Abstract
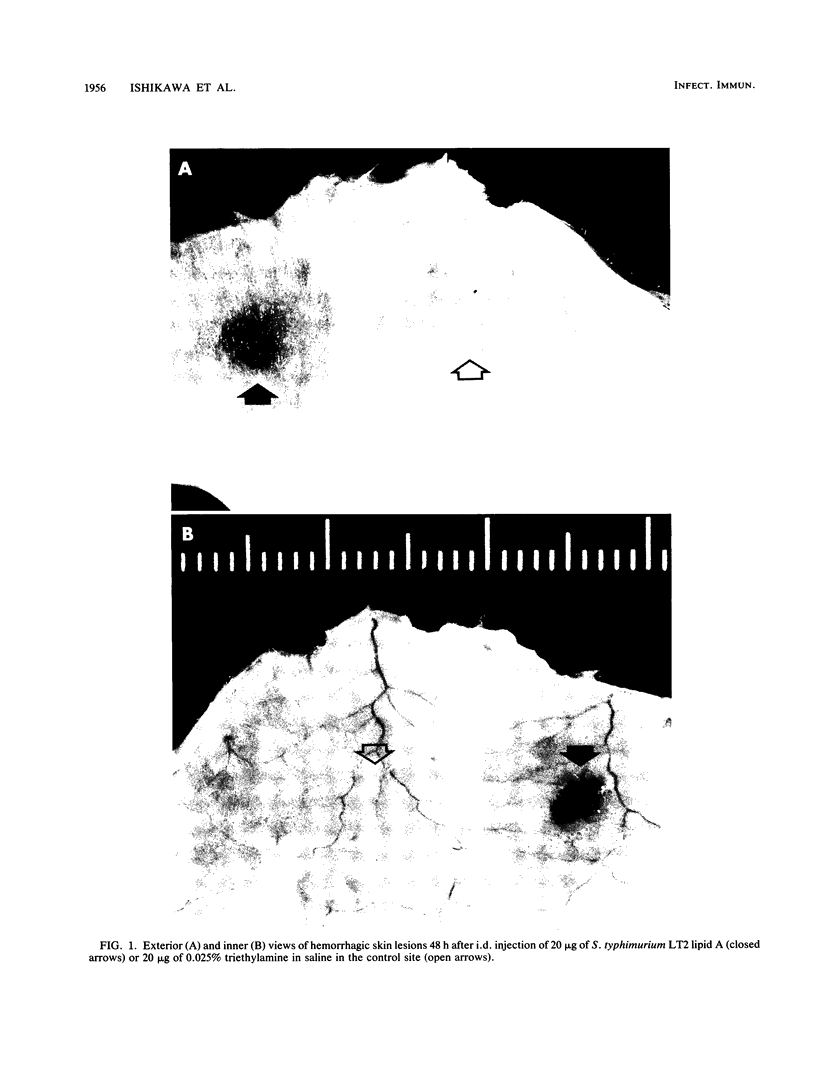
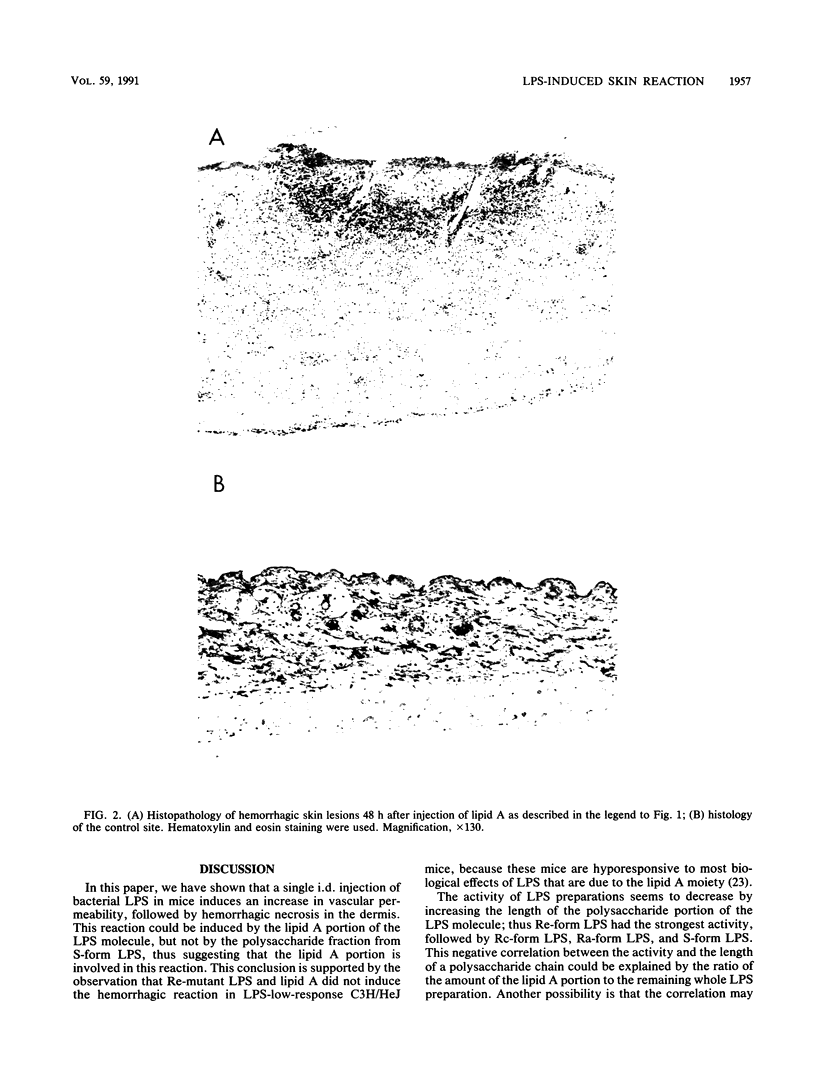
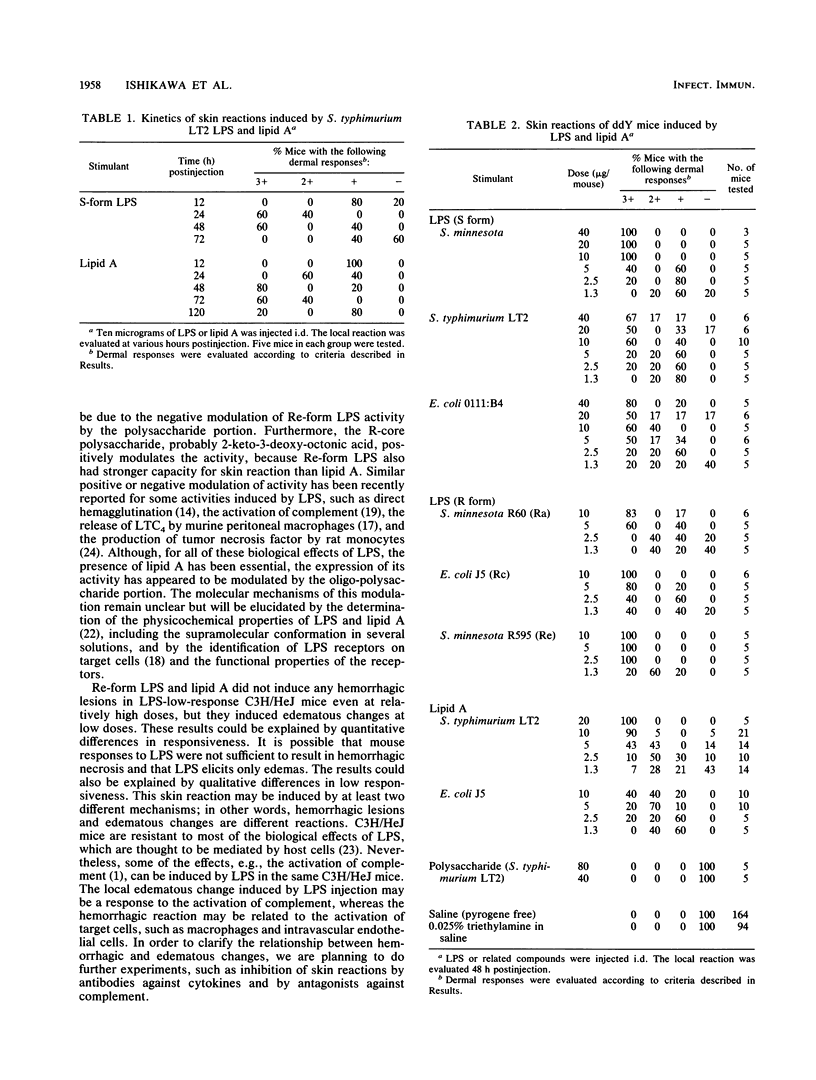
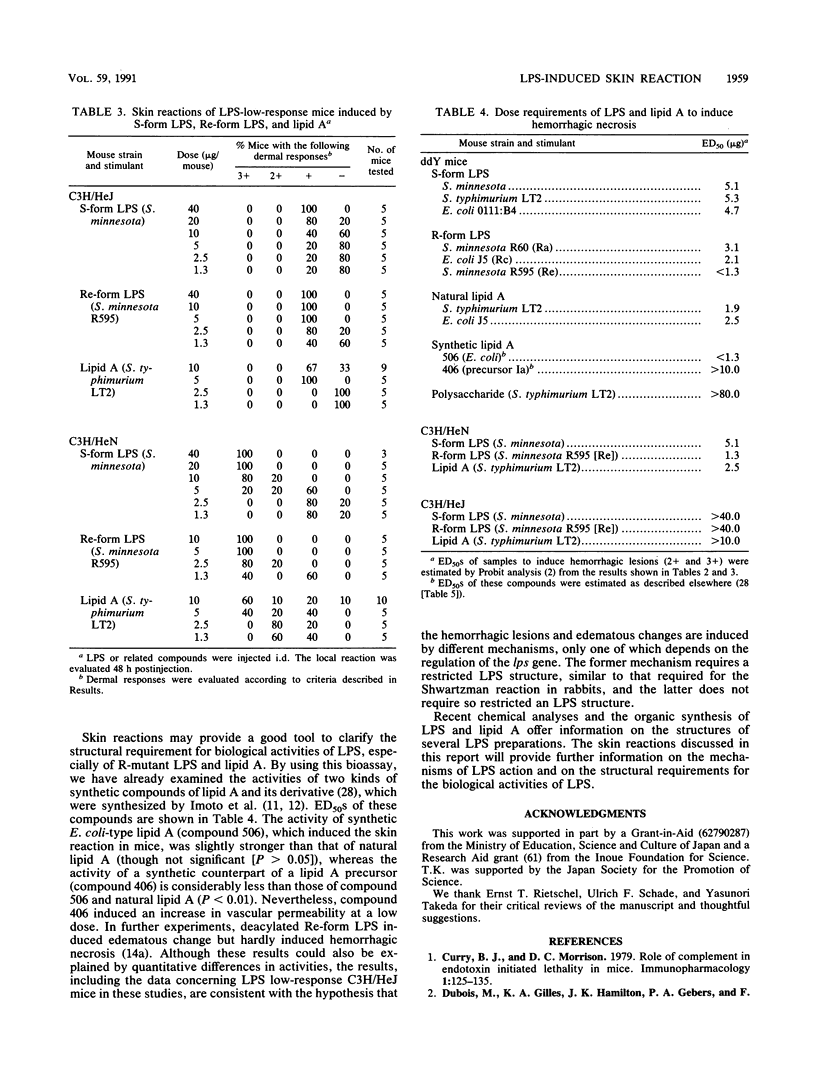
Dermal inflammation and hemorrhagic necrosis induced by bacterial lipopolysaccharide (LPS) and lipid A were studied in mice. In ddY mice, a single intradermal injection of Salmonella typhimurium S-form LPS and lipid A into the abdominal dermis elicited an edematous change due to an increase in local vascular permeability 12 h postinjection, followed by hemorrhagic necrosis from 24 to 72 h. This skin reaction was also induced in a dose-dependent manner by S-form LPS, R-mutant LPS, and lipid A of S. typhimurium and Escherichia coli, but not by polysaccharide from Salmonella S-form LPS. The dermal inflammation-inducing activities of LPS and lipid A were roughly in the following order (from highest to lowest): Re-form LPS, Rc-form LPS and lipid A, Ra-form LPS, and S-form LPS. These results suggest that the lipid A portion of the LPS molecule is responsible for the skin reaction. In C3H/HeN mice, Re-form LPS and lipid A induced the same intensity of skin reaction as that in ddY mice. In C3H/HeJ mice, which have a low response to LPS, Re-LPS and lipid A did not induce any hemorrhagic response but showed a distinct edematous change. Although hemorrhagic necrosis and edematous changes could be explained by quantitative differences in skin lesions, the other possible explanation is that hemorrhagic necrosis and the increase in local vascular permeability are induced by different mechanisms, only one of which depends on the regulation of the lps gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Curry B. J., Morrison D. C. Role of complement in endotoxin initiated lethality in mice. Immunopharmacology. 1979 Mar;1(2):125–135. doi: 10.1016/0162-3109(79)90049-3. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lehmann V., Lüderitz O., Rietschel E. T., Westphal O., Brade H., Brade L., Freudenberg M. A., Hansen-Hagge T., Lüderitz T. Endotoxic properties of chemically synthesized lipid A part structures. Comparison of synthetic lipid A precursor and synthetic analogues with biosynthetic lipid A precursor and free lipid A. Eur J Biochem. 1984 Apr 16;140(2):221–227. doi: 10.1111/j.1432-1033.1984.tb08090.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Rietschel E. T., Westphal O., Brade H., Brade L., Freudenberg M., Schade U., Imoto M., Yoshimura H. Synthetic and natural Escherichia coli free lipid A express identical endotoxic activities. Eur J Biochem. 1985 Apr 1;148(1):1–5. doi: 10.1111/j.1432-1033.1985.tb08798.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Rietschel E. T., Lüderitz O., Westphal O. Interaction of lipopolysaccharides and lipid A with complement. Eur J Biochem. 1971 Mar 1;19(1):143–152. doi: 10.1111/j.1432-1033.1971.tb01298.x. [DOI] [PubMed] [Google Scholar]
- Hisatsune K., Kondo S., Iguchi T., Machida M., Asou S., Inaguma M., Yamamoto F. Sugar composition of lipopolysaccharides of family Vibrionaceae. Absence of 2-keto-3-deoxyoctonate (KDO) except in Vibrio parahaemolyticus O6. Microbiol Immunol. 1982;26(8):649–664. doi: 10.1111/j.1348-0421.1982.tb00209.x. [DOI] [PubMed] [Google Scholar]
- KELLY M. G., SMITH N. H., WODINSKY I., RALL D. P. Strain differences in local hemorrhagic response (Shwartzman-like reaction) of mice to a single intradermal injection of bacterial polysaccharides. J Exp Med. 1957 Jun 1;105(6):653–654. doi: 10.1084/jem.105.6.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirikae T., Inada K., Hirata M., Yoshida M., Galanos C., Lüderitz O. Hemagglutination induced by lipopolysaccharides and lipid A. Microbiol Immunol. 1986;30(3):269–274. doi: 10.1111/j.1348-0421.1986.tb00942.x. [DOI] [PubMed] [Google Scholar]
- Kotani S., Takada H., Tsujimoto M., Ogawa T., Harada K., Mori Y., Kawasaki A., Tanaka A., Nagao S., Tanaka S. Immunobiologically active lipid A analogs synthesized according to a revised structural model of natural lipid A. Infect Immun. 1984 Jul;45(1):293–296. doi: 10.1128/iai.45.1.293-296.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S., Takada H., Tsujimoto M., Ogawa T., Takahashi I., Ikeda T., Otsuka K., Shimauchi H., Kasai N., Mashimo J. Synthetic lipid A with endotoxic and related biological activities comparable to those of a natural lipid A from an Escherichia coli re-mutant. Infect Immun. 1985 Jul;49(1):225–237. doi: 10.1128/iai.49.1.225-237.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüderitz T., Brandenburg K., Seydel U., Roth A., Galanos C., Rietschel E. T. Structural and physicochemical requirements of endotoxins for the activation of arachidonic acid metabolism in mouse peritoneal macrophages in vitro. Eur J Biochem. 1989 Jan 15;179(1):11–16. doi: 10.1111/j.1432-1033.1989.tb14514.x. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Kline L. F. Activation of the classical and properdin pathways of complement by bacterial lipopolysaccharides (LPS). J Immunol. 1977 Jan;118(1):362–368. [PubMed] [Google Scholar]
- Morrison D. C. The case for specific lipopolysaccharide receptors expressed on mammalian cells. Microb Pathog. 1989 Dec;7(6):389–398. doi: 10.1016/0882-4010(89)90019-3. [DOI] [PubMed] [Google Scholar]
- Rietschel E. T., Schade U., Jensen M., Wollenweber H. W., Lüderitz O., Greisman S. G. Bacterial endotoxins: chemical structure, biological activity and role in septicaemia. Scand J Infect Dis Suppl. 1982;31:8–21. [PubMed] [Google Scholar]
- Schlayer H. J., Karck U., Ganter U., Hermann R., Decker K. Enhancement of neutrophil adherence to isolated rat liver sinusoidal endothelial cells by supernatants of lipopolysaccharide-activated monocytes. Role of tumor necrosis factor. J Hepatol. 1987 Dec;5(3):311–321. doi: 10.1016/s0168-8278(87)80037-5. [DOI] [PubMed] [Google Scholar]
- Sultzer B. M. Genetic control of leucocyte responses to endotoxin. Nature. 1968 Sep 21;219(5160):1253–1254. doi: 10.1038/2191253a0. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Hirata M., Inada K., Tsunoda N., Kirikae T., Onodera T., Ishikawa Y., Sasaki O., Shiba T., Kusumoto S. Endotoxic properties of chemically synthesized lipid A analogs. Studies on six inflammatory reactions in vivo, and one reaction in vitro. Microbiol Immunol. 1989;33(10):797–810. doi: 10.1111/j.1348-0421.1989.tb00966.x. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Hirata M., Nemoto N., Wako H. Skin reaction produced by endotoxins and lactobacillus in mouse. Jpn J Exp Med. 1974 Jun;44(3):241–248. [PubMed] [Google Scholar]