Abstract
Context: Elevated glucose levels impair islet function and survival, and it has been proposed that intraislet expression of IL-1β contributes to glucotoxicity.
Objective: The objective was to investigate IL-1β mRNA expression in near-pure β-cells of patients with type 2 diabetes (T2DM) and study the regulation of IL-1β by glucose in isolated human islets.
Methods: Laser capture microdissection was performed to isolate β-cells from pancreas sections of 10 type 2 diabetic donors and nine controls, and IL-1β mRNA expression was analyzed using gene arrays and PCR. Cultured human islets and fluorescence-activated cell sorter-purified human β-cells were used to study the regulation of IL-1β expression by glucose and IL-1β.
Results: Gene array analysis of RNA from β-cells of individuals with T2DM revealed increased expression of IL-1β mRNA. Real-time PCR confirmed increased IL-1β expression in six of 10 T2DM samples, with minimal or no expression in nine control samples. In cultured human islets, IL-1β mRNA and protein expression was induced by high glucose and IL-1β autostimulation and decreased by the IL-1 receptor antagonist IL-1Ra. The glucose response was negatively correlated with basal IL-1β expression levels. Autostimulation was transient and nuclear factor-κB dependent. Glucose-induced IL-1β was biologically active and stimulated IL-8 release. Low picogram per milliliter concentrations of IL-1β up-regulated inflammatory factors IL-8 and IL-6.
Conclusion: Evidence that IL-1β mRNA expression is up-regulated in β-cells of patients with T2DM is presented, and glucose-promoted IL-1β autostimulation may be a possible contributor.
Pancreatic β-cells obtained by laser capture microdissection from individuals with type 2 diabetes display elevated expression of the inflammatory cytokine interleukin (IL)-1β. This elevation may be due to glucose-promoted IL-1β auto-stimulation.
Chronically elevated glucose levels impair islet function and proliferation and induce apoptosis, leading to the concept of islet glucotoxicity (1,2). Whereas it has been well documented over the last 20 yr that cytokines are crucial in the etiopathology of type 1 diabetes (3,4), only recently was it postulated that cytokines also play a role in islet dysfunction and death in type 2 diabetes (T2DM) (5,6,7). It has been proposed that intraislet expression of inflammatory cytokines, in particular of IL-1β, contributes to the pathogenesis of type 2 diabetes (6,8). This hypothesis was based on observations of increased IL-1β expression in pancreas sections of patients with T2DM by immunofluorescence and by in situ hybridization as well as in hyperglycemic Psammomys obesus (8). Furthermore, in vitro, high-glucose concentrations induced IL-1β release from some but not all human islet cell preparations (8). Nevertheless, the role of IL-1β in the deleterious effects of high glucose or the type 2 diabetes milieu on human pancreatic islets has been challenged based on ex vivo studies (9,10). However, a recent clinical study demonstrated that the blockade of IL-1 in type 2 diabetic patients with the IL-1 receptor antagonist (IL1-Ra) results in improved blood glucose levels and insulin secretion in the absence of changes in peripheral insulin resistance and body mass index (BMI) (11).
IL-1β has some unique features not shared by other chemokines and cytokines. Most notably, the signal transduction pathway via the IL-1 receptor (type I) is unusually effective and less than 10 molecules of IL-1β bound per cell can induce biological responses (12,13,14). Because of its effective signaling and cytotoxic effects on cells, processing, release, and receptor binding of IL-1β to target cells is tightly controlled. Unlike other secreted cytokines, IL-1β does not have a leader sequence and has to be processed from pro-IL-1β to IL-1β by inflammasomes before secretion (15). Secreted IL-1β associates with binding proteins such as the soluble form of the nonsignaling type II IL-1 receptor, thereby inhibiting its signaling to target cells (12,16). These IL-1β binding proteins are also the reason that conventional ELISA detection of IL-1β is problematic (16). Furthermore, IL-1β-producing cells also synthesize their own antagonist IL-1Ra, which binds to the IL-1 receptor without having agonistic properties and thereby modulates inflammatory responses (17,18). The low level of expression and the above described control mechanisms render IL-1β protein difficult to detect.
IL-1β is typically produced by activated immune cells, but it is also expressed at lower levels by many different cell types (19). In islets, IL-1β expression has been demonstrated in resident lymphoid cells (20,21), ductal cells, and vascular endothelial cells (22) as well as insulin producing β-cells (8,21,23).
To demonstrate increased IL-1β mRNA expression in pancreatic sections of type 2 diabetic patients, we used laser capture microdissection to obtain near pure samples of β-cells from 10 patients with T2DM and nine controls. To elucidate the regulation of IL-1β expression and to understand the observed variable effects of glucose on IL-1β induction, we examined IL-1β expression in vitro using 12 different human islet preparations. Furthermore, we studied the effects of IL-1β and glucose on islet derived inflammatory factors IL-8 and IL-6.
Subjects and Methods
Laser capture microdissection (LCM)
Nearly pure β-cells were obtained from human pancreatic frozen sections of 10 T2DM donors and nine controls by LCM. The technical details and β-cell purity were described previously (24). Briefly, LCM was performed under direct microscopic visualization of the autofluorescent signal positive areas. We performed 150–250 pulses per section on 20–30 sections per donor corresponding to a total of 5,000–15,000 cells. RNA was extracted (25), amplified, biotinylated, and hybridized to GeneChip human X3P array (Affymetrix, Santa Clara, CA) and analyzed as described (24).
RT-PCR of LCM-derived RNA was done using TaqMan reverse transcription reagents and universal PCR master mix (Applied Biosystems, Foster City, CA). Ribosomal L32 transcripts served as a reference and primers were designed using Primer Express software (Applied Biosystems). For triplicate PCR determinations, SYBR green (IL-1β, IL-8) or TaqMan technology (RPL32) was used. The relative amount of mRNA was calculated by the ΔΔ cycle threshold method. Forward and reverse primers were, respectively, for IL-1β (CCACGGCCACATTTGGTT and AGGGAAGCGGTTGCTCATC), IL-8, (GATCCACAAGTCCTTGTTCCA and GCTTCCACATGTCCTCACAA), and RPL32, (TCCTGGTCCACAACGTCAAG and AGCGATCTCGGCACAGTAAGA) with the internal detection primer, AGCTGGAAGTGCTGCTGATGTGCAAC.
Human islet cultures and treatment with glucose, IL-1β, and IL-1Ra
Islets were isolated from pancreata of organ donors aged between 35 and 70 yr with a BMI between 20.7 and 40.6 kg/m2. Islet purity ranged between 75 and 90% as judged by dithizone staining and glucose-stimulated insulin secretion was 3.3 ± 0.96-fold (mean ± sd, n = 8). Islets were plated on extracellular matrix-coated dishes (Novamed Ltd., Jerusalem, Israel) at a density of 150–200 islets per 35 mm Ø dish in CMRL 1066 medium containing 5.5 mm glucose and 10% fetal calf serum (8). After 3 d of preculture, experiments were started by adding medium with 5.5 or 33.3 mm glucose for a further 4 d without medium change. IL-1Ra (Amgen, Seattle, WA) was added at 1 μg/ml and rhIL-1β (R&D Systems Inc., Minneapolis, MN) at 0.2 ng/ml at the start of the experiments.
Human β-cell purification
The detailed protocol for purification of human β-cells is described elsewhere (26) and depends on the preferential labeling of β-cells with the fluorescent Zn2+-chelator Newport Green (27,28) and subsequent fluorescence-activated cell sorter (FACS) sorting. The resulting cell population was comprised of greater than 90% β-cells based on immunostaining with either antiinsulin or antipancreatic duodenal homeobox-1. Then 105 β-cells/dish were cultured and treated as described for the whole islets.
Quantitative PCR of cDNA from islet and β-cell cultures
RNA extraction and cDNA synthesis was done as described (29). For quantitative PCR, the real-time PCR system 7500 (Applied Biosystems) and the following commercial TaqMan assays were used: IL-1β, Hs00174097_m1; IL-1Ra, Hs00277299_m1; hypoxanthine-guanine phosphoribosyl transferase HPRT-1, Hs99999909_m1; IL-6, Hs00174131_m1; IL-8, Hs00174103_m1; CD31, Hs00169777_m1; insulin, Hs00154355_m1; CD68, Hs02741908_m1; caspase-1, Hs00236158_m1; prohormone convertase (PC)-1, Hs01026108_m1; PC2, Hs00159922_m1; and eukaryotic 18s rRNA, Hs99999901_s1 (Applied Biosystems, Rotkreuz, Switzerland). Triplicate cycle threshold values were normalized to 18s and values above cycle 36 were not used.
Detection of IL-8 and IL-6 with Luminex technology
Culture media were collected and stored at −20 C. IL-6 and IL-8 concentrations were assayed using a 2-Plex LINCO kit containing beads coupled with antibodies specific for IL-6 and IL-8 (Millipore, Billerica, MA). Recording was done with a Bioplex analyzer from Bio-Rad (Hercules, CA).
Nuclear factor-κB (NF-κB) inhibition
Islets were precultured for 3 d, media were removed, and cells were treated for 2 h with 5 μm BAY 11–7083 (ANAWA, Wangen, Switzerland) or solvent control [0.01% dimethylsulfoxide (DMSO)]. Cells were washed with media and stimulated for 24 h with or without 0.2 ng/ml IL-1β before RNA extraction.
Western blotting
Western blotting of protein extracts from islets was done as described (29). Anti-IL-1β antibody was from Xoma (Berkeley, CA), horseradish peroxidase-labeled goat antihuman IgG (Pierce Biotechnology, Rockford, IL), antiactin (C-2), and a horseradish peroxidase-labeled goat antimouse IgG (Santa Cruz Biotechnology, Santa Cruz, CA). Signal detection was assessed with a chemoluminescence imager (LAS-3000; Bucher Biotech, Basel, Switzerland).
Statistics
Data were analyzed with the GraphPad Prism program (GraphPad, San Diego, CA). Statistical significance was determined using the t test for normally distributed samples, the Mann Whitney for nonnormal distribution, and ANOVA with Bonferroni’s post hoc test for multiple comparisons. Significance was set at P < 0.05.
Results
IL-1β mRNA expression is increased in β-cells from patients with T2DM
IL-1β and IL-8 mRNA expression was analyzed from gene profiles of near-pure samples of β-cells obtained by LCM from frozen pancreatic tissue sections of 10 T2DM and nine nondiabetic cadaver donors. The control and diabetic groups had a matched BMI of 30.8 ± 6.0 kg/m2 in the diabetic and 30.9 ± 5.4 kg/m2 in the control group. The average duration for known diabetes was 5.3 ± 2.3 yr (n = 7), and the cause of death was in most cases a cardiovascular accident (supplemental Table 1, published as supplemental data on The Endocrine Society’s Journals Online Web site at http://jcem.endojournals.org).
Gene array analysis (Table 1) showed that IL-1β and IL-8 mRNA expression was higher in β-cell samples from patients with T2DM when compared with controls. Regression analysis of the gene array data revealed a significant correlation (r2 = 0.48, P = 0.0267) between the expression of IL-1β and IL-8 and no correlation between IL-1β and insulin expression (P = 0.155). Furthermore, there was no differential gene expression in samples from the control and T2DM groups for IL-1Ra, insulin, zinc transporter ZnT8 (SLC30A8), inflammatory/immune cell markers (CD68, CD163, CD3d, and tryptase-γ1), and endothelial cell markers (VE-cadherin, CD31, CD54, von Willebrand factor, CD34, CD51).
Table 1.
IL-1β mRNA expression in nearly pure β-cells obtained from patients with and without T2DM
| Gene array IL-1ß | PCR IL-1ß | Gene array IL-8 | PCR IL-8 | |
|---|---|---|---|---|
| Diabetic | ||||
| D2 | 154 | 241.35 | 76.8 | 17.2 |
| D3 | 107 | 51.55 | 148.7 | 35.14 |
| D1 | 29 | 44.83 | 31.4 | nd |
| DM2 | 78 | 117.47 | 27.5 | 0.65 |
| D6 | 28 | 3.65 | 16.6 | nd |
| D7 | 77 | 6.27 | 70.6 | 12.08 |
| D8 | 16 | nd | 34.4 | 22.5 |
| D9 | 47 | nd | 57.3 | nd |
| D11 | 30 | nd | 32.9 | 3.14 |
| D12 | 20 | nd | 11 | nd |
| Mean ± se | 58 ± 14.2 | 77.52 ± 36.84 | 50.7 ± 12.9 | 15.12 ± 5.23 |
| Nondiabetic | ||||
| H13 | 13 | 1.00 | 12.89 | nd |
| H16–2 | 30 | nd | 28.3 | 1.00 |
| H03 | 35 | 1.27 | 48.1 | nd |
| H18 | 23 | nd | 17.5 | 1.96 |
| H6 | 15 | nd | 25.4 | 0.87 |
| H7 | 26 | nd | 10.7 | nd |
| H8 | 39 | nd | 15.4 | nd |
| H14 | 50 | 1.88 | 35.5 | 8.93 |
| H16 | 21 | nd | 13.3 | nd |
| Mean ± se | 28.0 ± 3.96 | 1.39 ± 0.26 | 22.24 ± 3.81 | 3.19 ± 1.9 |
| P = 0.032 | P = 0.024 | P = 0.0337 | P = 0.086 |
IL-1β and IL-8 mRNA expression in laser-captured β-cell was analyzed by gene array and real-time PCR. Real-time PCR results were expressed as fold of a positive control sample arbitrarily defined as 1. Results passed the normality test. P, Controls vs. diabetics (t test); nd, not detectable.
Increased expression of IL-1β and IL-8 in samples from T2DM was confirmed by real-time quantitative PCR. We found strongly increased IL-1β mRNA levels (77.52 ± 36.84 fold) in six of 10 patients with T2DM, whereas minimal expression of IL-1β (1.39 ± 0.26 fold) was observed in three of nine controls (P = 0.021). IL-8 expression was also observed by real-time PCR in six of 10 T2DM samples and four of nine control samples (Table 1). The mean IL-8 mRNA levels of the diabetics of 15.12 ± 5.23-fold vs. 3.19 ± 1.9-fold in controls also showed a trend to increased expression (P = 0.086) in the diabetic group. The gene array data correlated with the PCR data for both IL-1β (r2 = 0.67, P = 0.007) and IL-8 (r2 = 0.73, P = 0.0016). There were no correlations for the IL-1β PCR data with the BMI or cold ischemia time. A weak correlation was found for blood glucose and the IL-8 gene array data (r2 = 0.358, P = 0.018) but no correlation with the IL-8 PCR results. However, the blood glucose levels were significantly correlated with both the IL-1β-positive PCR results (r2 = 0.784, P = 0.003) and the IL-1β gene array data (r2 = 0.565, P = 0.001).
IL-1β mRNA levels are induced by glucose and the responsiveness to glucose correlates with basal IL-1β levels
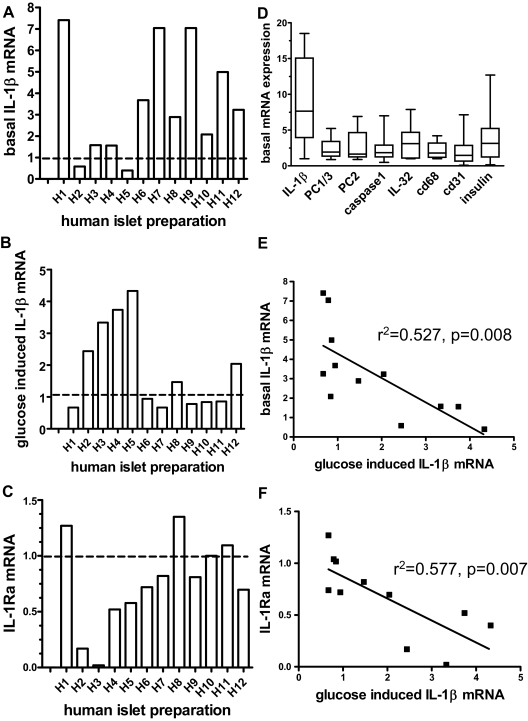
IL-1β mRNA expression and the effect of high glucose concentrations were evaluated in vitro using cultured human islets from 12 different donors. Untreated islets from different donors expressed IL-1β mRNA at unusually variable levels when compared with other basal transcript levels such as PC1/3 and PC2, insulin, macrophage marker CD68, and endothelial cell marker CD31 (Fig. 1, A and D). There was no correlation of basal IL-1β expression with basal insulin (P = 0.649), CD31 (P = 0.65), or CD68 (P = 0.179) expression. This excludes that a different cell composition in different islet isolations or a technical problem related to the RNA preparation is the cause for the variable basal IL-1β levels. Upon treatment of different islet preparations with high glucose concentrations, we observed that six of 12 islet preparations displayed a glucose-induced increase of IL-1β mRNA levels relative to the basal state (Fig. 1B). The mean increase in all 12 preparations was 2.0 ± 0.4-fold (Fig. 2A) and 3.02 ± 0.49-fold in those six preparations that did respond. Most islet preparations with low basal IL-1β levels displayed a glucose-induced increase in IL-1β expression. Conversely, in preparations with elevated basal IL-1β mRNA levels, glucose failed to further enhance IL-1β expression (Fig. 1, A and B, i.e. H1, H7, H9). There was a significant negative correlation between basal and glucose-stimulated IL-1β mRNA expression (Fig. 1E), suggesting that high basal IL-1β levels blunted the effects of glucose.
Figure 1.
Responsiveness of human islets to glucose-induced IL-1β expression and IL-1Ra inhibition depends on baseline IL-1β expression levels. Human islets were cultured for 4 d at 5.5 (basal) or 33.3 mm glucose. IL-1β and IL-1Ra mRNA levels were quantified using 18s RNA as an internal standard. The mean of triplicate determinations for each preparation is shown. A, Basal IL-1β mRNA levels expressed relative to a human islet preparation arbitrarily designated as H0 and defined as 1. B Effect of 33.3 vs. 5.5 mm glucose on IL-1β mRNA in different human islet preparations expressed as fold of basal levels (5.5 mm glucose). C, Effect of 33.3 mm glucose on IL-1Ra mRNA expressed as fold of basal levels, D, Variation of basal expression of different gene products in 11–12 human islet preparations. For each separate gene transcript, we defined the sample with the lowest expression as 1. E, Linear regression analysis of basal IL-1β mRNA vs. fold stimulation of IL-1β mRNA by glucose. F, Fold glucose-stimulated IL1β mRNA vs. fold glucose-inhibited IL-1Ra mRNA.
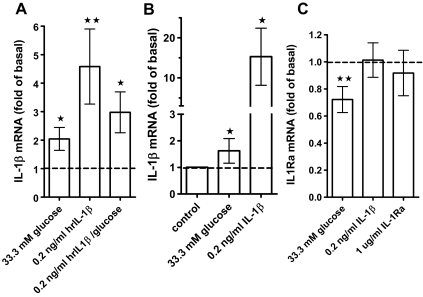
Figure 2.
IL-1β mRNA is induced by IL-1β autostimulation and glucose. A, Effect of 33.3 mm glucose (n = 12 separate donors), 0.2 ng/ml IL-1β (n = 11 separate donors), and a combination of 33.3 mm glucose and 0.2 ng/ml IL-1β (n = 8 separate donors) on IL-1β mRNA levels in whole-islet cultures relative to basal levels (5.5 mm glucose). Means ± se, significance vs. 5.5 mm glucose was determined with the Wilcoxon rank test. *, P < 0.05; **, P < 0.01. B, Effect of 0.2 ng/ml IL-1β or 33.3 mm glucose on IL-1β mRNA levels in purified β-cells (mean ± se, n = 3) relative to 5.5 mm glucose (Mann Whitney test). C, Effect of 33.3 mm glucose (n = 12), 0.2 ng/ml IL-1β (n = 10), or 1 μg/ml IL-1Ra (n = 6) on IL-1Ra mRNA levels relative to untreated islet control cultures. Means ± se, significance vs. untreated controls was determined with the Wilcoxon rank test, **, P < 0.01.
Glucose reduces IL-1Ra mRNA levels
We next analyzed the effects of glucose on IL-1Ra mRNA levels in the 12 human islet cultures (Fig. 1C). The mean IL-1Ra mRNA level of all human preparations was decreased by 33.3 mm glucose to 72.2 ± 9.57% of the control levels (Fig. 2C). Most islet preparations with glucose-induced IL-1β mRNA had diminished IL-1Ra mRNA levels, whereas nonresponding preparations had unchanged IL-1Ra levels, and there were significant correlations between glucose-stimulated IL-1β and glucose inhibited IL-1Ra mRNA levels (Fig. 1F) and between basal IL-1β and glucose-inhibited IL-1Ra (supplemental Fig. 1, published as supplemental data on The Endocrine Society’s Journals Online Web site at http://jcem.endojournals.org).
IL-1β expression is increased by IL-1β autostimulation
Induction of IL-1β expression by IL-1β itself (autostimulation) was reported for different cell types (30,31,32,33) but not yet for islets. Treatment of human islets with 0.2 ng/ml exogenous IL-1β [a concentration in the range released by islets (8,9)] significantly stimulated IL-1β mRNA expression by 4.6 ± 1.3-fold (Fig. 2A). By contrast, nonspecific cell death induced by staurosporine resulted in a dose-dependent inhibition of IL-1β mRNA expression (not shown). Autostimulation was positively correlated with glucose stimulated IL-1β expression in human islet preparations (supplemental Fig. 2). High glucose together with 0.2 ng/ml IL-1β did not further increase IL-1β mRNA (Fig. 2A ), and there was no effect of 0.2 ng/ml IL-1β on IL-1Ra expression (Fig. 2C).
Because primary islet cell cultures contain different cell types, we examined whether IL-1β autostimulation can be observed in FACS-purified human β-cells. IL-1β strongly induced autostimulation in purified β-cells (15.3 ± 4.11; Fig. 2B), whereas glucose stimulated IL-1β mRNA expression by 1.62 ± 0.27-fold (Fig. 2B).
Glucose induces biologically active IL-1β
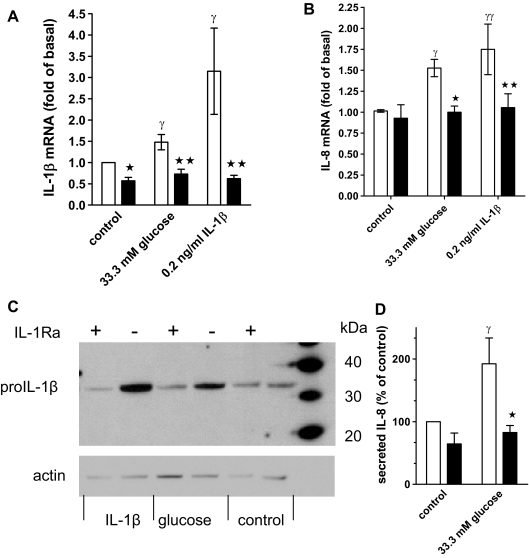
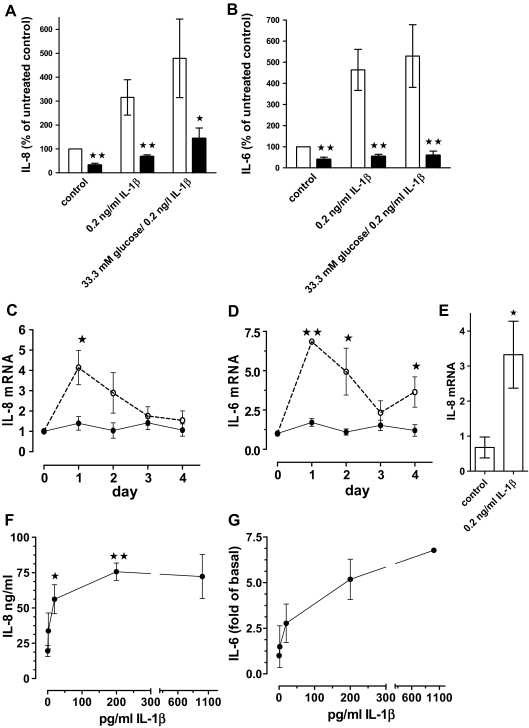
To test whether glucose treatment results in release of biologically active IL-1β, we used the antagonist IL-1Ra, which blocks ligand-induced IL-1 receptor activation without having agonistic properties (17,34). We incubated control and glucose- and IL-1β-treated human islet cultures with IL-1Ra and measured IL-1β and IL-8 mRNA and protein expression. Figure 3, A and B, shows that IL-1Ra blocked IL-1β-induced IL-1β and IL-8 mRNA, demonstrating complete antagonism of IL-1β action. Importantly, IL-1Ra also inhibited glucose-stimulated IL-1β and IL-8 mRNA expression (Fig. 3 , A and B).
Figure 3.
Glucose-induced IL-1β and IL-8 expression is antagonized with IL-1Ra. A, IL-1β mRNA expression in human islet cell cultures maintained at 5.5 mm glucose (control) or 33.3 mm glucose or treated with 0.2 ng/ml IL-1β in the presence (black bars) or absence (open bars) of 1 μg/ml IL-1Ra. Data are expressed relative to the untreated controls (means ± se, n = 7). Significance of the effect of IL-1Ra and glucose was determined with ANOVA and Bonferroni’s post hoc test. *, Control vs. IL-1Ra, P < 0.05 ; **, 33.3 mm glucose vs. IL-1Ra, P < 0.01 ; γ, control vs. 33.3 mm glucose or IL-1β P < 0.05. B, IL-8 mRNA and statistics determined as described for A (means ± se, n = 6). *, 33.3 mm glucose vs. IL-1Ra, P < 0.05; **, IL-1β vs. IL-1Ra, P < 0.01; γ, control vs. 33.3 mm glucose, P < 0.05; γγ, control vs. IL-1β, P < 0.01. C. Human islet cell cultures were treated as described under A, and islet cell extracts were subjected to Western blotting with an anti-IL-1β and an antiactin antibody. D, Secreted IL-8 in culture supernatants of cultures treated for 4 d with 33.3 mm glucose or 5.5 mm glucose in the presence (black bars) or absence (open bars) of 1 μg/ml IL-1Ra. Results from each islet preparation were expressed as percent of the untreated control (mean IL-8 concentration ± sd: 12.5 ± 4.4 ng/ml, n = 5). *, 33.3 mm glucose vs. IL-1Ra, P < 0.05 ; γ, control vs. 33.3 mm glucose, P < 0.05.
Autostimulation and glucose-induced IL-1β and IL-8 production was also demonstrated at the protein level by measuring released IL-8 and performing Western blot analysis of pro-IL-1β (32 kDa). The latter enabled us to distinguish islet cell-produced IL-1β from exogenously added IL-1β. A representative blot with islet extracts from six different donors is shown in Fig. 3C. In all islet preparations, IL-1β strongly induced and IL-1Ra inhibited pro-IL-1β expression. Glucose increased pro-IL-1β expression in three of six islet donors, a frequency expected based on the results of Fig. 1 . Furthermore, glucose increased the level of IL-8 in culture supernatants (Fig. 3D). IL-1Ra antagonized glucose-induced pro-IL-1β and IL-8 (Fig. 3, C and D).
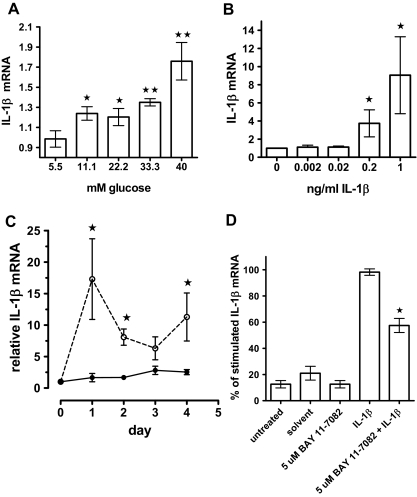
Dose response, kinetics, and NF-κB dependence of IL-1β expression
Next, we determined the IL-1β and glucose dose dependence of IL-1β mRNA induction (Fig. 4, A and B) and observed significantly increased IL-1β expression with 11.1 mm glucose (Fig. 4A) and 0.2 and 1 ng/ml IL-1β (Fig. 4B). The kinetics of IL-1β-induced IL-1β mRNA expression showed it to be transient (Fig. 4C). Basal IL-1β mRNA levels increased only minimally during the 4-d culture period, whereas 0.2 ng/ml exogenous IL-1β induced a peak of IL-1β mRNA expression at d 1, followed by a decline and another increase at d 4. By contrast, glucose-induced IL-1β mRNA stimulation was slower, and a significant increase was evident only at d 4 (not shown). To test whether NF-κB is involved in IL-1β autostimulation, we pretreated islets with the irreversible inhibitor of inhibitory-κBα phosphorylation BAY 11–7082 (35). Figure 4D shows that inhibition of NF-κB activation reduced IL-1β autostimulation by 41.2 ± 6.2%.
Figure 4.
Glucose and IL-1β dose dependence of IL-1β expression and kinetics and NF-κB dependence of IL-1β autostimulation. A, IL-1β mRNA levels of human islet cultures treated for 4 d in the presence of increasing concentrations of glucose. Each data point shows the mean (± se) of quadruplicate cultures from two donors analyzed in triplicate. Significance relative to the lowest glucose value was determined with Mann Whitney. *, P < 0.05; **, P < 0.01. B, Human islet cell cultures were treated for 4 d without (0) or with increasing concentrations of recombinant human IL-1β. Results were expressed as fold of the untreated control and represent the mean of three islet preparations ± se. Significance relative to the value without IL-1β treatment was determined with Mann Whitney. *, P < 0.05. C, Kinetics of IL-1β mRNA stimulation. Islet cultures were treated with (dashed lines) or without (solid lines) 0.2 ng/ml IL-1β; results were expressed as fold of the 0 time point, and the mean ± se of three different islet preparations is shown. Significance was determined for control vs. IL-1β-treated cultures for each individual time point with Mann Whitney. *, P < 0.05. D, Cultured human islets were treated for 2 h with 5 μm BAY 11–7082 or 0.01% DMSO (solvent) alone. After washing, the islet cultures were incubated for 24 h with or without 0.2 ng/ml IL-1β. Data were expressed as percent of the cultures stimulated with 0.2 ng/ml IL-1β together with 0.01% DMSO and are means ± se of four different islet preparations. Statistical significance was determined using the Mann Whitney test. **, P < 0.01 for BAY 11–7082/ IL-1β vs. IL-1β alone.
Low picogram per milliliter concentrations of IL-1β regulate IL-6 and IL-8 expression in human islets
IL-1β is a master regulator of various inflammatory factors including IL-8 and IL-6 (19). To test that this holds true in islets, we antagonized IL-1β-induced IL-6 and IL-8 protein release with IL-1Ra (Fig. 5, A and B). Time-course experiments showed that IL-1β-induced IL-6 and IL-8 mRNA with kinetics similar to those of IL-1β autoinduction (Fig. 5, C and D, compared with Fig. 4C). IL-1β also increased IL-8 mRNA expression in FACS-purified β-cells by 4.95 ± 0.95-fold (Fig. 5E).
Figure 5.
IL-1β induces IL-8 and IL-6 expression and release from human islets. A and B, Human islet cell cultures were treated for 4 d without (control) or with 0.2 ng/ml IL-β, or IL-1β and 33.3 mm glucose in the presence (black bars) or absence (open bars) of 1 μg/ml IL-1Ra. IL-8 (A) and IL-6 (B) concentrations in culture supernatants (200 islets per 2 ml media) were determined and expressed as percent of the untreated controls (100%). Mean ± se for four different islet preparations are shown in A and for six islet preparations in B. Significance was determined with Mann Whitney. *, P < 0.05; **, P < 0.01 of cultures with vs. without IL-1Ra. C and D, Kinetics of IL-8 (C) and IL-6 (D) mRNA induction by 0.2 ng/ml IL-1β. Results were expressed as fold of the 0 time point, the mean ± se of three different islet preparations is shown, and significance was determined with Mann Whitney. *, P < 0.05; **, P < 0.01 of control vs. IL-1β-treated cultures for each time point. E, IL-8 mRNA levels of FACS-purified β-cells treated with or without 0.2 ng/ml IL-1β (n = 3 separate β-cell purifications; *, P < 0.05, Mann Whitney). F and G, Dose responses of IL-1β-induced IL-8 and IL-6 released into culture media from human islets treated for 4 d with various concentrations of IL-1β. For IL-8 (F), the mean ± se of three islet preparations is shown. Significance vs. the data point without IL-1β was determined by ANOVA with the Dunnett’s posttest. *, P < 0.05; **, P < 0.01. The dose response of IL-1β-induced IL-6 release (G) is expressed as fold of untreated control (0.7–5.4 ng/ml) and the mean ± sd of two islet preparations is shown.
The concentrations of IL-1β released by islets were in the low picogram per milliliter range (8,9). A dose dependence with picogram per milliliter concentrations of exogenous IL-1β demonstrated that these low amounts stimulated the release of IL-8 and IL-6 protein in human islet cultures (Fig. 5, F and G). Already 20 pg/ml of IL-1β significantly induced the release of nanogram per milliliter concentrations of IL-8, and maximal effects were observed at 200 pg/ml of IL-1β. Similarly low doses of IL-1β also induced nanogram per milliliter concentrations of rodent CXC-chemokine ligand 1 in INS-1E cells (data not shown).
Discussion
In vivo expression of cytokines, in particular the tightly regulated and transiently expressed IL-1β, is difficult to assess. In the present study, the gene expression profiles of β-cells captured by LCM of pancreas sections from patients with T2DM were analyzed, and IL-1β expression was confirmed by real-time PCR. These samples contain β-cell selected tissue strongly depleted of glucagon, somatostatin, acinar, and duct cells when compared with isolated whole islets (24). We observed IL-1β mRNA expression in β-cells of six of 10 patients with T2DM and, at a much lower level, in three of nine controls. Because only low amounts of material can be obtained by LCM, analysis of IL-1β protein was not possible.
In a recent study with whole islets isolated from patients with T2DM, no significant difference in IL-1β expression could be observed, compared with nondiabetic islets (9). These islets were cultured at 5.5 mm glucose for 3–4 d before analysis, and IL-1β expression may have returned to lower levels during that time. Furthermore, the isolation stress and the high number of non-β-cells are additional confounding factors, particularly in T2DM islets displaying reduced numbers of β-cells, compared with controls.
The observed IL-1β expression pattern was very variable in individuals with T2DM. In the array screening, we observed no differences between control and T2DM samples for β-cell specific genes as well as markers for macrophage and endothelial cells. It is thus unlikely that the variability of IL-1β expression is due to a technical problem related to RNA isolation or variable contamination by non-β-cells. Furthermore, we found no correlation between IL-1β expression and age, BMI, cause of death, or cold ischemia time. We did, however, find significant correlation of IL-1β expression with glycemia, suggesting that variable blood glucose levels could contribute to the variable IL-1β expression levels. A further possible cause for the variable IL-1β levels could be its mode of expression. In other cell types, IL-1β expression oscillated and was amplified by autostimulation (30,31,32,33). In keeping with this, we indeed observed transient IL-1β expression and autostimulation in islets in vitro. With such changing expression levels, constant measurements can hardly be expected when sampling is done at just one time point. Highly variable circulating IL-1β concentrations were recently also reported in patients with type 1 diabetes, whereas other cytokines did not show such fluctuations (36). Furthermore, it is possible that only some of the patients with T2DM presented with an inflammatory phenotype.
We also analyzed the effects of elevated glucose on IL-1β mRNA expression in vitro using 12 different islet preparations. In contrast to the ex vivo samples obtained by LCM, normal untreated islets always expressed IL-1β. However, basal expression levels were unusually variable in the different islet preparations, compared with other genes that did not show such a high variability. The most constant expression was observed with the macrophage marker CD68. The variability was also not due to IL-1β induction during the 6- to 7-d culture period because IL-1β levels changed only by a factor of 2 within subjects, and this cannot account for the up to 20-fold between-subject difference observed among different preparations. Variable basal IL-1β expression could be the result of the stress of islet isolation. In previous studies various inflammatory cyto- and chemokines including IL-1β were induced upon islet isolation (24,37,38) and persisted 2–11 d after isolation. Also, differences in the attachment of the islets on the extracellular matrix, which has been shown to induce IL-1β (23), or differences in the genetic background of the donors may contribute to the variability. Independent of the underlying cause, variable basal IL-1β could be the reason that we did not observe glucose stimulated IL-1β in all islet preparations. This notion is supported by the finding of a negative correlation between basal IL-1β and glucose-stimulated IL-1β. Furthermore, in the presence of exogenously added IL-1β, glucose did not further stimulate IL-1β mRNA. Taken together, this suggests that elevated basal IL-1β levels blunt the effects of glucose on IL-1β expression in some but not all islet preparations.
A glucose dose response revealed that a concentration as low as 11 mm glucose already significantly increased IL-1β mRNA expression. Elevated glucose concentrations also increased IL-1β at the protein level and up-regulated inflammatory factor IL-8 mRNA and protein expression. IL-1Ra blocked glucose-induced IL-1β and IL-8, indicating that the effect of glucose requires IL-1 receptor activation via increased secretion of biologically active IL-1β.
Islet IL-1β expression was partly mediated by NF-κB, which is a known activator of the IL-1β promoter (33).
A variety of different cell types present within islets can produce IL-1β (8,20,21,22,23). Here we demonstrate autostimulation in FACS-purified β-cells. This finding together with IL-1β expression in samples obtained by LCM supports the idea that human β-cells themselves can produce IL-1β. IL-1β expression was recently also demonstrated in sorted rat β-cells (23). This does not rule out that additional islet cells (e.g. macrophages) also produce IL-1β in inflamed islets of patients with T2DM (39).
The LCM data also revealed that IL-1β and IL-8 expression was correlated, and in vitro, we found that glucose induced IL-8 mRNA and protein release by an IL-1-dependent mechanism. IL-1β concentrations measured in islet culture supernatants are in the low picogram per milliliter range (8,9), and we show that these low concentrations were sufficient to stimulate IL-8 and IL-6, which were released in nanogram per milliliter concentrations. IL-8 produced by cultured human islets is functional and mediates chemoattraction of macrophages (40). The induction of proinflammatory mediators together with IL-1β autostimulation, which may act in an auto- and/or paracrine way, implicates IL-1β in the mediation and amplification of intraislet inflammatory processes. We hypothesize that glucose-induced intraislet IL-1β may contribute to an inflammatory state in at least some patients with T2DM.
Supplementary Material
Acknowledgments
We thank G. Sigfried-Kellenberger for excellent technical assistance. Some batches of human islets of Langerhans were provided by the Cell Isolation and Transplantation Center at the University of Geneva School of Medicine, thanks to the ECIT Islets for Research distribution program sponsored by the Juvenile Diabetes Research Foundation. We thank P. Marchetti for providing us pancreas sections from T2D donors (supported by a European Foundation for the Study of Diabetes/Pfizer Resource Grant).
Footnotes
The work of M.B.-S., J.A.E., and M.Y.D. was supported by grants from the Swiss National Science Foundation, the Juvenile Diabetes Research Foundation, the University Research Priority Program Integrative Human Physiology at the University of Zürich, and a European Foundation for the Study of Diabetes/Merck Sharp & Dohme basic research grant. The work of G.C.W., L.M., and J.T. was supported by grants from the National Institutes of Health (NCRR ICR U4Z RR 16606 and U19DK6125), the American Diabetes Association, and the Diabetes Research and Wellness Foundation. P.A.H. was supported by Grant 7-2005-1158 from the Juvenile Diabetes Research Foundation.
Disclosure Summary: M.B.-S., J.T., G.P., L.M., J.A.E., J.K.-C., F.P., P.A.H., G.C.W., and M.Y.D. have nothing to declare.
First Published Online July 29, 2008
Abbreviations: BMI, Body mass index; DMSO, dimethylsulfoxide; FACS, fluorescence-activated cell sorter; IL1-Ra, IL-1 receptor antagonist; LCM, laser capture microdissection; NF-κB, nuclear factor-κB; PC, prohormone convertase; T2DM, type 2 diabetes.
References
- Leahy JL, Cooper HE, Deal DA, Weir GC 1986 Chronic hyperglycemia is associated with impaired glucose influence on insulin secretion. A study in normal rats using chronic in vivo glucose infusions. J Clin Invest 77:908–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donath MY, Gross DJ, Cerasi E, Kaiser N 1999 Hyperglycemia-induced β-cell apoptosis in pancreatic islets of Psammomys obesus during development of diabetes. Diabetes 48:738–744 [DOI] [PubMed] [Google Scholar]
- Mandrup-Poulsen T 1996 The role of interleukin-1 in the pathogenesis of IDDM. Diabetologia 39:1005–1029 [DOI] [PubMed] [Google Scholar]
- Eizirik DL, Mandrup-Poulsen T 2001 A choice of death—the signal-transduction of immune-mediated β-cell apoptosis. Diabetologia 44:2115–2133 [DOI] [PubMed] [Google Scholar]
- Donath MY, Storling J, Maedler K, Mandrup-Poulsen T 2003 Inflammatory mediators and islet β-cell failure: a link between type 1 and type 2 diabetes. J Mol Med 81:455–470 [DOI] [PubMed] [Google Scholar]
- Donath MY, Ehses JA, Maedler K, Schumann DM, Ellingsgaard H, Eppler E, Reinecke M 2005 Mechanisms of β-cell death in type 2 diabetes. Diabetes 54(Suppl 2):S108–S113 [DOI] [PubMed] [Google Scholar]
- Kolb H, Mandrup-Poulsen T 2005 An immune origin of type 2 diabetes? Diabetologia 48:1038–1050 [DOI] [PubMed] [Google Scholar]
- Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY 2002 Glucose-induced β-cell production of interleukin-1β contributes to glucotoxicity in human pancreatic islets. J Clin Invest 110:851–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh N, Cnop M, Kharroubi I, Bugliani M, Lupi R, Marchetti P, Eizirik DL 2005 Is there a role for locally produced interleukin-1 in the deleterious effects of high glucose or the type 2 diabetes milieu to human pancreatic islets? Diabetes 54:3238–3244 [DOI] [PubMed] [Google Scholar]
- Jorns A, Rath KJ, Bock O, Lenzen S 2006 β Cell death in hyperglycaemic Psammomys obesus is not cytokine-mediated. Diabetologia 49:2704–2712 [DOI] [PubMed] [Google Scholar]
- Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY 2007 Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 356:1517–1526 [DOI] [PubMed] [Google Scholar]
- Slack J, McMahan CJ, Waugh S, Schooley K, Spriggs MK, Sims JE, Dower SK 1993 Independent binding of interleukin-1α and interleukin-1β to type I and type II interleukin-1 receptors. J Biol Chem 268:2513–2524 [PubMed] [Google Scholar]
- Orencole SF, Dinarello CA 1989 Characterization of a subclone (D10S) of the D10.G4.1 helper T-cell line which proliferates to attomolar concentrations of interleukin-1 in the absence of mitogens. Cytokine 1:14–22 [DOI] [PubMed] [Google Scholar]
- Dinarello CA 1996 Biologic basis for interleukin-1 in disease. Blood 87:2095–2147 [PubMed] [Google Scholar]
- Martinon F, Tschopp J 2007 Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ 14:10–22 [DOI] [PubMed] [Google Scholar]
- Arend WP, Malyak M, Smith Jr MF, Whisenand TD, Slack JL, Sims JE, Giri JG, Dower SK 1994 Binding of IL-1α, IL-1β, and IL-1 receptor antagonist by soluble IL-1 receptors and levels of soluble IL-1 receptors in synovial fluids. J Immunol 153:4766–4774 [PubMed] [Google Scholar]
- Arend WP, Guthridge CJ 2000 Biological role of interleukin 1 receptor antagonist isoforms. Ann Rheum Dis 59(Suppl 1):i60–i64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA 2000 The role of the interleukin-1-receptor antagonist in blocking inflammation mediated by interleukin-1. N Engl J Med 343:732–734 [DOI] [PubMed] [Google Scholar]
- Dinarello CA 1994 The interleukin-1 family: 10 years of discovery. FASEB J 8:1314–1325 [PubMed] [Google Scholar]
- Arnush M, Scarim AL, Heitmeier MR, Kelly CB, Corbett JA 1998 Potential role of resident islet macrophage activation in the initiation of autoimmune diabetes. J Immunol 160:2684–2691 [PubMed] [Google Scholar]
- Heitmeier MR, Arnush M, Scarim AL, Corbett JA 2001 Pancreatic β-cell damage mediated by β-cell production of IL-1: a novel mechanism for virus-induced diabetes. J Biol Chem 276:11151–11158 [DOI] [PubMed] [Google Scholar]
- Matsuda T, Omori K, Vuong T, Pascual M, Valiente L, Ferreri K, Todorov I, Kuroda Y, Smith CV, Kandeel F, Mullen Y 2005 Inhibition of p38 pathway suppresses human islet production of pro-inflammatory cytokines and improves islet graft function. Am J Transplant 5:484–493 [DOI] [PubMed] [Google Scholar]
- Ribaux P, Ehses JA, Lin-Marq N, Carrozzino F, Boni-Schnetzler M, Hammar E, Irminger JC, Donath MY, Halban PA 2007 Induction of CXCL1 by extracellular matrix and autocrine enhancement by interleukin-1 in rat pancreatic β-cells. Endocrinology 148:5582–5590 [DOI] [PubMed] [Google Scholar]
- Marselli L, Thorne J, Ahn YB, Omer A, Sgroi DC, Libermann T, Otu HH, Sharma A, Bonner-Weir S, Weir GC 2008 Gene expression of purified β-cell tissue obtained from human pancreas with laser capture microdissection. J Clin Endocrinol Metab 93:1046–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmert-Buck MR, Bonner RF, Smith PD, Chuaqui RF, Zhuang Z, Goldstein SR, Weiss RA, Liotta LA 1996 Laser capture microdissection. Science 274:998–1001 [DOI] [PubMed] [Google Scholar]
- Parnaud G, Bosco D, Berney T, Pattou F, Kerr-Conte J, Donath MY, Bruun C, Mandrup-Poulsen T, Billestrup N, Halban PA 2008 Proliferation of sorted human and rat β cells. Diabetologia 51:91–100 [DOI] [PubMed] [Google Scholar]
- Gmyr V, Belaich S, Muharram G, Lukowiak B, Vandewalle B, Pattou F, Kerr-Conte J 2004 Rapid purification of human ductal cells from human pancreatic fractions with surface antibody CA19–9. Biochem Biophys Res Commun 320:27–33 [DOI] [PubMed] [Google Scholar]
- Lukowiak B, Vandewalle B, Riachy R, Kerr-Conte J, Gmyr V, Belaich S, Lefebvre J, Pattou F 2001 Identification and purification of functional human β-cells by a new specific zinc-fluorescent probe. J Histochem Cytochem 49:519–528 [DOI] [PubMed] [Google Scholar]
- Schumann DM, Maedler K, Franklin I, Konrad D, Storling J, Boni-Schnetzler M, Gjinovci A, Kurrer MO, Gauthier BR, Bosco D, Andres A, Berney T, Greter M, Becher B, Chervonsky AV, Halban PA, Mandrup-Poulsen T, Wollheim CB, Donath MY 2007 The Fas pathway is involved in pancreatic β cell secretory function. Proc Natl Acad Sci USA 104:2861–2866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA, Ikejima T, Warner SJ, Orencole SF, Lonnemann G, Cannon JG, Libby P 1987 Interleukin 1 induces interleukin 1. I. Induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J Immunol 139:1902–1910 [PubMed] [Google Scholar]
- Libby P, Ordovas JM, Birinyi LK, Auger KR, Dinarello CA 1986 Inducible interleukin-1 gene expression in human vascular smooth muscle cells. J Clin Invest 78:1432–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner SJ, Auger KR, Libby P 1987 Human interleukin 1 induces interleukin 1 gene expression in human vascular smooth muscle cells. J Exp Med 165:1316–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda Y, Tsukada J, Misago M, Kominato Y, Auron PE, Tanaka Y 2002 Autocrine induction of the human pro-IL-1β gene promoter by IL-1β in monocytes. J Immunol 168:1984–1991 [DOI] [PubMed] [Google Scholar]
- Arend WP, Malyak M, Guthridge CJ, Gabay C 1998 Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol 16:27–55 [DOI] [PubMed] [Google Scholar]
- Strieter RM, Burdick MD, Gomperts BN, Belperio JA, Keane MP 2005 CXC chemokines in angiogenesis. Cytokine Growth Factor Rev 16:593–609 [DOI] [PubMed] [Google Scholar]
- Pfleger C, Mortensen HB, Hansen L, Herder C, Roep BO, Hoey H, Aanstoot HJ, Kocova M, Schloot NC 2008 Association of IL-1ra and adiponectin with C-peptide and remission in patients with type 1 diabetes. Diabetes 57:929–937 [DOI] [PubMed] [Google Scholar]
- Johansson U, Olsson A, Gabrielsson S, Nilsson B, Korsgren O 2003 Inflammatory mediators expressed in human islets of Langerhans: implications for islet transplantation. Biochem Biophys Res Commun 308:474–479 [DOI] [PubMed] [Google Scholar]
- Bottino R, Balamurugan AN, Tse H, Thirunavukkarasu C, Ge X, Profozich J, Milton M, Ziegenfuss A, Trucco M, Piganelli JD 2004 Response of human islets to isolation stress and the effect of antioxidant treatment. Diabetes 53:2559–2568 [DOI] [PubMed] [Google Scholar]
- Weksler-Zangen S, Raz I, Lenzen S, Jorns A, Ehrenfeld S, Amir G, Oprescu A, Yagil Y, Yagil C, Zangen DH, Kaiser N 2008 Impaired glucose-stimulated insulin secretion is coupled with exocrine pancreatic lesions in the Cohen diabetic rat. Diabetes 57:279–287 [DOI] [PubMed] [Google Scholar]
- Ehses JA, Perren A, Eppler E, Ribaux P, Pospisilik JA, Maor-Cahn R, Gueripel X, Ellingsgaard H, Schneider MK, Biollaz G, Fontana A, Reinecke M, Homo-Delarche F, Donath MY 2007 Increased number of islet-associated macrophages in type 2 diabetes. Diabetes 56:2356–2370 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.