Abstract
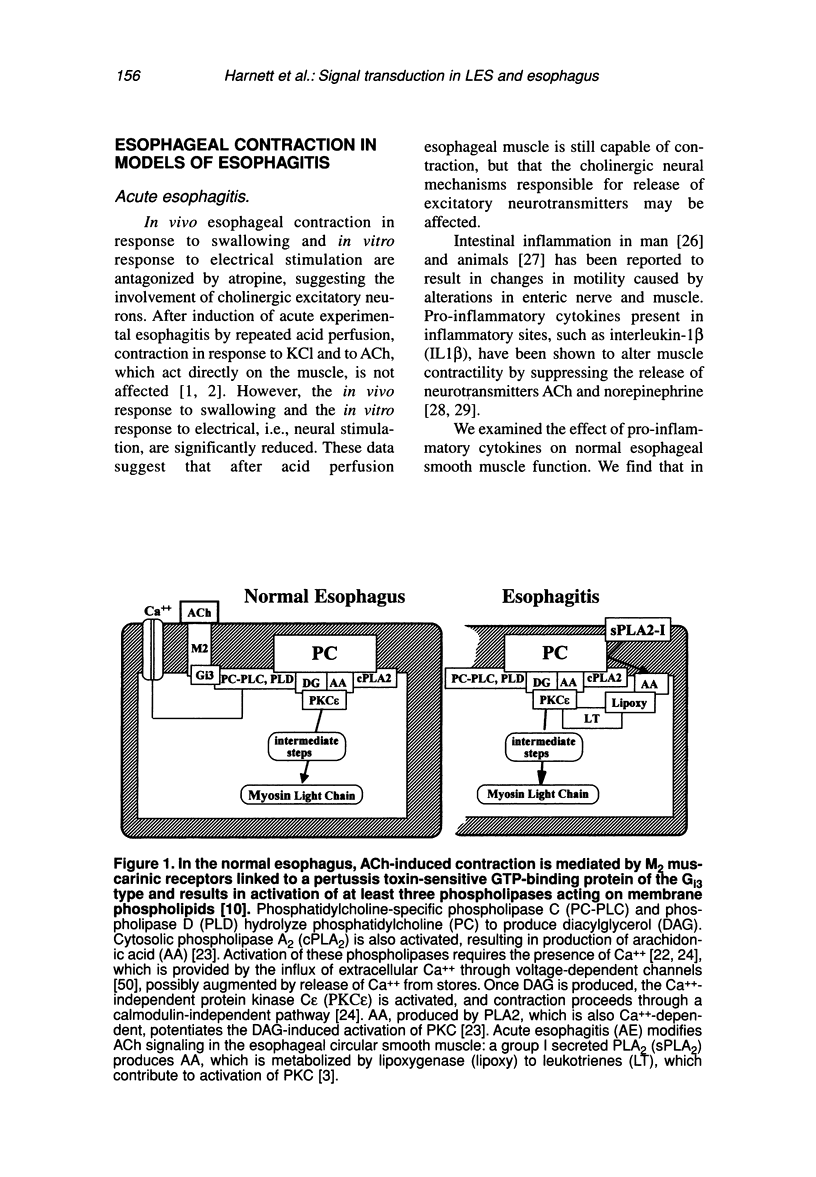
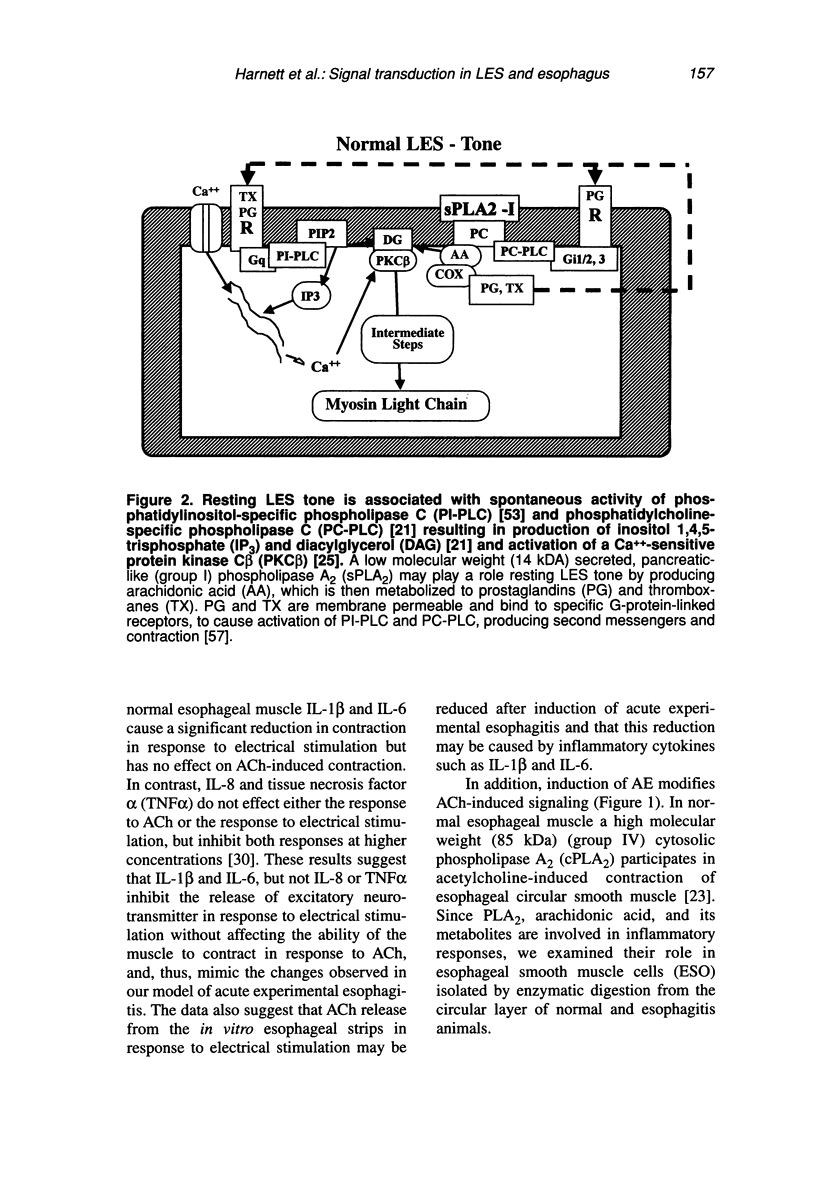
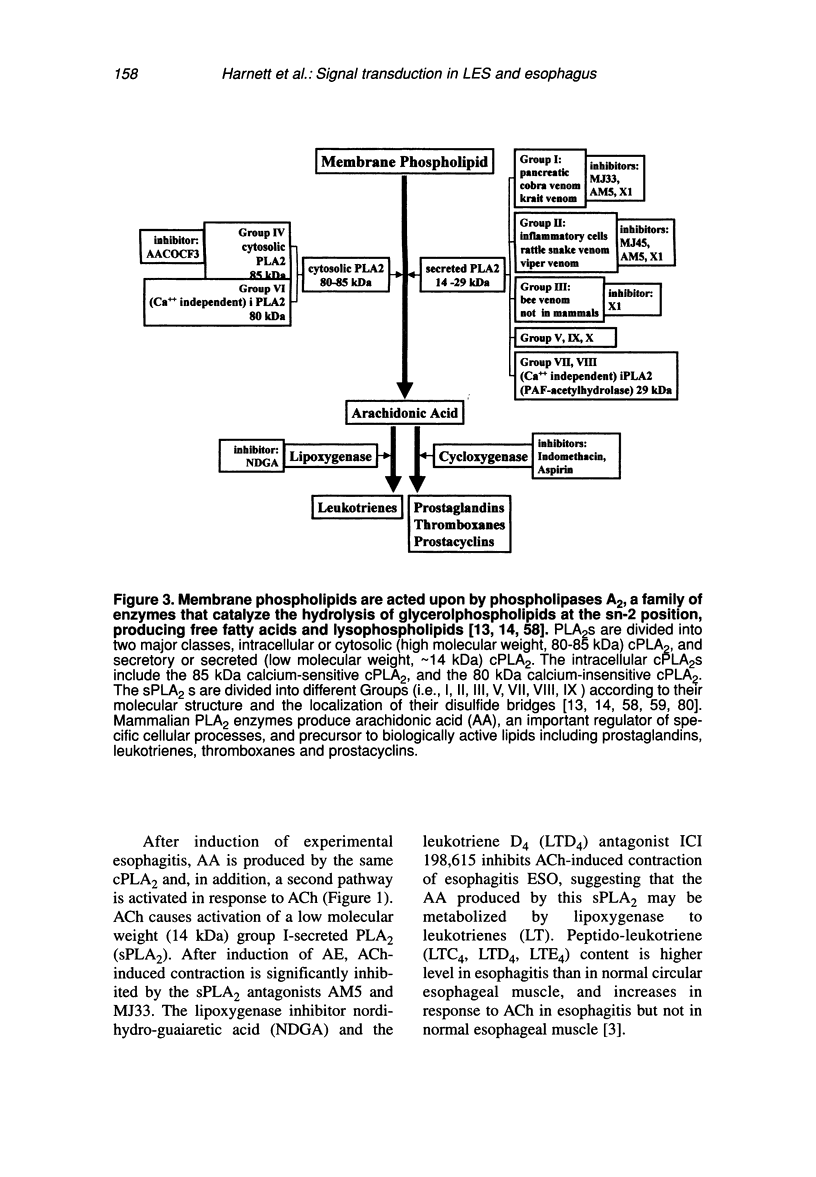
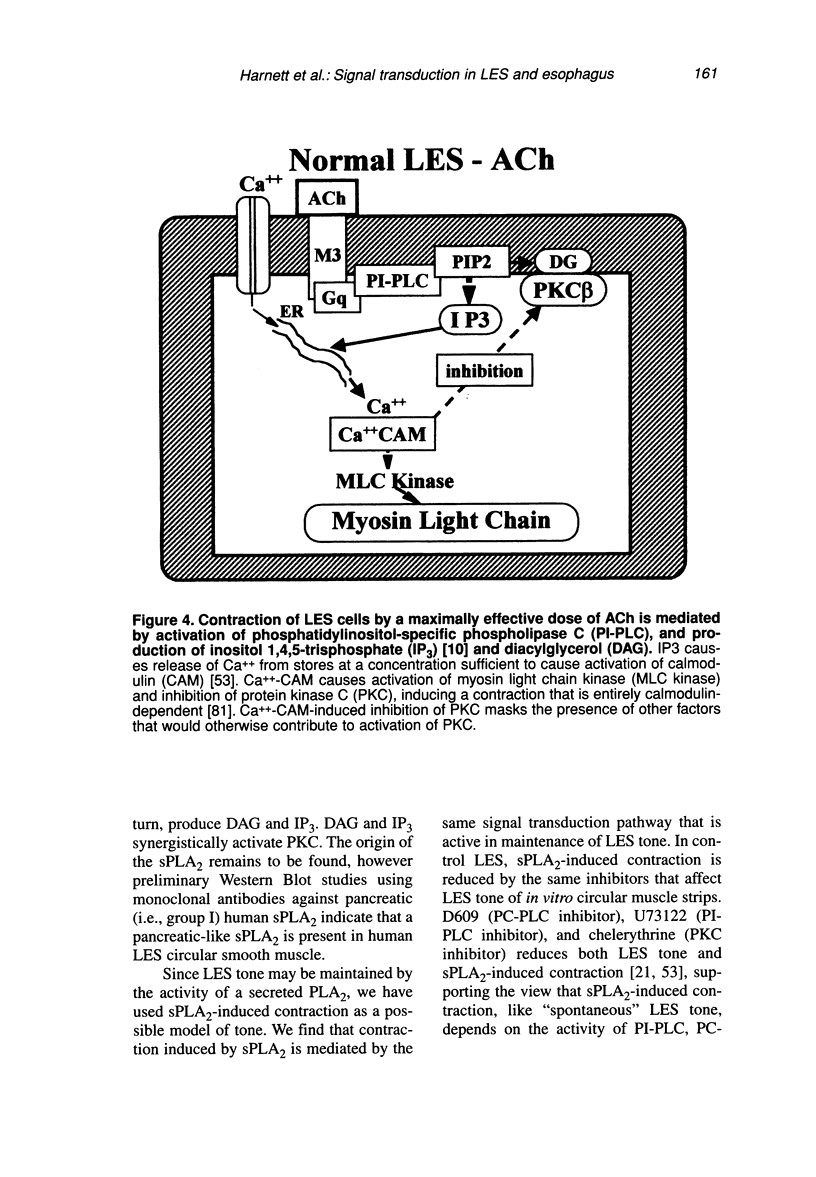
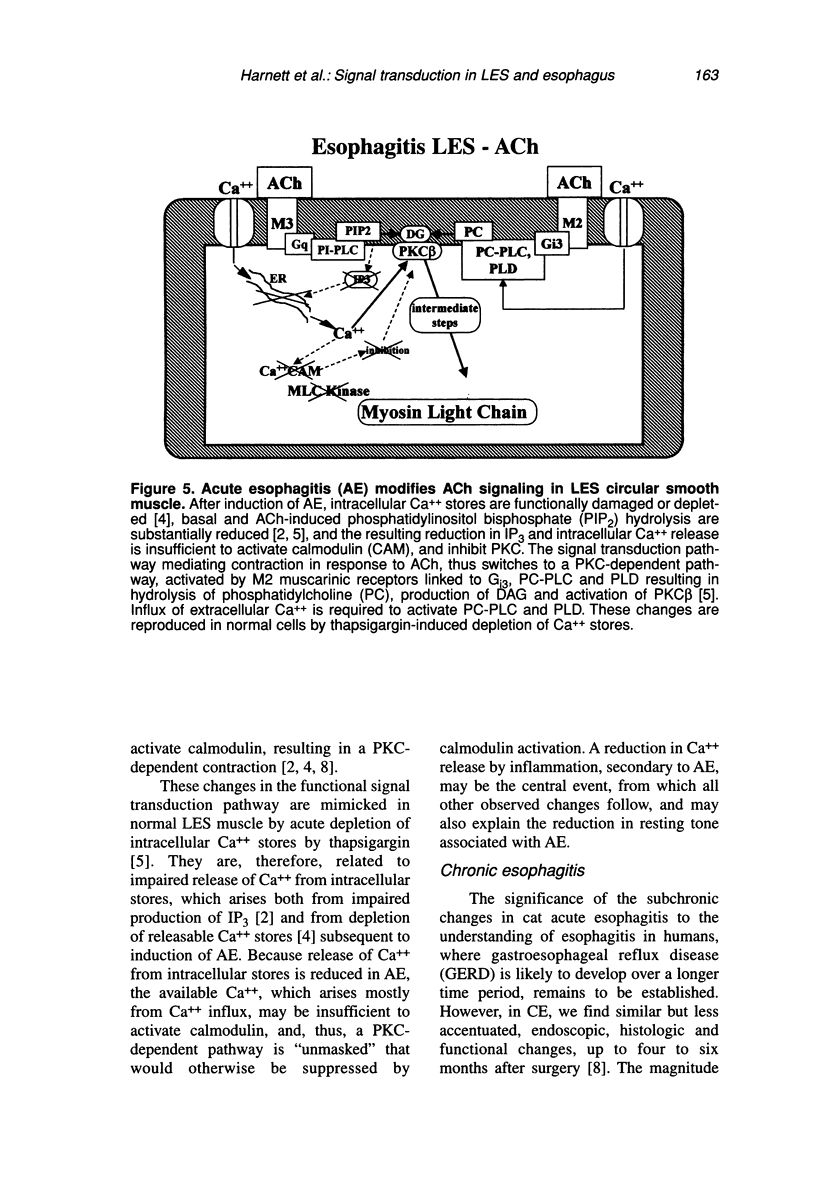
Contraction of normal esophageal circular muscle (ESO) in response to acetylcholine (ACh) is linked to M2 muscarinic receptors activating at least three intracellular phospholipases, i.e., phosphatidylcholine-specific phospholipase C (PC-PLC), phospholipase D (PLD), and the high molecular weight (85 kDa) cytosolic phospholipase A2 (cPLA2) to induce phosphatidylcholine (PC) metabolism, production of diacylglycerol (DAG) and arachidonic acid (AA), resulting in activation of a protein kinase C (PKC)-dependent pathway. In contrast, lower esophageal sphincter (LES) contraction induced by maximally effective doses of ACh is mediated by muscarinic M3 receptors, linked to pertussis toxin-insensitive GTP-binding proteins of the G(q/11) type. They activate phospholipase C, which hydrolyzes phosphatidylinositol bisphosphate (PIP2), producing inositol 1,4,5-trisphosphate (IP3) and DAG. IP3 causes release of intracellular Ca++ and formation of a Ca++-calmodulin complex, resulting in activation of myosin light chain kinase and contraction through a calmodulin-dependent pathway. Signal transduction pathways responsible for maintenance of LES tone are quite distinct from those activated during contraction in response to maximally effective doses of agonists (e.g., ACh). Resting LES tone is associated with activity of a low molecular weight (approximately 14 kDa) pancreatic-like (group 1) secreted phospholipase A2 (sPLA2) and production of arachidonic acid (AA), which is metabolized to prostaglandins and thromboxanes. These AA metabolites act on receptors linked to G-proteins to induce activation of PI- and PC-specific phospholipases, and production of second messengers. Resting LES tone is associated with submaximal PI hydrolysis resulting in submaximal levels of inositol trisphosphate (IP3-induced Ca++ release, and interaction with DAG to activate PKC. In an animal model of acute esophagitis, acid-induced inflammation alters the contractile pathway of ESO and LES. In LES circular muscle, after induction of experimental esophagitis, basal levels of PI hydrolysis are substantially reduced and intracellular Ca++ stores are functionally damaged, resulting in a reduction of resting tone. The reduction in intracellular Ca++ release causes a switch in the signal transduction pathway mediating contraction in response to ACh. In the normal LES, ACh causes release of Ca++ from intracellular stores and activation of a calmodulin-dependent pathway. After esophagitis, ACh-induced contraction depends on influx of extracellular Ca++, which is insufficient to activate calmodulin, and contraction is mediated by a PKC-dependent pathway. These changes are reproduced in normal LES cells by thapsigargin-induced depletion of Ca++ stores, suggesting that the amount of Ca++ available for release from intracellular stores defines the signal transduction pathway activated by a maximally effective dose of ACh.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarsman A. J., de Jong J. G., Arnoldussen E., Neys F. W., van Wassenaar P. D., Van den Bosch H. Immunoaffinity purification, partial sequence, and subcellular localization of rat liver phospholipase A2. J Biol Chem. 1989 Jun 15;264(17):10008–10014. [PubMed] [Google Scholar]
- Ancian P., Lambeau G., Mattéi M. G., Lazdunski M. The human 180-kDa receptor for secretory phospholipases A2. Molecular cloning, identification of a secreted soluble form, expression, and chromosomal localization. J Biol Chem. 1995 Apr 14;270(15):8963–8970. doi: 10.1074/jbc.270.15.8963. [DOI] [PubMed] [Google Scholar]
- Anderson G. D., Hauser S. D., McGarity K. L., Bremer M. E., Isakson P. C., Gregory S. A. Selective inhibition of cyclooxygenase (COX)-2 reverses inflammation and expression of COX-2 and interleukin 6 in rat adjuvant arthritis. J Clin Invest. 1996 Jun 1;97(11):2672–2679. doi: 10.1172/JCI118717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita H., Hanasaki K., Nakano T., Oka S., Teraoka H., Matsumoto K. Novel proliferative effect of phospholipase A2 in Swiss 3T3 cells via specific binding site. J Biol Chem. 1991 Oct 15;266(29):19139–19141. [PubMed] [Google Scholar]
- Biancani P., Barwick K., Selling J., McCallum R. Effects of acute experimental esophagitis on mechanical properties of the lower esophageal sphincter. Gastroenterology. 1984 Jul;87(1):8–16. [PubMed] [Google Scholar]
- Biancani P., Billett G., Hillemeier C., Nissensohn M., Rhim B. Y., Szewczak S., Behar J. Acute experimental esophagitis impairs signal transduction in cat lower esophageal sphincter circular muscle. Gastroenterology. 1992 Oct;103(4):1199–1206. doi: 10.1016/0016-5085(92)91504-w. [DOI] [PubMed] [Google Scholar]
- Biancani P., Harnett K. M., Sohn U. D., Rhim B. Y., Behar J., Hillemeier C., Bitar K. N. Differential signal transduction pathways in cat lower esophageal sphincter tone and response to ACh. Am J Physiol. 1994 May;266(5 Pt 1):G767–G774. doi: 10.1152/ajpgi.1994.266.5.G767. [DOI] [PubMed] [Google Scholar]
- Biancani P., Hillemeier C., Bitar K. N., Makhlouf G. M. Contraction mediated by Ca2+ influx in esophageal muscle and by Ca2+ release in the LES. Am J Physiol. 1987 Dec;253(6 Pt 1):G760–G766. doi: 10.1152/ajpgi.1987.253.6.G760. [DOI] [PubMed] [Google Scholar]
- Biancani P., Zabinski M., Kerstein M., Behar J. Lower esophageal sphincter mechanics: anatomic and physiologic relationships of the esophagogastric junction of cat. Gastroenterology. 1982 Mar;82(3):468–475. [PubMed] [Google Scholar]
- Billah M. M., Anthes J. C. The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochem J. 1990 Jul 15;269(2):281–291. doi: 10.1042/bj2690281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah M. M., Eckel S., Mullmann T. J., Egan R. W., Siegel M. I. Phosphatidylcholine hydrolysis by phospholipase D determines phosphatidate and diglyceride levels in chemotactic peptide-stimulated human neutrophils. Involvement of phosphatidate phosphohydrolase in signal transduction. J Biol Chem. 1989 Oct 15;264(29):17069–17077. [PubMed] [Google Scholar]
- Chakravarthy B. R., Isaacs R. J., Morley P., Durkin J. P., Whitfield J. F. Stimulation of protein kinase C during Ca(2+)-induced keratinocyte differentiation. Selective blockade of MARCKS phosphorylation by calmodulin. J Biol Chem. 1995 Jan 20;270(3):1362–1368. doi: 10.1074/jbc.270.3.1362. [DOI] [PubMed] [Google Scholar]
- Chakravarthy B. R., Isaacs R. J., Morley P., Whitfield J. F. Ca2+ x calmodulin prevents myristoylated alanine-rich kinase C substrate protein phosphorylation by protein kinase Cs in C6 rat glioma cells. J Biol Chem. 1995 Oct 20;270(42):24911–24916. doi: 10.1074/jbc.270.42.24911. [DOI] [PubMed] [Google Scholar]
- Christensen J., Conklin J. L., Freeman B. W. Physiologic specialization at esophagogastric junction in three species. Am J Physiol. 1973 Dec;225(6):1265–1270. doi: 10.1152/ajplegacy.1973.225.6.1265. [DOI] [PubMed] [Google Scholar]
- Christensen J., Freeman B. W., Miller J. K. Some physiological characteristics of the esophagogastric junction in the opossum. Gastroenterology. 1973 Jun;64(6):1119–1125. [PubMed] [Google Scholar]
- Christensen J., Roberts R. L. Differences between esophageal body and lower esophageal sphincter in mitochondria of smooth muscle in opossum. Gastroenterology. 1983 Sep;85(3):650–656. [PubMed] [Google Scholar]
- Conti P., Panara M. R., Barbacane R. C., Placido F. C., Bongrazio M., Reale M., Dempsey R. A., Fiore S. Blocking the interleukin-1 receptor inhibits leukotriene B4 and prostaglandin E2 generation in human monocyte cultures. Cell Immunol. 1992 Nov;145(1):199–209. doi: 10.1016/0008-8749(92)90323-h. [DOI] [PubMed] [Google Scholar]
- Davidson F. F., Dennis E. A. Evolutionary relationships and implications for the regulation of phospholipase A2 from snake venom to human secreted forms. J Mol Evol. 1990 Sep;31(3):228–238. doi: 10.1007/BF02109500. [DOI] [PubMed] [Google Scholar]
- Dennis E. A. Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem. 1994 May 6;269(18):13057–13060. [PubMed] [Google Scholar]
- Dennis E. A., Rhee S. G., Billah M. M., Hannun Y. A. Role of phospholipase in generating lipid second messengers in signal transduction. FASEB J. 1991 Apr;5(7):2068–2077. doi: 10.1096/fasebj.5.7.1901288. [DOI] [PubMed] [Google Scholar]
- Dennis E. A. The growing phospholipase A2 superfamily of signal transduction enzymes. Trends Biochem Sci. 1997 Jan;22(1):1–2. doi: 10.1016/s0968-0004(96)20031-3. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- Gelb M. H., Jain M. K., Berg O. G. Inhibition of phospholipase A2. FASEB J. 1994 Sep;8(12):916–924. doi: 10.1096/fasebj.8.12.8088457. [DOI] [PubMed] [Google Scholar]
- Glaser K. B., Mobilio D., Chang J. Y., Senko N. Phospholipase A2 enzymes: regulation and inhibition. Trends Pharmacol Sci. 1993 Mar;14(3):92–98. doi: 10.1016/0165-6147(93)90071-q. [DOI] [PubMed] [Google Scholar]
- Heinrikson R. L., Krueger E. T., Keim P. S. Amino acid sequence of phospholipase A2-alpha from the venom of Crotalus adamanteus. A new classification of phospholipases A2 based upon structural determinants. J Biol Chem. 1977 Jul 25;252(14):4913–4921. [PubMed] [Google Scholar]
- Hillemeier C., Bitar K. N., Sohn U., Biancani P. Protein kinase C mediates spontaneous tone in the cat lower esophageal sphincter. J Pharmacol Exp Ther. 1996 Apr;277(1):144–149. [PubMed] [Google Scholar]
- Hinson R. M., Williams J. A., Shacter E. Elevated interleukin 6 is induced by prostaglandin E2 in a murine model of inflammation: possible role of cyclooxygenase-2. Proc Natl Acad Sci U S A. 1996 May 14;93(10):4885–4890. doi: 10.1073/pnas.93.10.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homaidan F. R., Zhao L., Burakoff R. IL-1 beta induces synthesis of phospholipase A2-activating protein in rabbit distal colon. Am J Physiol. 1997 Jun;272(6 Pt 1):G1338–G1346. doi: 10.1152/ajpgi.1997.272.6.G1338. [DOI] [PubMed] [Google Scholar]
- Jacques C., Béréziat G., Humbert L., Olivier J. L., Corvol M. T., Masliah J., Berenbaum F. Posttranscriptional effect of insulin-like growth factor-I on interleukin-1beta-induced type II-secreted phospholipase A2 gene expression in rabbit articular chondrocytes. J Clin Invest. 1997 Apr 15;99(8):1864–1872. doi: 10.1172/JCI119353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERN F., Jr, ALMY T. P., ABBOT F. K., BOGDONOFF M. D. The motility of the distal colon in nonspecific ulcerative colitis. Gastroenterology. 1951 Nov;19(3):492–503. [PubMed] [Google Scholar]
- Kim N., Sohn U. D., Mangannan V., Rich H., Jain M. K., Behar J., Biancani P. Leukotrienes in acetylcholine-induced contraction of esophageal circular smooth muscle in experimental esophagitis. Gastroenterology. 1997 May;112(5):1548–1558. doi: 10.1016/s0016-5085(97)70036-2. [DOI] [PubMed] [Google Scholar]
- Komatsu H., Yaju H., Chiba K., Okumoto T. Inhibition by cyclo-oxygenase inhibitors of interleukin-6 production by human peripheral blood mononuclear cells. Int J Immunopharmacol. 1991;13(8):1137–1146. doi: 10.1016/0192-0561(91)90165-4. [DOI] [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983 Feb 17;301(5901):621–623. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- Krüger H., Schröder W., Buchner K., Hucho F. Protein kinase C inhibition by calmodulin and its fragments. J Protein Chem. 1990 Aug;9(4):467–473. doi: 10.1007/BF01024623. [DOI] [PubMed] [Google Scholar]
- Kudo I., Murakami M., Hara S., Inoue K. Mammalian non-pancreatic phospholipases A2. Biochim Biophys Acta. 1993 Nov 3;1170(3):217–231. doi: 10.1016/0005-2760(93)90003-r. [DOI] [PubMed] [Google Scholar]
- Kuwata H., Nakatani Y., Murakami M., Kudo I. Cytosolic phospholipase A2 is required for cytokine-induced expression of type IIA secretory phospholipase A2 that mediates optimal cyclooxygenase-2-dependent delayed prostaglandin E2 generation in rat 3Y1 fibroblasts. J Biol Chem. 1998 Jan 16;273(3):1733–1740. doi: 10.1074/jbc.273.3.1733. [DOI] [PubMed] [Google Scholar]
- Lambeau G., Ancian P., Barhanin J., Lazdunski M. Cloning and expression of a membrane receptor for secretory phospholipases A2. J Biol Chem. 1994 Jan 21;269(3):1575–1578. [PubMed] [Google Scholar]
- Lambeau G., Ancian P., Nicolas J. P., Beiboer S. H., Moinier D., Verheij H., Lazdunski M. Structural elements of secretory phospholipases A2 involved in the binding to M-type receptors. J Biol Chem. 1995 Mar 10;270(10):5534–5540. doi: 10.1074/jbc.270.10.5534. [DOI] [PubMed] [Google Scholar]
- Lambeau G., Barhanin J., Lazdunski M. Identification of different receptor types for toxic phospholipases A2 in rabbit skeletal muscle. FEBS Lett. 1991 Nov 18;293(1-2):29–33. doi: 10.1016/0014-5793(91)81145-x. [DOI] [PubMed] [Google Scholar]
- Lambeau G., Barhanin J., Schweitz H., Qar J., Lazdunski M. Identification and properties of very high affinity brain membrane-binding sites for a neurotoxic phospholipase from the taipan venom. J Biol Chem. 1989 Jul 5;264(19):11503–11510. [PubMed] [Google Scholar]
- Lambeau G., Lazdunski M., Barhanin J. Properties of receptors for neurotoxic phospholipases A2 in different tissues. Neurochem Res. 1991 Jun;16(6):651–658. doi: 10.1007/BF00965551. [DOI] [PubMed] [Google Scholar]
- Lambeau G., Schmid-Alliana A., Lazdunski M., Barhanin J. Identification and purification of a very high affinity binding protein for toxic phospholipases A2 in skeletal muscle. J Biol Chem. 1990 Jun 5;265(16):9526–9532. [PubMed] [Google Scholar]
- Leisten J. C., Gaarde W. A., Scholz W. Interleukin-6 serum levels correlate with footpad swelling in adjuvant-induced arthritic Lewis rats treated with cyclosporin A or indomethacin. Clin Immunol Immunopathol. 1990 Jul;56(1):108–115. doi: 10.1016/0090-1229(90)90174-o. [DOI] [PubMed] [Google Scholar]
- Ma Z., Ramanadham S., Corbett J. A., Bohrer A., Gross R. W., McDaniel M. L., Turk J. Interleukin-1 enhances pancreatic islet arachidonic acid 12-lipoxygenase product generation by increasing substrate availability through a nitric oxide-dependent mechanism. J Biol Chem. 1996 Jan 12;271(2):1029–1042. doi: 10.1074/jbc.271.2.1029. [DOI] [PubMed] [Google Scholar]
- Murakami M., Austen K. F., Arm J. P. The immediate phase of c-kit ligand stimulation of mouse bone marrow-derived mast cells elicits rapid leukotriene C4 generation through posttranslational activation of cytosolic phospholipase A2 and 5-lipoxygenase. J Exp Med. 1995 Jul 1;182(1):197–206. doi: 10.1084/jem.182.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M., Kuwata H., Amakasu Y., Shimbara S., Nakatani Y., Atsumi G., Kudo I. Prostaglandin E2 amplifies cytosolic phospholipase A2- and cyclooxygenase-2-dependent delayed prostaglandin E2 generation in mouse osteoblastic cells. Enhancement by secretory phospholipase A2. J Biol Chem. 1997 Aug 8;272(32):19891–19897. doi: 10.1074/jbc.272.32.19891. [DOI] [PubMed] [Google Scholar]
- Nakajima M., Hanasaki K., Ueda M., Arita H. Effect of pancreatic type phospholipase A2 on isolated porcine cerebral arteries via its specific binding sites. FEBS Lett. 1992 Sep 14;309(3):261–264. doi: 10.1016/0014-5793(92)80785-f. [DOI] [PubMed] [Google Scholar]
- Nassar G. M., Montero A., Fukunaga M., Badr K. F. Contrasting effects of proinflammatory and T-helper lymphocyte subset-2 cytokines on the 5-lipoxygenase pathway in monocytes. Kidney Int. 1997 May;51(5):1520–1528. doi: 10.1038/ki.1997.209. [DOI] [PubMed] [Google Scholar]
- Ogle C. K., Guo X., Szczur K., Hartmann S., Ogle J. D. Production of tumor necrosis factor, interleukin-6 and prostaglandin E2 by LPS-stimulated rat bone marrow macrophages after thermal injury: effect of indomethacin. Inflammation. 1994 Apr;18(2):175–185. doi: 10.1007/BF01534558. [DOI] [PubMed] [Google Scholar]
- Palmer J. M., Weisbrodt N. W., Castro G. A. Trichinella spiralis: intestinal myoelectric activity during enteric infection in the rat. Exp Parasitol. 1984 Apr;57(2):132–141. doi: 10.1016/0014-4894(84)90072-9. [DOI] [PubMed] [Google Scholar]
- Portanova J. P., Zhang Y., Anderson G. D., Hauser S. D., Masferrer J. L., Seibert K., Gregory S. A., Isakson P. C. Selective neutralization of prostaglandin E2 blocks inflammation, hyperalgesia, and interleukin 6 production in vivo. J Exp Med. 1996 Sep 1;184(3):883–891. doi: 10.1084/jem.184.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruzanski W., Stefanski E., Vadas P., Kennedy B. P., van den Bosch H. Regulation of the cellular expression of secretory and cytosolic phospholipases A2, and cyclooxygenase-2 by peptide growth factors. Biochim Biophys Acta. 1998 May 27;1403(1):47–56. doi: 10.1016/s0167-4889(98)00029-9. [DOI] [PubMed] [Google Scholar]
- Qian Z., Drewes L. R. A novel mechanism for acetylcholine to generate diacylglycerol in brain. J Biol Chem. 1990 Mar 5;265(7):3607–3610. [PubMed] [Google Scholar]
- Qian Z., Drewes L. R. Cross-talk between receptor-regulated phospholipase D and phospholipase C in brain. FASEB J. 1991 Mar 1;5(3):315–319. doi: 10.1096/fasebj.5.3.2001791. [DOI] [PubMed] [Google Scholar]
- Rich H., Sohn U. D., Behar J., Kim N., Biancani P. Experimental esophagitis affects intracellular calcium stores in the cat lower esophageal sphincter. Am J Physiol. 1997 Jun;272(6 Pt 1):G1523–G1529. doi: 10.1152/ajpgi.1997.272.6.G1523. [DOI] [PubMed] [Google Scholar]
- Rühl A., Berezin I., Collins S. M. Involvement of eicosanoids and macrophage-like cells in cytokine-mediated changes in rat myenteric nerves. Gastroenterology. 1995 Dec;109(6):1852–1862. doi: 10.1016/0016-5085(95)90752-1. [DOI] [PubMed] [Google Scholar]
- Sohn U. D., Chiu T. T., Bitar K. N., Hillemeier C., Behar J., Biancani P. Calcium requirements for acetylcholine-induced contraction of cat esophageal circular muscle cells. Am J Physiol. 1994 Feb;266(2 Pt 1):G330–G338. doi: 10.1152/ajpgi.1994.266.2.G330. [DOI] [PubMed] [Google Scholar]
- Sohn U. D., Han B., Tashjian A. H., Jr, Behar J., Biancani P. Agonist-independent, muscle-type-specific signal transduction pathways in cat esophageal and lower esophageal sphincter circular smooth muscle. J Pharmacol Exp Ther. 1995 Apr;273(1):482–491. [PubMed] [Google Scholar]
- Sohn U. D., Harnett K. M., Cao W., Rich H., Kim N., Behar J., Biancani P. Acute experimental esophagitis activates a second signal transduction pathway in cat smooth muscle from the lower esophageal sphincter. J Pharmacol Exp Ther. 1997 Dec;283(3):1293–1304. [PubMed] [Google Scholar]
- Sohn U. D., Harnett K. M., De Petris G., Behar J., Biancani P. Distinct muscarinic receptors, G proteins and phospholipases in esophageal and lower esophageal sphincter circular muscle. J Pharmacol Exp Ther. 1993 Dec;267(3):1205–1214. [PubMed] [Google Scholar]
- Sohn U. D., Kim D. K., Bonventre J. V., Behar J., Biancani P. Role of 100-kDa cytosolic PLA2 in ACh-induced contraction of cat esophageal circular muscle. Am J Physiol. 1994 Sep;267(3 Pt 1):G433–G441. doi: 10.1152/ajpgi.1994.267.3.G433. [DOI] [PubMed] [Google Scholar]
- Sohn U. D., Zoukhri D., Dartt D., Sergheraert C., Harnett K. M., Behar J., Biancani P. Different protein kinase C isozymes mediate lower esophageal sphincter tone and phasic contraction of esophageal circular smooth muscle. Mol Pharmacol. 1997 Mar;51(3):462–470. [PubMed] [Google Scholar]
- Sommers C. D., Bobbitt J. L., Bemis K. G., Snyder D. W. Porcine pancreatic phospholipase A2-induced contractions of guinea pig lung pleural strips. Eur J Pharmacol. 1992 May 27;216(1):87–96. doi: 10.1016/0014-2999(92)90213-n. [DOI] [PubMed] [Google Scholar]
- Tischfield J. A. A reassessment of the low molecular weight phospholipase A2 gene family in mammals. J Biol Chem. 1997 Jul 11;272(28):17247–17250. doi: 10.1074/jbc.272.28.17247. [DOI] [PubMed] [Google Scholar]
- Williams J. A., Shacter E. Regulation of macrophage cytokine production by prostaglandin E2. Distinct roles of cyclooxygenase-1 and -2. J Biol Chem. 1997 Oct 10;272(41):25693–25699. doi: 10.1074/jbc.272.41.25693. [DOI] [PubMed] [Google Scholar]
- Yu P., Harnett K. M., Biancani P., De Petris G., Behar J. Interaction between signal transduction pathways contributing to gallbladder tonic contraction. Am J Physiol. 1993 Dec;265(6 Pt 1):G1082–G1089. doi: 10.1152/ajpgi.1993.265.6.G1082. [DOI] [PubMed] [Google Scholar]
- Zhao D. Y., Hollenberg M. D., Severson D. L. Calmodulin inhibits the protein kinase C-catalysed phosphorylation of an endogenous protein in A10 smooth-muscle cells. Biochem J. 1991 Jul 15;277(Pt 2):445–450. doi: 10.1042/bj2770445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carle D. J., Christensen J., Szabo A. C., Templeman D. C., McKinley D. R. Calcium dependence of neuromuscular events in esophageal smooth muscle of the opossum. Am J Physiol. 1977 Jun;232(6):E547–E552. doi: 10.1152/ajpendo.1977.232.6.E547. [DOI] [PubMed] [Google Scholar]


