Abstract
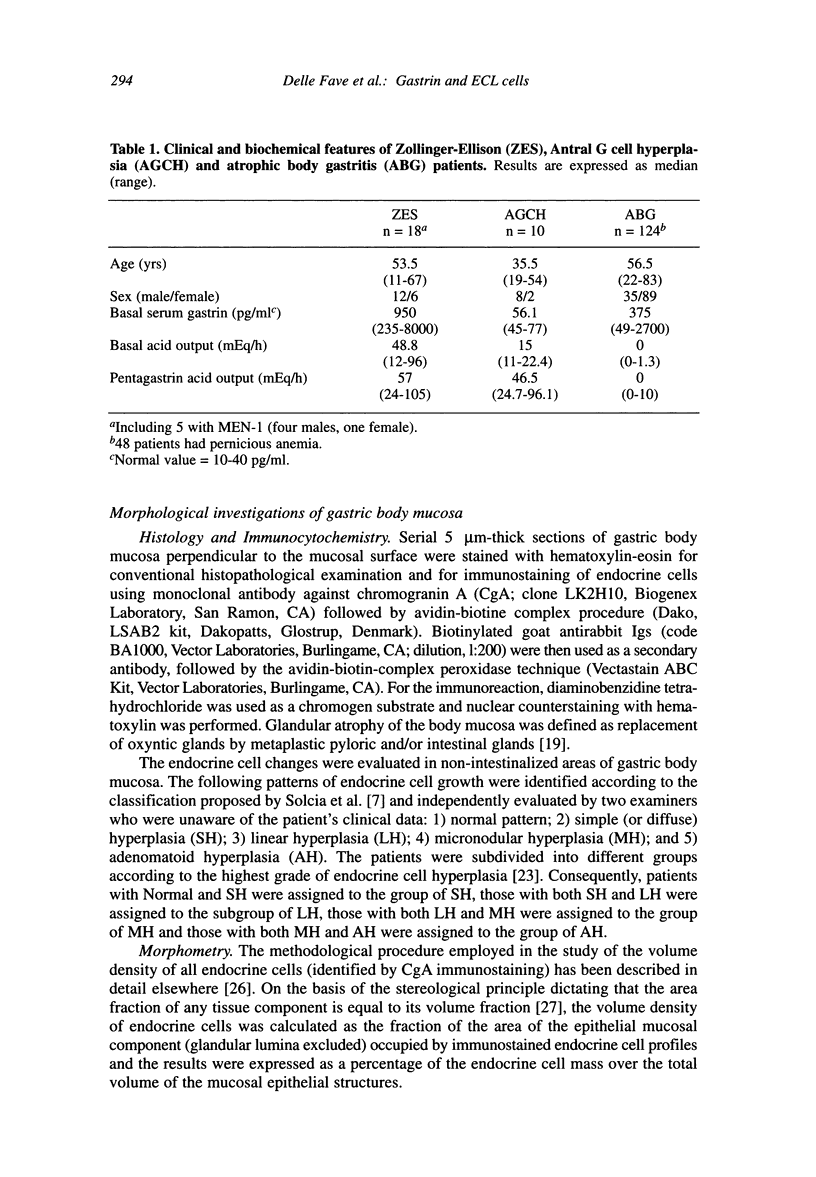
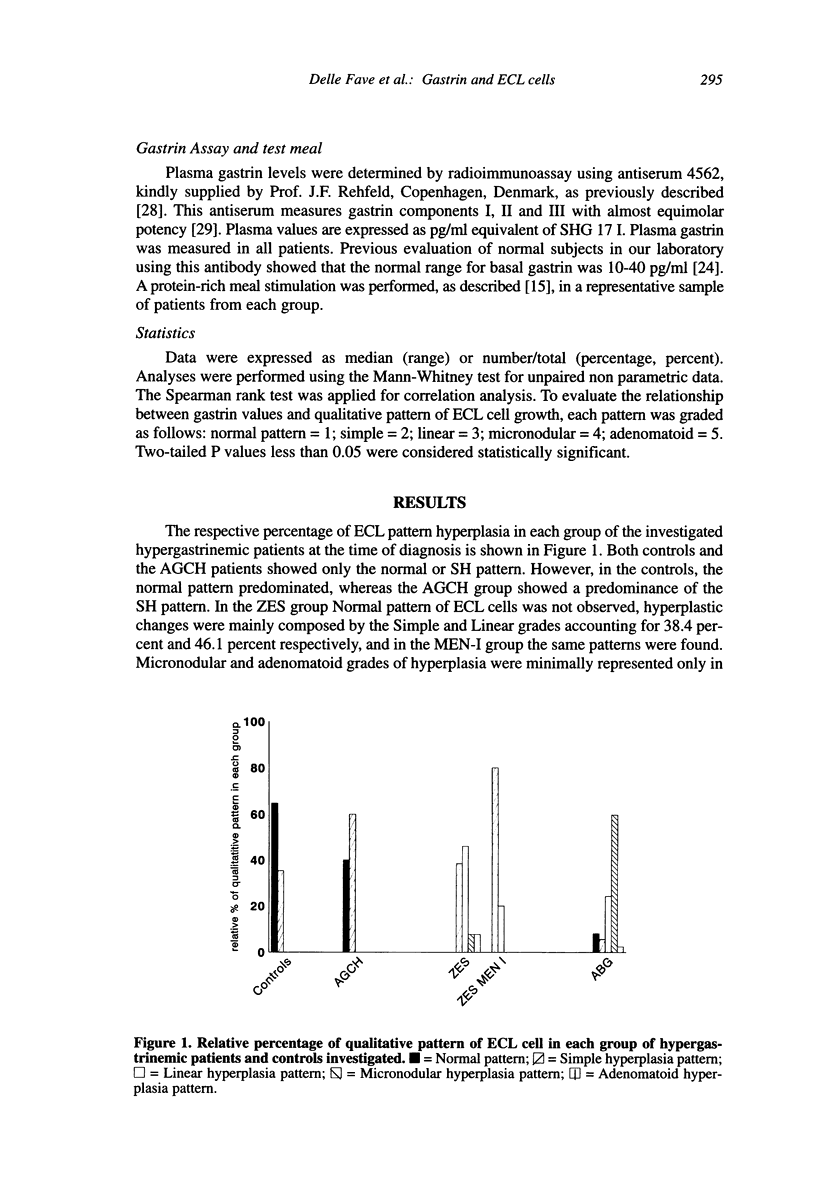
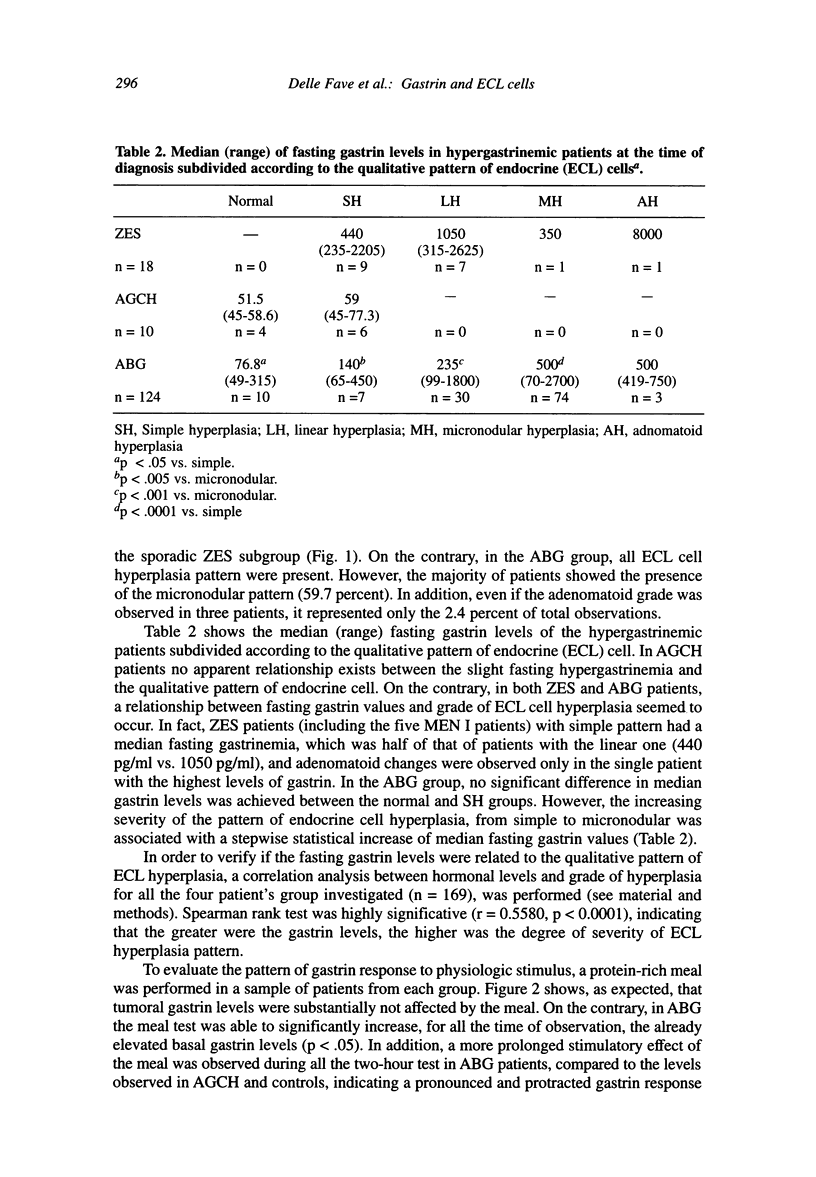
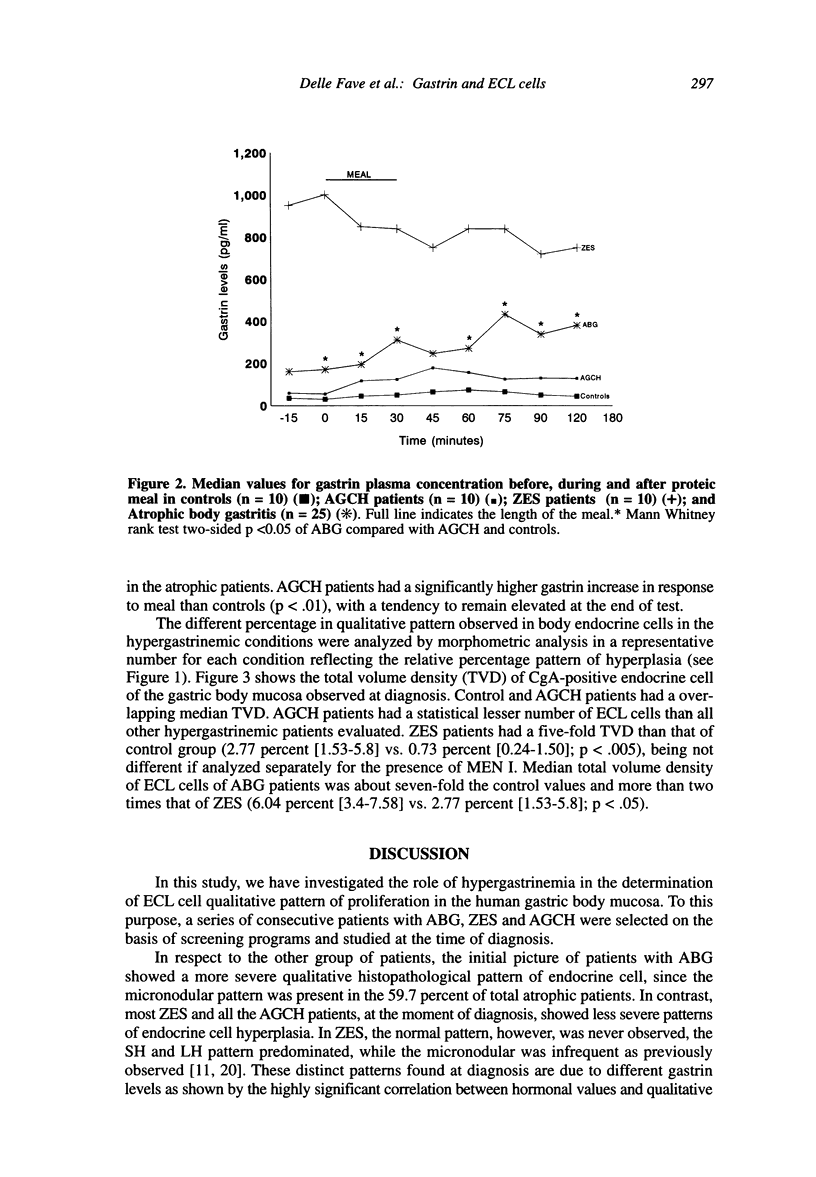
The enterochromaffin-like (ECL) cells, the most frequent endocrine cells of the oxyntic mucosa of the stomach, are under the trophic stimulus of gastrin. These cells undergo a hyperplastic increase in variety of hypergastrinemic diseases. The most widely accepted nomenclature for the description of hyperplastic proliferation has been retrospectively arranged in a sequence presumed to reflect a temporal evolution of the proliferative process. A comparative, prospective study aimed to verify, in human hypergastrinemic diseases such as atrophic body gastritis (ABG), Zollinger-Ellison syndrome (ZES) and antral gastrin cell hyperfunction (AGCH), the effect of exposure of ECL cells to different pattern of gastrin hypersecretion, is lacking. To this purpose, we studied a series of consecutive patients with ABG, ZES and AGCH at the time of first diagnosis. Material and Methods: The patients included in this study (124 ABG, 18 ZES and 10 AGCH) were selected on the basis of two previously performed screening studies aimed to diagnose these diseases. All patients at the time of diagnosis underwent gastroscopy, with multiple biopsies of the gastric body mucosa for the evaluation of qualitative pattern of ECL cells hyperplasia, and basal fasting gastrin determination. A sample of hypergastrinemic patients from each group was further investigated by meal-stimulation of gastrin secretion and quantitative morphometry for CgA positive gastric body endocrine cells. Results: AGCH patients showed only the normal or simple hyperplasia pattern. In the ZES group, simple and linear grades accounted for 38.4 percent and 46.1 percent, respectively. MEN-I patients showed only these two patterns. The majority of ABG patients showed the presence of micronodular pattern (59.7 percent). A correlation analysis between fasting gastrin levels and grade of hyperplasia (r = 0.5580, p < 0.0001), indicates that the greater the gastrin levels, the higher is the degree of severity of ECL hyperplasia pattern. In conclusion, our data support the role of gastrin as the selective contributor to the progression of ECL cell hyperplasia in humans.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Annibale B., Bonamico M., Rindi G., Villani L., Ferrante E., Vania A., Solcia E., Delle Fave G. Antral gastrin cell hyperfunction in children. A functional and immunocytochemical report. Gastroenterology. 1991 Dec;101(6):1547–1551. doi: 10.1016/0016-5085(91)90390-7. [DOI] [PubMed] [Google Scholar]
- Annibale B., De Magistris L., Corleto V., D'Ambra G., Marignani M., Iannoni C., Delle Fave G. Zollinger-Ellison syndrome and antral G-cell hyperfunction in patients with resistant duodenal ulcer disease. Aliment Pharmacol Ther. 1994 Feb;8(1):87–93. doi: 10.1111/j.1365-2036.1994.tb00164.x. [DOI] [PubMed] [Google Scholar]
- Annibale B., Marignani M., Azzoni C., D'Ambra G., Caruana P., D'Adda T., Delle Fave G., Bordi C. Atrophic body gastritis: distinct features associated with Helicobacter pylori infection. Helicobacter. 1997 Jun;2(2):57–64. doi: 10.1111/j.1523-5378.1997.tb00060.x. [DOI] [PubMed] [Google Scholar]
- Annibale B., Rindi G., D'Ambra G., Marignani M., Solcia E., Bordi C., Delle Fave G. Antral gastrin cell hyperfunction and Helicobacter pylori infection. Aliment Pharmacol Ther. 1996 Aug;10(4):607–615. doi: 10.1046/j.1365-2036.1996.31173000.x. [DOI] [PubMed] [Google Scholar]
- Borch K., Renvall H., Liedberg G., Andersen B. N. Relations between circulating gastrin and endocrine cell proliferation in the atrophic gastric fundic mucosa. Scand J Gastroenterol. 1986 Apr;21(3):357–363. doi: 10.3109/00365528609003087. [DOI] [PubMed] [Google Scholar]
- Borch K., Renvall H., Liedberg G. Gastric endocrine cell hyperplasia and carcinoid tumors in pernicious anemia. Gastroenterology. 1985 Mar;88(3):638–648. doi: 10.1016/0016-5085(85)90131-3. [DOI] [PubMed] [Google Scholar]
- Bordi C., Azzoni C., Pilato F. P., Robutti F., D'Ambra G., Caruana P., Rindi G., Corleto V. D., Annibale B., Delle Fave G. Morphometry of gastric endocrine cells in hypergastrinemic patients treated with the somatostatin analogue octreotide. Regul Pept. 1993 Sep 22;47(3):307–318. doi: 10.1016/0167-0115(93)90397-q. [DOI] [PubMed] [Google Scholar]
- Bordi C., Costa A., Missale G. Letter: ECL cell proliferation and gastrin levels. Gastroenterology. 1975 Jan;68(1):205–206. [PubMed] [Google Scholar]
- Brenna E., Waldum H. L. Trophic effect of gastrin on the enterochromaffin like cells of the rat stomach: establishment of a dose response relationship. Gut. 1992 Oct;33(10):1303–1306. doi: 10.1136/gut.33.10.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadiot G., Lehy T., Ruszniewski P., Bonfils S., Mignon M. Gastric endocrine cell evolution in patients with Zollinger-Ellison syndrome. Influence of gastrinoma growth and long-term omeprazole treatment. Dig Dis Sci. 1993 Jul;38(7):1307–1317. doi: 10.1007/BF01296083. [DOI] [PubMed] [Google Scholar]
- Correa P., Yardley J. H. Grading and classification of chronic gastritis: one American response to the Sydney system. Gastroenterology. 1992 Jan;102(1):355–359. doi: 10.1016/0016-5085(92)91820-t. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt W., Lamberts R. Inter-relationship between serum gastrin levels, gastric mucosal histology and gastric endocrine cell growth. Digestion. 1992;51 (Suppl 1):76–81. doi: 10.1159/000200920. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt W. The achlorhydria-carcinoid sequence: role of gastrin. Digestion. 1988;39(2):61–79. doi: 10.1159/000199609. [DOI] [PubMed] [Google Scholar]
- D'Adda T., Annibale B., Delle Fave G., Bordi C. Oxyntic endocrine cells of hypergastrinaemic patients. Differential response to antrectomy or octreotide. Gut. 1996 May;38(5):668–674. doi: 10.1136/gut.38.5.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delle Fave G., Annibale B., Puoti M., Giordano E., Corleto V., de Magistris L., Torsoli A. Medical treatment of antral gastrin cell hyperfunction: role of nonantisecretory therapy. Digestion. 1990;46(2):65–71. doi: 10.1159/000200334. [DOI] [PubMed] [Google Scholar]
- Delle Fave G., Kohn A., De Magistris L., Annibale B., Bruzzone R., Sparvoli C., Severi C., Torsoli A. Effects of bombesin on gastrin and gastric acid secretion in patients with duodenal ulcer. Gut. 1983 Mar;24(3):231–235. doi: 10.1136/gut.24.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro G., Annibale B., Marignani M., Azzoni C., D'Adda T., D'Ambra G., Bordi C., delle Fave G. Effectiveness of octreotide in controlling fasting hypergastrinemia and related enterochromaffin-like cell growth. J Clin Endocrinol Metab. 1996 Feb;81(2):677–683. doi: 10.1210/jcem.81.2.8636288. [DOI] [PubMed] [Google Scholar]
- Lamberts R., Creutzfeldt W., Strüber H. G., Brunner G., Solcia E. Long-term omeprazole therapy in peptic ulcer disease: gastrin, endocrine cell growth, and gastritis. Gastroenterology. 1993 May;104(5):1356–1370. doi: 10.1016/0016-5085(93)90344-c. [DOI] [PubMed] [Google Scholar]
- Loud A. V., Anversa P. Morphometric analysis of biologic processes. Lab Invest. 1984 Mar;50(3):250–261. [PubMed] [Google Scholar]
- Maton P. N., Lack E. E., Collen M. J., Cornelius M. J., David E., Gardner J. D., Jensen R. T. The effect of Zollinger-Ellison syndrome and omeprazole therapy on gastric oxyntic endocrine cells. Gastroenterology. 1990 Oct;99(4):943–950. doi: 10.1016/0016-5085(90)90611-4. [DOI] [PubMed] [Google Scholar]
- Modlin I. M., Tang L. H. The gastric enterochromaffin-like cell: an enigmatic cellular link. Gastroenterology. 1996 Sep;111(3):783–810. doi: 10.1053/gast.1996.v111.agast961110783. [DOI] [PubMed] [Google Scholar]
- Ryberg B., Tielemans Y., Axelson J., Carlsson E., Håkanson R., Mattson H., Sundler F., Willems G. Gastrin stimulates the self-replication rate of enterochromaffinlike cells in the rat stomach. Effects of omeprazole, ranitidine, and gastrin-17 in intact and antrectomized rats. Gastroenterology. 1990 Oct;99(4):935–942. doi: 10.1016/0016-5085(90)90610-d. [DOI] [PubMed] [Google Scholar]
- Solcia E., Bordi C., Creutzfeldt W., Dayal Y., Dayan A. D., Falkmer S., Grimelius L., Havu N. Histopathological classification of nonantral gastric endocrine growths in man. Digestion. 1988;41(4):185–200. doi: 10.1159/000199786. [DOI] [PubMed] [Google Scholar]
- Solcia E., Fiocca R., Villani L., Luinetti O., Capella C. Hyperplastic, dysplastic, and neoplastic enterochromaffin-like-cell proliferations of the gastric mucosa. Classification and histogenesis. Am J Surg Pathol. 1995;19 (Suppl 1):S1–S7. [PubMed] [Google Scholar]
- Strickland R. G., Mackay I. R. A reappraisal of the nature and significance of chronic atrophic gastritis. Am J Dig Dis. 1973 May;18(5):426–440. doi: 10.1007/BF01071995. [DOI] [PubMed] [Google Scholar]
- Wolfe M. M., Jensen R. T. Zollinger-Ellison syndrome. Current concepts in diagnosis and management. N Engl J Med. 1987 Nov 5;317(19):1200–1209. doi: 10.1056/NEJM198711053171907. [DOI] [PubMed] [Google Scholar]
- de Magistris L., Rehfeld J. F. A simple enzymatic procedure for radioimmunochemical quantitation of the large molecular forms of gastrin and cholecystokinin. Anal Biochem. 1980 Feb;102(1):126–133. doi: 10.1016/0003-2697(80)90327-9. [DOI] [PubMed] [Google Scholar]


