg protein) |
|---|---|---|---|
| Control | 0.066±0.058 | 0.0 | 14.55±0.65 |
| Asc/BH4 | 1.33±0.71a | 104.4±47.6 | 801.0±315.3a |
| Asc/BH4 + LPS | 0.297±0.092b | 172.66±54.3 | 501.9±58.5 |
| Asc/BH4 + TNF-α + IFN-γ | 0.609±0.138b | 196.0±21.8 | 814.0±124.2 |
Adult cardiomyocytes were pre-treated with ascorbate and BH4 for 6 hr before addition of LPS and cytokines for 12 hr. Conditions include: LPS (1μg/ml); TNF-α (20 ng/ml); IFN-γ (50 ng/ml); ascorbate (75 μM)/BH4 (10 μM). Values represent the mean±SD of at least 3 experiments.
P<0.01 vs control;
P<0.05 vs Asc/BH4.
These findings contradict other studies reporting increased BH4 levels and NO production in endothelial cells [24] and hearts [25] upon supplementation with the same BH4 concentrations used here. This difference is likely due to the significant accumulation of BH2 in adult cardiomyocytes. The exact reason for increased BH2 accumulation in isolated cardiomyocytes, however, is not clear. It has been hypothesized that oxidative stress may increase the formation of BH2 via stimulated oxidation of BH4. Since ascorbate protects cells from oxidative stress and stabilizes BH4 [26], its effect on cardiomyocytes was examined. As shown in Table 2, supplementation of cardiomyocytes with ascorbate for 6 hours increased intracellular ascorbate levels by >50-fold but did not decrease BH2 accumulation. Therefore the significant accumulation of BH2 in adult cardiomyocytes is unlikely due to a direct non-enzymatic oxidation of BH4.
BH4 deficiency and superoxide formation from iNOS expressing cardiomyocytes
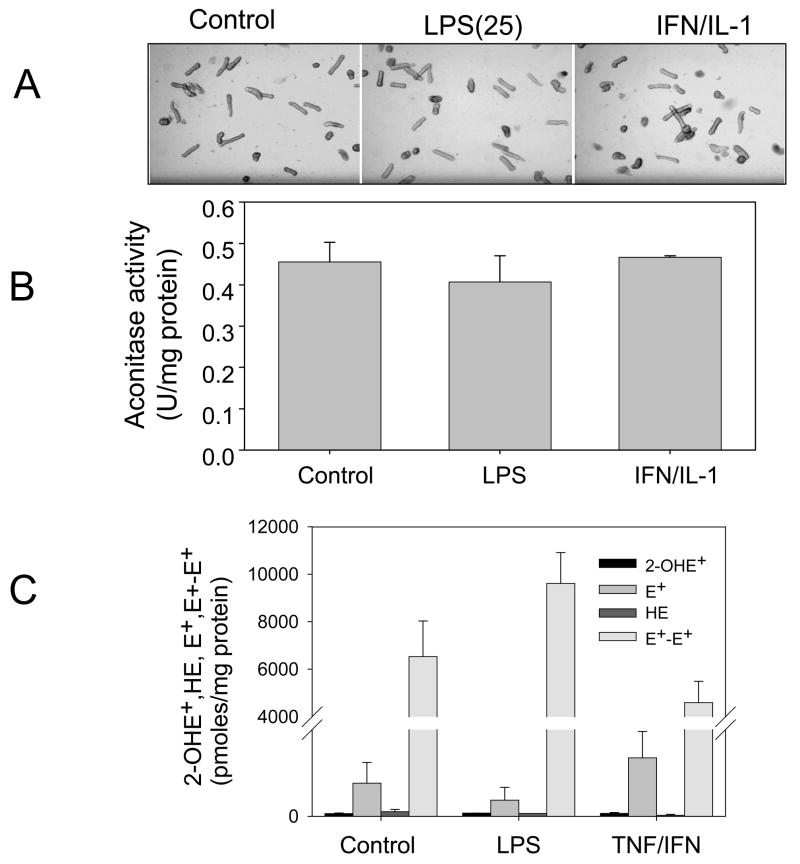
Increased expression of iNOS dissociated from BH4 upregulation could potentially promote uncoupled iNOS activity. This was examined by following mitochondrial aconitase which is recognized as a target for superoxide causing loss of aconitase activity [27]. Cardiomyocytes treated with LPS or IFN-γ (20 ng/ml) and IL-1α (0.125 ng/ml) did not significantly alter mitochondrial aconitase activity suggesting that iNOS is not uncoupled to form superoxide under these conditions (Fig. 6). It was also shown that these cells do not show changes in their normal rod-shape phenotype.
Figure 6. Superoxide detection by aconitase activity and 2-hydroxyethidium in stimulated adult cardiomyocytes.
Cells treated with LPS (1 μg/ml) or IFN-γ (20 ng/ml) plus IL-1α (0.125 ng/ml) for 12 h were analyzed for: (A) Rod-shape in contrast phase microscopy and (B) activity of mitochondrial aconitase. (C) Cells were treated with LPS (25 μg/ml) and TNF/IFN for 12 h and treated with HE for 20 min to test 2-hydroxyethidium and ethidium formation. Results are expressed as mean±SD (n=3).
A more specific assay for superoxide with hydroethidine (HE) has been developed that is based on the detection of 2-hydroxyethidium (2-OHE+), the specific product of the reaction of superoxide with HE [28]. The 2-OHE+ can be analyzed by HPLC-EC with improved sensitivity. Thus we analyzed the products of HE in cell lysates from cardiomyocytes treated as described in the previous experiments followed by incubation with HE. It was shown that cardiomyocytes fail to increase 2-OHE+ formation while HE was preferentially oxidized to ethidium (E+) and ethidium dimers (E+-E+) (Fig. 6C). We conclude that stimulation of cardiomyocytes with LPS and TNF/IFN is not conducive to stimulate superoxide production in the cells.
Discussion
It has been thought that increased expression of iNOS protein and NO production in cardiomyocytes initiates a cascade of events leading to myocyte dysfunction and/or injury. Here we show, however, that adult cardiomyocytes stimulated with different agonists (i.e. LPS or cytokines) have a defective capacity to generate NO despite a robust increase in iNOS expression. This response involves a deficient BH4 synthesis due to lack of GTPCH activity. These findings clearly indicate the necessity to reconsider the role of iNOS in adult cardiomyocytes in response to inflammatory mediators.
There are few studies on the relationship between BH4 synthetic enzymes and NO production in adult cardiomyocytes. Early studies reported that both iNOS and GTPCH mRNA were found in cardiomyocytes isolated from LPS-treated adult rats. Two fragments consisting of 1.1 and 3.5 Kb were identified for GTPCH mRNA but no information on GTPCH protein levels and enzyme activity was provided [29]. GTPCH is the first enzyme in the de novo biosynthetic pathway of BH4 and is thought to regulate the production of BH4. It has been shown that high GCH-gene expression follows enhanced enzyme activity and consequently increased BH4 production in several cell types including neonatal cardiomyocytes [30]. Inflammatory mediators such as LPS and cytokines are potent inducers of GTPCH, but this response is distinctively repressed in adult cardiomyocytes. These cells have been shown to express constitutive levels of GTPCH protein with low enzyme activity that is not stimulated by LPS [9]. These observations are further confirmed in this study by showing that adult cardiomyocytes stimulated with LPS and various combinations of cytokines did not increase GTPCH protein levels and enzyme activity. In contrast, RAW264.7 cells increased GTPCH expression several folds which also translated into a much higher activity than that detected in cardiomyocytes. The exact mechanisms limiting GTPCH activity in adult cardiomyocytes, however, are not currently known.
One possibility to explain the refractoriness of the BH4 synthetic pathway to LPS or cytokines treatment is that adult cardiomyocytes express a splice variant of GTPCH which causes loss of enzyme activity [31]. Alternative splicing of pre-mRNA transcripts have been implicated in the differential regulation of some protein levels in cardiomyocytes during differentiation. Since neonatal cardiomyocytes are able to increase biopterin production upon stimulation with LPS and cytokines, it is possible that expression of GTPCH variant forms to decrease BH4 levels is part of their maturation process. Recent reports indicate that GTPCH activity is extremely sensitive to variant expression causing significant loss of activity [31]. This possibility is consistent with the detection of constitutive GTPCH protein levels but low activity. Allosteric regulation is another mechanism important in the tuning of GTPCH activity. In hepatocytes this mechanism is essential to efficiently increase BH4 production upon phenylalanine load or terminate enzyme activation as BH4 levels increase several fold over basal levels. This allosteric regulation is mediated by the partner protein GTP cyclohydrolase feedback regulatory protein (GFRP) [32, 33]. Previous studies have identified GFRP mRNA in the heart [34] suggesting that a possible mechanism for the low levels of BH4 takes place via GFRP-mediated inhibition of GTPCH. This regulation, however, is mediated by the binding of either BH4 or BH2 [32]. We show that formation of BH4 and biopterin levels are very low which makes it more challenging to consider a role for GFRP in the allosteric inhibition of GTPCH activity in adult cardiomyocytes.
Concentrations of BH4 were measured by two independent HPLC methods. One using fluorescence detection of total biopterin (BH4+ BH2+ biopterin) after oxidation of the (BH4+BH2) to biopterin by KI/I2 reagent in acidified cell lysates. The other applies electrochemical detection to directly quantify BH4 and BH2 but not biopterin, which is not electrochemically active in the 0–450 mV serial coularray working range. In this case, cell lysates are prepared in acidic media and supplemented with DTPA as a metal chelator and DTE as a reducing agent to prevent the degradation of the cofactor. While these two methods agree in that cardiomyocytes present low biopterin and BH4 levels, the electrochemical method indicated that BH4 levels are negligible. Since recovery of BH4 in cell extracts was shown to be >98%, we hypothesize that the differences between the two methodologies may be consequent of biopterin accumulated in the cells. The most salient information, however, is that BH4 levels in adult cardiomyocytes are low in order of 0.01–1.5 pmoles/mg protein, which agrees well with the low levels of cofactor published in mouse-enriched myocytes preparation (0.4±0.3 pmol/mg protein) [25]. Also using the same electrochemical technique, we obtain values of ~2–3 pmoles/mg protein both in intact rat and mouse heart (unpublished results) which agrees favorably with the values reported previously for rat heart [35].
Increased iNOS monomers in adult cardiomyocytes can be explained by the limited production of BH4 that destabilizes iNOS quaternary protein resulting in lack of NO-forming activity. This behavior is analogous to iNOS expression in a NIH3T3 cell system which is deficient in BH4 [36]. Under these conditions, iNOS presents low enzyme activity simultaneous with increased monomer formation as shown by gel-filtration chromatography in conjunction with Western blot analysis. While it is recognized that BH4-deficient iNOS monomers lack the capacity to increase NO formation, it is not known if they retain the capability of reducing oxygen to generate superoxide anion (O2•−) from iNOS expressed in activated macrophages has been radical. Release of O2•− from iNOS expressed in activated macrophages has been linked to limiting L-arginine availability [37] but the specific role of BH4 was not addressed in these studies. The critical role of BH4 in the regulation of oxygen activation by NOS has been clearly established [38,39]. Thus, we anticipated that adult cardiomyocytes represent an important scenario that could favor the uncoupling of iNOS. Using the activity of aconitase as an endogenous reporter for O2•− production from iNOS, we did not find evidence to support uncoupled iNOS activity in any of the treatments. It is possible to argue that aconitase inactivation may be less sensitive to O2•− from extra-mitochondrial origin (i.e. iNOS) which may not be as efficient as intra- mitochondrial O2•− at inhibiting aconitase [40]. Thus we applied the cell permeable probe hydroethidine for a more specific detection of superoxide. The reaction of superoxide with HE generates 2-OHE+ that can be quantified by HPLC with electrochemical detection [12]. We found that stimulated cardiomyocytes did not increase 2-OHE+ formation. A significant increase in ethidium (E+) and HE-dimeric products (HE-E+ and E+-E+) however were detected. This result was attributed to the reaction of HE with heme proteins including cytochrome c, which has been shown to catalyze the conversion of HE to other oxidation products in vitro [41,42]. The preferential oxidation of HE to E+ may be a concern for the lack of 2-OHE+ formation in cardiomyocytes, although a small free unreacted amount of HE was found in the cells. Additionally, the detection of HE-dimeric products indicates that the intermediate HE radical is generated. Since the reaction of superoxide with HE radical precedes the formation of 2-OHE+, we conclude that HE is likely available for reaction with superoxide. The pattern of HE reactivity in cardiomyocytes, however, is intriguing as it may reflect both distribution of the probe in the different cell compartments and also relative abundance of oxidant proteins. A more comprehensive study on the reactions of HE in cardiomyocytes is underway. In conclusion, although aconitase inactivation does not categorically exclude the possibility that low levels of O2•− could be generated from cardiomyocytes overexpressing iNOS, this data in combination with lack of 2-OHE+ indicates that this can at least be considered a minor process.
In summary, our study shows that adult cardiomyocytes do not increase NO production despite the indisputable increase in iNOS protein levels via either cytokine or LPS stimulation. We have found that this response correlates with defective increases in BH4 cofactor production. Therefore, adult cardiomyocytes are an unlikely source of high NO production in conditions associated with inflammatory stimuli in the adult heart. In contrast, previous studies have shown that neonatal cardiomyocytes exhibit a phenotype commonly seen in inflammatory cells manifested by significant increases in iNOS expression coupled with increases in both BH4 and NO production. Such a remarkable difference between neonatal and adult cardiomyocytes needs to be further explored in regard to our current understanding of timing and sources of NO in the whole heart in vivo. This difference suggests that inflammatory, neonatal and perhaps stem cells might be better targets than resident adult cardiomyocytes to various inflammatory signals that occur in several cardiac disease states including myocardial infarction, myocarditis, cardiac transplant rejection and septic shock.
Acknowledgments
Funding: This work was supported by National Institutes of Health; National Heart Blood and Lung Institute [HL067244 to JVV and HL078937 to GMP]
References
- 1.Cannon PJ, Yang X, Szabolcs MJ, Ravalli S, Sciacca RR, Michler RE. The role of inducible nitric oxide synthase in cardiac allograft rejection. Cardiovasc Res. 1998;38:6–15. doi: 10.1016/s0008-6363(98)00022-4. [DOI] [PubMed] [Google Scholar]
- 2.Szabolcs MJ, Ravalli S, Minanov O, Sciacca RR, Michler RE, Cannon PJ. Apoptosis and increased expression of inducible nitric oxide synthase in human allograft rejection. Transplantation. 1998;65:804–812. doi: 10.1097/00007890-199803270-00007. [DOI] [PubMed] [Google Scholar]
- 3.Terblanche M, Almog Y, Rosenson RS, Smith TS, Hackam DG. Statins and sepsis: multiple modifications at multiple levels. Lancet Infect Dis. 2007;7:358–368. doi: 10.1016/S1473-3099(07)70111-1. [DOI] [PubMed] [Google Scholar]
- 4.Luss HL, Watkins SC, Freeswick PD, Imro AK, Nussler AK, Billiar TR, Simmons RL, del Nido PJ, McGowan FX., Jr Characterization of inducible nitric oxide synthase expression in endotoxemic rat cardiomyocyte in vivo following cytokine exposure in vitro. J Molec Cell Cardiol. 1995;27:2015–2029. doi: 10.1016/0022-2828(95)90023-3. [DOI] [PubMed] [Google Scholar]
- 5.Lewis NP, Tsao PS, Rickenbacher PR, Xue C, Johns RA, Haywood GA, von der Leyen H, Trindade PT, Cooke JP, Hunt SA, Billingham ME, Valantine HA, Fowler MB. Induction of nitric oxide synthase in the human cardiac allograft is associated with contractile dysfunction of the left ventricle. Circulation. 1996;93:720–729. doi: 10.1161/01.cir.93.4.720. [DOI] [PubMed] [Google Scholar]
- 6.Razavi HM, Hamilton JA, Feng Q. Modulation of apoptosis by nitric oxide: implications in myocardial ischemia and heart failure. Pharmacol Ther. 2005;106:147–162. doi: 10.1016/j.pharmthera.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Albakri QA, Stuehr DJ. Intracellular assembly of inducible NO synthase is limited by nitric oxide-mediated changes in heme insertion and availability. J Biol Chem. 1996;271:5414–5421. doi: 10.1074/jbc.271.10.5414. [DOI] [PubMed] [Google Scholar]
- 8.Baek KJ, Thiel BA, Lucas S, Stuehr DJ. Macrophage nitric oxide synthase subunits. Purification, characterization and role of prosthetic groups and substrate in regulating their association into a dimeric enzyme. J Biol Chem. 1993;268:21120–21129. [PubMed] [Google Scholar]
- 9.Kalivendi S, Hatakeyama K, Whitsett J, Konorev E, Kalyanaraman B, Vásquez-Vivar J. Changes in tetrahydrobiopterin levels in endothelial cells and adult cardiomyocytes induced by LPS and hydrogen peroxide--a role for GFRP? Free Radic Biol Med. 2005;38:481–491. doi: 10.1016/j.freeradbiomed.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Schimke I, Schikora M, Meyer R, Dubel HP, Modersohn D, Kleber FX, Baumann G. Oxidative stress in the human heart is associated with changes in the antioxidative defense as shown after heart transplantation. Mol Cell Biochem. 2000;204:89–96. doi: 10.1023/a:1007030322514. [DOI] [PubMed] [Google Scholar]
- 11.Akizuki E, Akaike T, Okamoto S, Fujii S, Yamaguchi Y, Ogawa M, Maeda H. Role of nitric oxide and superoxide in acute cardiac allograft rejection in rats. Proc Soc Exp Biol Med. 2000;225:151–159. doi: 10.1046/j.1525-1373.2000.22519.x. [DOI] [PubMed] [Google Scholar]
- 12.Whitsett J, Picklo MJ, Sr, Vasquez-Vivar J. 4-Hydroxy-2-nonenal increases superoxide anion radical in endothelial cells via stimulated GTP cyclohydrolase proteasomal degradation. Arterioscler Thromb Vasc Biol. 2007;27:2340–2347. doi: 10.1161/ATVBAHA.107.153742. [DOI] [PubMed] [Google Scholar]
- 13.Zielonka J, Vasquez-Vivar J, Kalyanaraman B. Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat Protoc. 2008;3:8–21. doi: 10.1038/nprot.2007.473. [DOI] [PubMed] [Google Scholar]
- 14.Kaur K, Sharma AK, Dhingra S, Singal PK. Interplay of TNF-α and IL-10 in regulating oxidative stress in isolated cardiac myocytes. J Mol Cell Cardiol. 2006;41:1023–1030. doi: 10.1016/j.yjmcc.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Tatsumi T, Matoba S, Kawahara A, Keira N, Shiraishi J, Akashi K, Kobara M, Tanaka T, Katamura M, Nakagawa C, Ohta B, Shirayama T, Takeda K, Asayama J, Fliss H, Nakagawa M. Cytokine-induced nitric oxide production inhibits mitochondrial energy production and impairs contractile function in rat cardiac myocytes. J Am Coll Cardiol. 2000;35:1338–1346. doi: 10.1016/s0735-1097(00)00526-x. [DOI] [PubMed] [Google Scholar]
- 16.Balligand J-L, Ungureanu D, Kelly RA, Kobzik L, Pimental D, Michel T, Smith TW. Abnormal contractile function due to induction of nitric oxide synthesis in rat cardiac myocytes follows exposure to activated macrophage-conditioned medium. J Clin Invest. 1993;91:2314–2319. doi: 10.1172/JCI116461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinsky DJ, Aji W, Szabolcs M, Athan ES, Liu Y, Yang YM, Kline RP, Olson KE, Cannon PJ. Nitric oxide triggers programmed cell death (apoptosis) of adult rat ventricular myocytes in culture. Am J Physiol Heart Circ Physiol. 1999;277:H1189–H1199. doi: 10.1152/ajpheart.1999.277.3.H1189. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Son NH, Szabolcs MJ, Ma N, Sciacca RR, Albala A, Edwards N, Cannon PJ. Effects of inhibition of poly(adenosine) diphosphate-ribose) synthase on acute cardiac allograft rejection. Transplantation. 2004;78:668–674. doi: 10.1097/01.tp.0000131662.01491.2e. [DOI] [PubMed] [Google Scholar]
- 19.Luss H, Li RK, Shapiro RA, Tzeng E, McGowan FX, Yoneyama T, Hatakeyama K, Geller DA, Mickle DA, Simmons RL, Billiar TR. Dedifferentiated human ventricular cardiac myocytes express inducible nitric oxide synthase mRNA but not protein in response to IL-1, TNF, IFN-γ, and LPS. J Mol Cell Cardiol. 1997;29:1153–1165. doi: 10.1006/jmcc.1996.0349. [DOI] [PubMed] [Google Scholar]
- 20.Zhu JX, Liu MY, Kennedy RH, Liu SJ. TNF-α-induced impairment of mitochondrial integrity and apoptosis mediated by caspase-8 in adult ventricular myocytes. Cytokine. 2006;34:96–105. doi: 10.1016/j.cyto.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Ing DJ, Zang J, Dzau VJ, Webster KA, Bishopric AH. Modulation of cytokine-induced cardiac myocyte apoptosis by nitric oxide, Bak and Bcl-x. Circ Res. 1999;84:21–33. doi: 10.1161/01.res.84.1.21. [DOI] [PubMed] [Google Scholar]
- 22.Kan HK, Xie Z, Finkel MS. TNF-α enhances cardiac myocyte NO production through MAP kinase-mediated NF-κB activation. Am J Physiol Heart Circ Physiol. 1999;46:H1641–H1646. doi: 10.1152/ajpheart.1999.277.4.H1641. [DOI] [PubMed] [Google Scholar]
- 23.Roman LJ, Martasek P, Masters BS. Intrinsic and extrinsic modulation of nitric oxide synthase activity. Chem Rev. 2002;102:1179–1190. doi: 10.1021/cr000661e. [DOI] [PubMed] [Google Scholar]
- 24.Bevers LM, Braam B, Post JA, van Zonneveld AJ, Rabelink TJ, Koomans HA, Verhaar MC, Joles JA. Tetrahydrobiopterin, but not L-arginine, decreases NO synthase uncoupling in cells expressing high levels of endothelial NO synthase. Hypertension. 2006;47:87–94. doi: 10.1161/01.HYP.0000196735.85398.0e. [DOI] [PubMed] [Google Scholar]
- 25.Dumitrescu C, Biondi R, Xia Y, Cardounel AJ, Druhan LJ, Ambrosio G, Zweier JL. Myocardial ischemia results in tetrahydrobiopterin (BH4) oxidation with impaired endothelial function ameliorated by BH4. Proc Natl Acad Sci U S A. 2007;104:15081–15086. doi: 10.1073/pnas.0702986104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heller R, Unbehaun A, Schellenberg B, Mayer B, Werner-Felmayer G, Werner ER. L-ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J Biol Chem. 2001;276:40–47. doi: 10.1074/jbc.M004392200. [DOI] [PubMed] [Google Scholar]
- 27.Beinert H, Kennedy MC, Stout CD. Aconitase as iron-sulfur protein, enzyme, and iron-regulatory protein. Chem Rev. 1996;96:2335–2374. doi: 10.1021/cr950040z. [DOI] [PubMed] [Google Scholar]
- 28.Zhao H, Joseph J, Fales HM, Sokoloski EA, Levine RL, Vasquez-Vivar J, Kalyanaraman B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc Natl Acad Sci USA. 2005;102:5727–5732. doi: 10.1073/pnas.0501719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balligand J-L, Ungureanu-Langrois D, Simmons WW, Pimental D, Malinski TA, Kapturczak M, Taha Z, Lowesnstein CJ, Davidoff AJ, Kelly RA, Smith TW, Michel T. Cytokine-inducible nitric oxide synthase (iNOS) expression in cardiac myocytes. J Biol Chem. 1994;269:27580–27588. [PubMed] [Google Scholar]
- 30.Kasai K, Hattori Y, Banba N, Hattori S, Motohashi S, Shimoda S-I, Kasai K, Shimoda S, Nakanishi N, Gross SS. Induction of tetrahydrobiopterin synthesis in rat cardiac myocytes on cytokine-induced NO generation. Am J Physiol Heart Circ Physiol. 1997;273:H665–672. doi: 10.1152/ajpheart.1997.273.2.H665. [DOI] [PubMed] [Google Scholar]
- 31.Pandya MJ, Golderer G, Werner ER, Werner-Felmayer G. Interaction of human GTP cyclohydrolase I with its splice variants. Biochem J. 2006;400:75–80. doi: 10.1042/BJ20060765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maita N, Hatakeyama K, Okada K, Hakoshima T. Structural basis of biopterin-induced inhibition of GTP cyclohydrolase I by GFRP, its feedback regulatory protein. J Biol Chem. 2004;279:51534–51540. doi: 10.1074/jbc.M409440200. [DOI] [PubMed] [Google Scholar]
- 33.Yoneyama T, Wislon LM, Hatakeyama K. GTP cyclohydrolase I feedback regulatory protein-dependent and independent inhibitors of GTP Cyclohydrolase I. Arch Biochem Biophys. 2001;388:67–73. doi: 10.1006/abbi.2001.2288. [DOI] [PubMed] [Google Scholar]
- 34.Gesierich A, Niroomand F, Tiefenbacher CP. Role of human GTP cyclohydrolase I and its regulatory protein in tetrahydrobiopterin metabolism. Basic Res Cardiol. 2003;98:69–75. doi: 10.1007/s00395-003-0394-y. [DOI] [PubMed] [Google Scholar]
- 35.Alp NJ, Mussa S, Khoo J, Cai S, Guzik T, Jefferson A, Goh N, Rockett KA, Channon KM. Tetrahydrobiopterin-dependent preservation of nitric oxide-mediated endothelial function in diabetess by targeted GTP-cyclohydrolase I overexpression. J Clin Invest. 112:725–735. doi: 10.1172/JCI17786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tzeng E, Billiar TR, Robbins PD, Loftus M, Stuehr DJ. Expression of human inducible nitric oxide synthase in a tetrahydrobiopterin (H4-B)-deficient cell line: H4B promotes assembly of enzyme subunits into an active dimer. Proc Natl Acad Sci USA. 1995;92:11771–11775. doi: 10.1073/pnas.92.25.11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia Y, Zweier JL. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proc Natl Acad Sci USA. 1997;94:6954–6958. doi: 10.1073/pnas.94.13.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vásquez-Vivar J, Kalyanaraman B, Martásek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA., Jr Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci USA. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vásquez-Vivar J, Hogg N, Martásek P, Karoui H, Pritchard KA, Jr, Kalyanaraman B. Tetrahydrobiopterin-dependent inhibition of superoxide generation from neuronal nitric oxide synthase. J Biol Chem. 1999;274:26736–26742. doi: 10.1074/jbc.274.38.26736. [DOI] [PubMed] [Google Scholar]
- 40.Missirlis F, Hu J, Kirby K, Hilliker AJ, Rouault TA, Phillips JP. Compartment-specific protection of iron-sulfur proteins by superoxide dismutase. J Biol Chem. 2003;278:47365–47369. doi: 10.1074/jbc.M307700200. [DOI] [PubMed] [Google Scholar]
- 41.Zielonka J, Srinivasan S, Hardy M, Ouari O, Lopez M, Vasquez-Vivar J, Avadhani NG, Kalyanaraman B. Cytochrome c-mediated oxidation of hydroethidine and mito-hydroethidine in mitochondria: identification of homo- and heterodimers. Free Radic Biol Med. 2008;44:835–846. doi: 10.1016/j.freeradbiomed.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papapostolou I, Patsoukis N, Georgiou CD. The fluorescence detection of superoxide radical using hydroethidine could be complicated by the presence of heme proteins. Anal Biochem. 2004;332:290–298. doi: 10.1016/j.ab.2004.06.022. [DOI] [PubMed] [Google Scholar]