Abstract
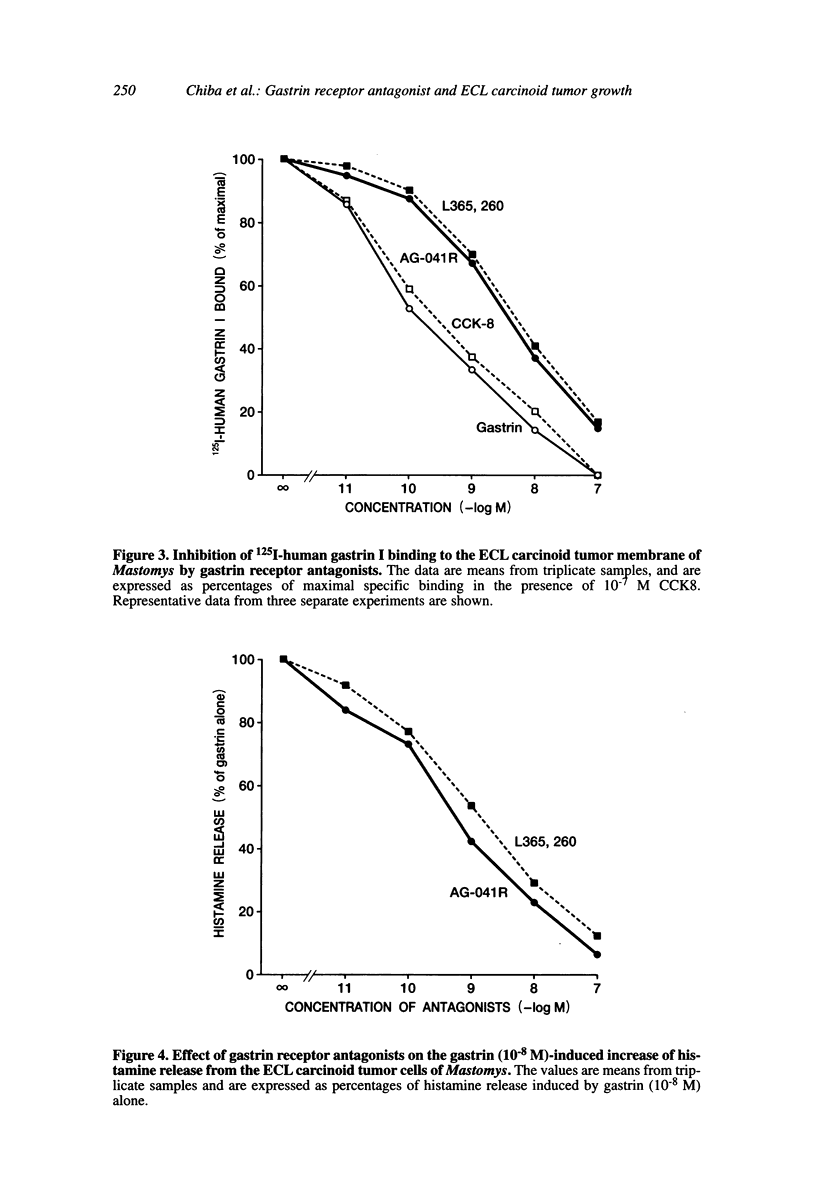
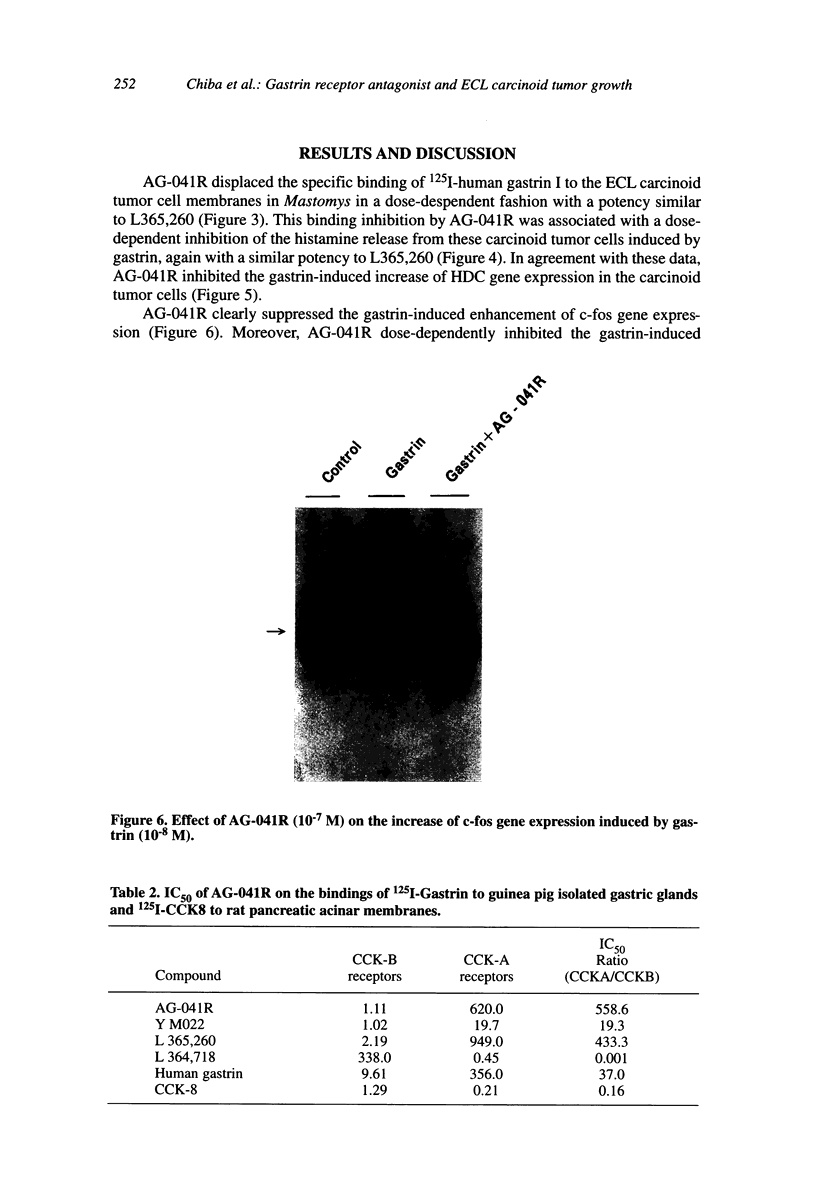
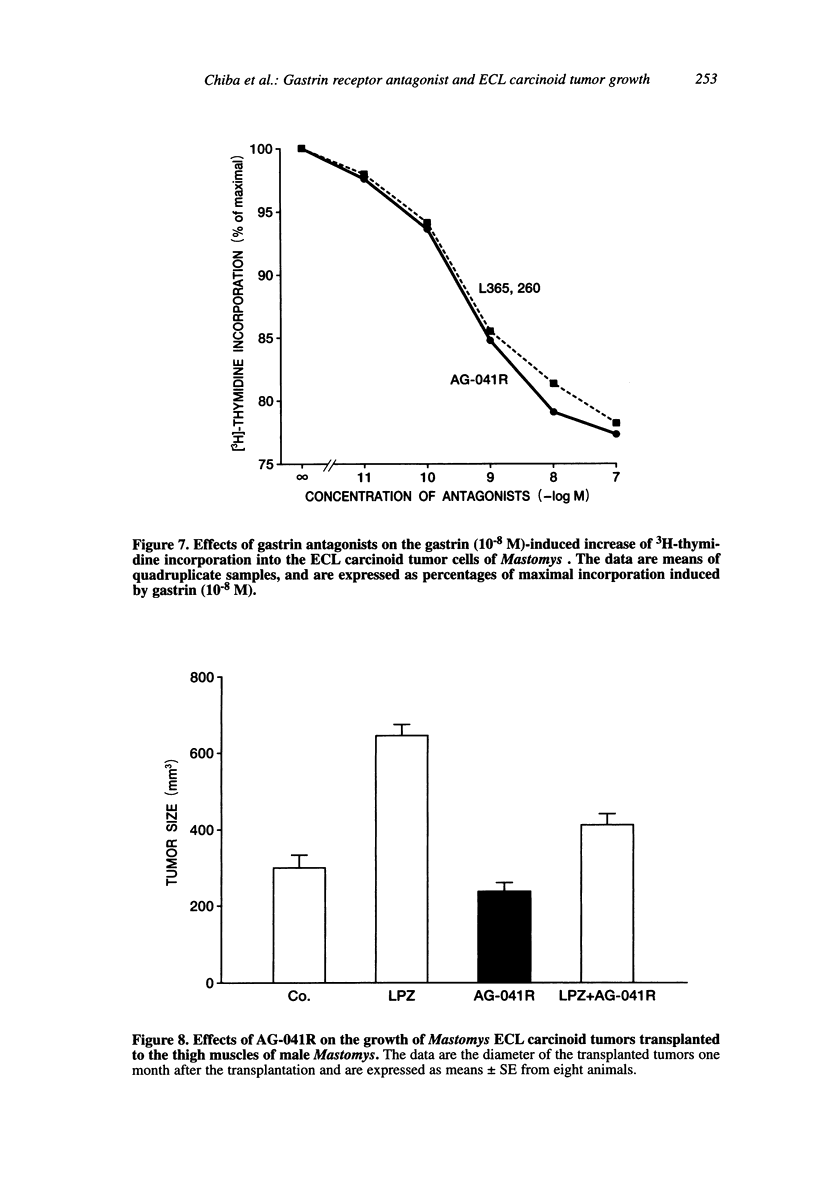
We examined the effects of a newly synthesized gastrin receptor antagonist, AG-041R, on the growth of enterochromaffin-like (ECL) carcinoid tumors in Mastomys natalensis both in vitro and in vivo. AG-041R was as potent as the well known gastrin antagonist L365,260 in inhibiting not only the gastrin-induced release of histamine from but also histidine decarboxylase (HDC) gene expression in the ECL carcinoid tumor cells. AG-041R also inhibited gastrin-induced DNA synthesis and c-fos gene expression in the tumor cells. Furthermore, AG-041R significantly inhibited the growth of the transplanted Mastomys ECL carcinoid tumors in vivo. From these data, it is concluded that endogenous gastrin is involved in the growth of ECL carcinoid tumors in Mastomys natalensis. Moreover, AG-041R is shown to have a potential as an anti-neoplastic agent for ECL carcinoid tumor of the stomach.
Full text
PDF
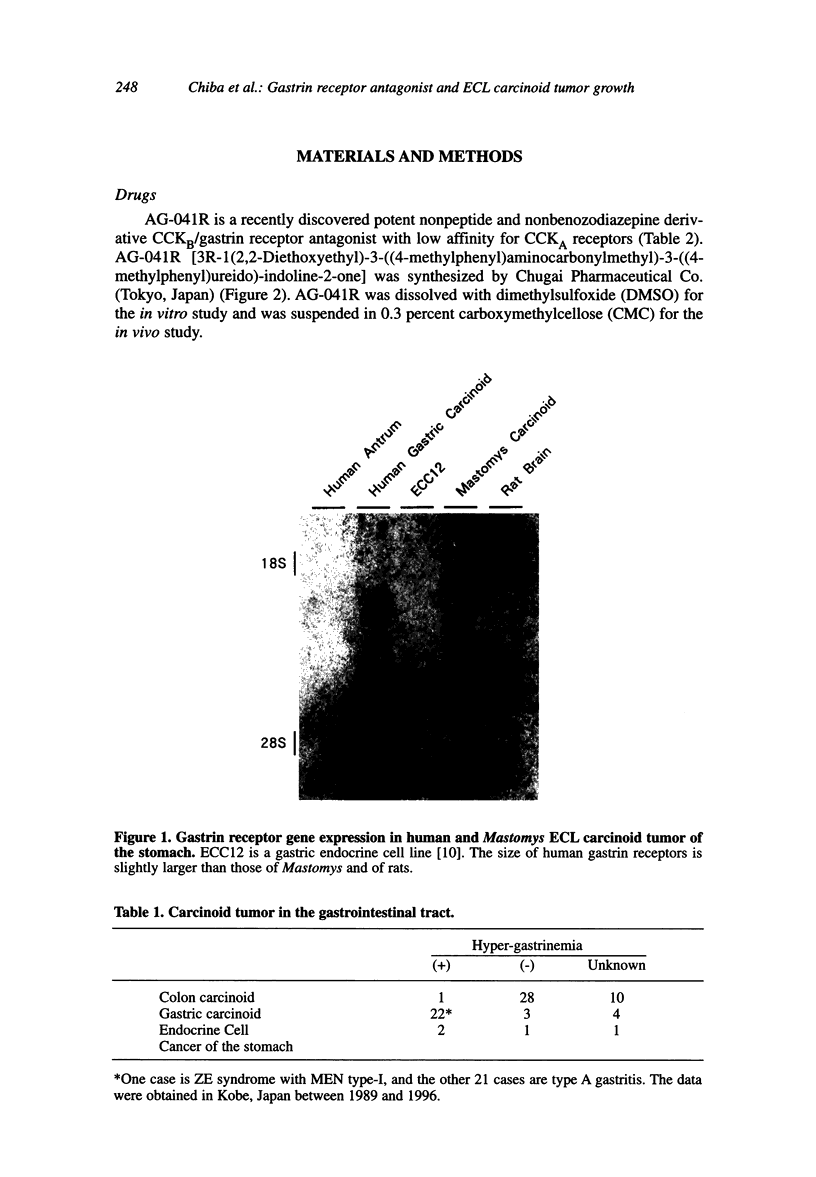




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chiba T., Kinoshita Y., Morishita T., Nakata H., Nakamura A., Hosoda S. Receptors for gastrin on gastric carcinoid tumor membrane of Mastomys natalensis. Biochem Biophys Res Commun. 1991 Jun 14;177(2):739–744. doi: 10.1016/0006-291x(91)91850-c. [DOI] [PubMed] [Google Scholar]
- Gilligan C. J., Lawton G. P., Tang L. H., West A. B., Modlin I. M. Gastric carcinoid tumors: the biology and therapy of an enigmatic and controversial lesion. Am J Gastroenterol. 1995 Mar;90(3):338–352. [PubMed] [Google Scholar]
- Kinoshita Y., Nakata H., Hassan S., Asahara M., Kawanami C., Matsushima Y., Naribayashi-Inomoto Y., Ping C. Y., Min D., Nakamura A. Gene expression of keratinocyte and hepatocyte growth factors during the healing of rat gastric mucosal lesions. Gastroenterology. 1995 Oct;109(4):1068–1077. doi: 10.1016/0016-5085(95)90564-2. [DOI] [PubMed] [Google Scholar]
- Lehy T., Cadiot G., Mignon M., Ruszniewski P., Bonfils S. Influence of multiple endocrine neoplasia type 1 on gastric endocrine cells in patients with the Zollinger-Ellison syndrome. Gut. 1992 Sep;33(9):1275–1279. doi: 10.1136/gut.33.9.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima Y., Kinoshita Y., Nakata H., Inomoto-Naribayashi Y., Asahara M., Kawanami C., Nakamura A., Ito M., Matsui T., Fujiwara T. Gastrin receptor gene expression in several human carcinomas. Jpn J Cancer Res. 1994 Aug;85(8):819–824. doi: 10.1111/j.1349-7006.1994.tb02953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naribayashi-Inomoto Y., Ding M., Nakata H., Narumiya S., Sugimoto Y., Honda A., Ichikawa A., Chiba T., Kinoshita Y. Copresence of prostaglandin EP2 and EP3 receptors on gastric enterochromaffin-like cell carcinoid in African rodents. Gastroenterology. 1995 Aug;109(2):341–347. doi: 10.1016/0016-5085(95)90319-4. [DOI] [PubMed] [Google Scholar]
- Rappel S., Altendorf-Hofmann A., Stolte M. Prognosis of gastric carcinoid tumours. Digestion. 1995;56(6):455–462. doi: 10.1159/000201276. [DOI] [PubMed] [Google Scholar]
- Suyama M., Saito T., Hara K., Kito H., Hosoda S. Production of serotonin by a transplantable strain of histamine-producing primary gastric carcinoid of Mastomys natalensis. J Natl Cancer Inst. 1984 Mar;72(3):751–757. [PubMed] [Google Scholar]