Abstract
Background and purpose:
α1-Adrenoceptor agonists induce Ca2+-transients in endothelial cells (ECs) of arterioles. However, the presence of α1-adrenoceptors on arteriolar ECs has not been excluded, and the identity of α1-adrenoceptor subtypes in arterioles only has been inferred from pharmacology. Therefore, we determined which subtypes were expressed by vascular smooth muscle cells (VSMCs) and ECs, and which subtype mediated α1-adrenoceptor-induced constriction.
Experimental approach:
EC Ca2+-transients in isolated, cannulated hamster cremasteric arterioles or freshly isolated ECs were studied using Fura 2. Arteriolar diameter was measured by video microscopy. α1-Adrenoceptor expression was assessed by western blot of whole-arteriolar homogenates and real-time RT-PCR on enzymatically isolated VSMCs and ECs.
Key results:
Phenylephrine-induced constriction and EC Ca2+-transients were abolished by the α1-adrenoceptor antagonist prazosin (30 nM) in arterioles. Phenylephrine-induced constriction was inhibited by the α1D-adrenoceptor antagonist BMY 7378 (KB=2.96 nM) and the α1A-adrenoceptor antagonist 5-methylurapidil (KB=4.08 nM), suggesting a significant role for α1D-adrenoceptors. Western blots confirmed α1D-adrenoceptor expression, but did not detect α1A-adrenoceptors. VSMCs expressed α1D- and α1A-, but not α1B-, adrenoceptor transcripts. No α1-adrenoceptor transcripts were detected in ECs. Neither phenylephrine (10 μM) nor noradrenaline (0.1–1 μM) elicited Ca2+-transients in freshly isolated ECs, whereas the endothelium-dependent vasodilators methacholine (1 μM) and substance P (100 nM) consistently increased Ca2+.
Conclusions and implications:
We reject the hypothesis that hamster cremasteric arteriolar ECs express α1-adrenoceptors and conclude that α1-adrenoceptor agonists predominantly act on VSMC α1D-adrenoceptors to cause vasoconstriction and a subsequent rise in EC Ca2+.
Keywords: microcirculation, endothelium, Ca2+ signalling, vasoconstriction, cell–cell communication, prazosin, BMY 7378, 5-methylurapidil, L-765314
Introduction
Vasoconstriction of arterioles and resistance arteries induced by α1-adrenergic agonists is associated not only with an increase in the concentration of intracellular Ca2+ ([Ca2+]in) in vascular smooth muscle cells (VSMCs) but also with an increase in [Ca2+]in in the endothelial cells (ECs) lining these vessels (Dora et al., 1997; Schuster et al., 2001; Tuttle and Falcone, 2001). Dora et al. (1997) first postulated that the α1-adrenergic agonist-induced increase in endothelial [Ca2+]in resulted from the movement of Ca2+ from smooth muscle cells to ECs through myoendothelial junctions. More recently, Isakson et al. (2007) have suggested that VSMC inositol 1,4,5-trisphosphate (IP3) may also play a role in this phenomenon. At odds with these hypotheses are the observations of Tuttle and Falcone (2001) who found that arteriolar vasoconstriction induced by prostaglandin F2α and the α2-adrenergic agonist UK-14304, both of which increase vascular smooth muscle [Ca2+]in, was not associated with a rise in EC [Ca2+]in, whereas EC [Ca2+]in did increase when the vessels were constricted with the α1-adrenergic agonists phenylephrine and noradrenaline. These investigators concluded that the rise in EC [Ca2+]in in response to α1-adrenergic agonists resulted from the expression of functional α1-adrenoceptors in ECs. This speculation is supported by studies demonstrating the expression or function of α1-adrenoceptors on vascular ECs (Vinet et al., 2000; Filippi et al., 2001; Heijnen et al., 2002; Vinci et al., 2007).
The α1-adrenoceptor subtypes expressed by, and functioning in, cells in the wall of skeletal muscle arterioles have not been extensively studied. Leech and Faber (1996) suggested, based on pharmacological evidence, that noradrenaline-induced constriction of first-order rat cremasteric arterioles is predominantly mediated by α1D-adrenoceptors. However, the apparent dissociation constant they reported for the antagonist BMY 7378 (pKB=6.86) does not really match with their conclusion that α1D-adrenoceptors play a major role in these arterioles (see Discussion and Table 2). In addition, more recent studies of skeletal muscle resistance arteries in rats (Zacharia et al., 2004) and humans (Jarajapu et al., 2001a) suggest that α1A-adrenoceptors are also important and, in fact, predominate in these vessels. Furthermore, the mRNA and protein expressions of α1-adrenoceptor subtypes have not been studied in skeletal muscle arterioles.
Table 2.
KB values for α1-adrenoceptor antagonists
| Antagonist | Control log EC50 | Control slope | Log EC50 with antagonist | Antagonist slope | Conc. ratio | [Antag]a (nM) | KB (nM) | pKB | pKB for rat aortab | pKB for rat tail arteryc | pKi for cloned α1Dd | pKi for cloned α1Ae |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMY 7378 | −7.21±0.05 | 0.55±0.03 | −6.57±0.06* | 0.76±0.07 | 4.38 | 10 | 2.96 | 8.53 | 8.57 (8.09–9) | 6.26 (5.82–6.5) | 8.84 (8.2–9.4) | 6.61 (6.11–7.16) |
| 5-Methylurapidil | −7.21±0.06 | 0.54±0.04 | −6.68±0.04* | 0.70±0.04* | 3.45 | 10 | 4.08 | 8.39 | 7.57 (6.82–8.1) | 8.58 (7.99–9) | 7.47 (6.76–8.21) | 8.62 (8.1–9.2) |
| L-765314 | −7.19±0.06 | 0.63±0.05 | −7.20±0.06 | 0.67±0.06 | 0.97 | 60 | NA | NA | NA | NA | NA | NA |
*Significantly different from control value, P<0.05.
Concentration of antagonist used to estimate the dissociation constant (KB).
Mean and range of pKB values for rat aorta from the following references: Goetz et al. (1995); Deng et al. (1996); Hussain and Marshall (1997); Carroll et al. (2001); Yoshio et al. (2001); Gisbert et al. (2003); Marti et al. (2005).
Mean and range of pKB values for rat tail artery from the following references: Lachnit et al. (1997); Yoshio et al. (2001); Gisbert et al. (2003); Marti et al. (2005).
Mean and range of pKi values for heterologously expressed α1D-adrenoceptors from the following references: Goetz et al. (1995); Kenny et al. (1995); Schwinn et al. (1995); Ford et al. (1997); Hussain and Marshall (1997); Zhu et al. (1997); Carroll et al. (2001); Yoshio et al. (2001).
Mean and range of pKi values for heterologously expressed α1A-adrenoceptors from the following references: Ford et al. (1997); Goetz et al. (1995); Kenny et al. (1995); Schwinn et al. (1995); Hussain and Marshall (1997); Zhu et al. (1997); Carroll et al. (2001); Yoshio et al. (2001).
The purpose of the present study was threefold. First, we wished to define the α1-adrenoceptors expressed by both smooth muscle cells and ECs in hamster cremasteric arterioles using enzymatically dissociated cells to precisely define cell-specific expression. Second, we wanted to determine the α1-adrenoceptor subtype that was responsible for α1-adrenoceptor-mediated vasoconstriction and the subsequent increase in EC [Ca2+]in. Finally, we wanted to test the hypothesis that endothelial α1-adrenoceptors were responsible for this increase in endothelial [Ca2+]in. Our results indicated that arteriolar VSMCs expressed transcripts for α1A- and α1D-adrenoceptors, whereas endothelial expression of α1-adrenoceptor mRNA was below the limits of detection. Based on these data, protein expression and pharmacology, we conclude that vascular smooth muscle α1D-adrenoceptors importantly mediated α1-adrenergic vasoconstriction and the subsequent increase in EC [Ca2+]in in hamster cremasteric arterioles.
Methods
Animal and tissue preparation
All animal use was approved by the Institutional Animal Care and Use Committees at Western Michigan University or Michigan State University. Male golden hamsters were killed by asphyxiation with CO2. Cremaster muscles and other tissues (heart and brain, see below) were rapidly removed, and second- and third-order arterioles were hand dissected from the cremaster muscles for protein, reverse transcription (RT)-PCR and functional studies (Burns et al., 2004; Cohen and Jackson, 2005) or enzymatic dissociation (Jackson et al., 1997; Cohen and Jackson, 2005), as described previously.
Arteriolar cell isolation
Dissected arterioles were enzymatically dissociated to yield both smooth muscle cells (Jackson et al., 1997) and endothelial tubes (Cohen and Jackson, 2005), as described previously. Freshly isolated cells were used for RT-PCR (see below) or for Ca2+-imaging experiments (Cohen and Jackson, 2005). We have previously documented the integrity and viability of these preparations (Jackson et al., 1997; Cohen and Jackson, 2005).
Arteriole cannulation
Cremasteric arterioles were cannulated with glass micropipettes and pressurized with no luminal flow (except as indicated) for in vitro studies, as described previously (Burns et al., 2004; Cohen and Jackson, 2005; Brekke et al., 2006). All vessels developed substantial myogenic tone (that is, resting diameter <80% of maximal diameter) at an intraluminal pressure of 80 cm H2O (59 mm Hg). Maximal diameter was obtained in extravascular solutions of 0 mM Ca2+.
Measurement of intracellular Ca2+
Emitted fluorescence of the ratiometric Ca2+-sensitive dye Fura 2 was used as an index of [Ca2+]in. As previously described, fluorescence was measured using a microscope-based photometry system (Photon Technologies International, Birmingham, NJ, USA) (Burns et al., 2004; Cohen and Jackson, 2005; Brekke et al., 2006). Fura 2 was excited with alternating 340 and 380 nm wavelength light with a DeltaRam X high-speed multi-wavelength illuminator, and Fura 2 emission was measured at 510 nm with a D-104 photomultiplier photometer system (Photon Technologies International) at 0.8 Hz. The illuminator and photometer were mounted on a Nikon TE 300 inverted microscope (Nikon Instruments Inc., Melville, NY, USA) equipped with × 20 (N.A. 0.5) and × 40 (N.A. 0.75) Plan Fluor long working-distance objectives. FeliX software (Photon Technologies International) was used to control the illuminator and photometer as well as for data acquizition.
Freshly isolated ECs were loaded with the calcium-sensitive dye Fura 2 using the acetoxymethyl ester of this indicator, Fura 2-AM (1 μM). Cells were incubated with Fura 2-AM for 30 min and an additional 30 min was allowed to washout and de-esterify the dye, as described previously (Cohen and Jackson, 2005).
ECs or VSMCs in isolated, cannulated cremasteric arterioles were selectively loaded with Fura 2-AM as described in detail previously by other groups (Falcone et al., 1993; Dora et al., 1997; Marrelli, 2000; Yashiro and Duling, 2000; Tuttle and Falcone, 2001). To preferentially load the ECs (Falcone et al., 1993; Dora et al., 1997; Marrelli, 2000; Yashiro and Duling, 2000; Tuttle and Falcone, 2001), Fura 2-AM (5 μM) was perfused through the lumen of the arteriole for 5 min. The vessel lumen was then washed free of Fura 2-AM for 20 min by back perfusion. An additional 30 min was allowed for de-esterification of Fura 2-AM.
In separate experiments, 5 μM Fura 2-AM was added to the bath for 60 min to preferentially load the VSMC layer (Falcone et al., 1993; Dora et al., 1997; Marrelli, 2000; Yashiro and Duling, 2000; Tuttle and Falcone, 2001). After the Fura 2-AM was washed out of the vessel chamber, 30 min were allowed for dye de-esterification. Cell-type specificity of loading was functionally verified by the diameter and calcium response elicited by the muscarinic acetylcholine receptor agonist acetyl-β-methylcholine chloride (methacholine, 1 μM), as described previously (Cohen and Jackson, 2005) (see Results).
Concentration–response protocols in cannulated vessels
Concentration–response experiments were conducted by adding increasing concentrations of phenylephrine in half-log increments to 20 mL of physiological saline solution. The heated physiological saline solution was re-circulated through the vessel chamber using a pump (model no. 7620-35; Cole Parmer, Vernon Hills, IL, USA). Each concentration was maintained until the arteriolar diameter reached steady state, as shown by the diameter trace using DIAMTRAK 3.1 software (T.O. Neild, Flinders University, NSW, Australia). When phenylephrine was tested in the presence of antagonists, the antagonist was added 30 min before the first concentration of phenylephrine was applied. Phenylephrine concentrations were increased from 1 nM to 10 μM in the presence and absence of the following antagonists: BMY 7378 (10 nM; α1D-selective; Goetz et al., 1995; Carroll et al., 2001; Yoshio et al., 2001), 5-methylurapidil (10 nM; α1A-selective; Gross et al., 1988; Ford et al., 1997; Yoshio et al., 2001), L-765314 (60 nM; α1B-selective; Patane et al., 1998) and prazosin (30 nM; inhibits all α1-receptor subtypes; Ford et al., 1997). Antagonist concentrations were selected to yield at least 90% receptor occupancy of the specific subtype of receptor based on published apparent dissociation constants (KB or Ki) for each antagonist (see references above), with the smallest possible occupancy of other α1-adrenoceptor subtypes.
Western blots
Dissected arterioles and hamster heart were placed in 2 mL microfuge tubes, flash frozen in liquid nitrogen and resuspended in lysis buffer (pH 7.5) containing 20 mM Tris base, 0.5 mM EGTA/EDTA, 0.1% SDS, 0.5% sodium deoxycholate, 1.5% NP-40, 25 μg mL−1 aprotinin/leupeptin, 20 μg mL−1 pepstatin A and 100 mM phenylmethanesulphonylfluoride. Vessels were sonicated in a set of 4 × 3 s bursts with vortexing and ice in between. Heart was minced, followed by Tissue Tearor (Biospec Products Inc., Bartlesville, OK, USA) homogenization in a set of 3 × 10 s bursts (setting 7) on ice. Then, arteriolar and heart tissue homogenates were flash frozen in liquid nitrogen and thawed on ice before centrifugation for 10 min at 13 400 g and 4 °C, and for 10 min at 1000 g and 4 °C; the resultant supernatants were used for protein quantification. Protein concentration was determined using the bicinchoninic acid assay, according to the manufacturer's protocol (Sigma, St Louis, MO, USA). Samples were boiled for 5 min and then loaded onto precast 10% Tris-glycine gels (Invitrogen, Carlsbad, CA, USA) along with MagicMark molecular weight marker (Invitrogen) and subjected to electrophoresis (125 V constant, 2.5 h on ice) in an XCell Surelock mini-cell using a PowerEase 500 power supply (Invitrogen). Proteins were transferred to Immobilon-P polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA) (125 mA constant, 1.5 h on ice) according to the manufacturer's protocol (Invitrogen). The membranes were incubated in Ponceau S solution (Sigma) (5 min) and rinsed briefly with distilled H2O to ensure successful transfer and uniformity of proteins. The membranes were then incubated in a series of solutions (rocking shaker at room temperature) starting with blocking solution (Tris-buffered saline containing 0.05% Tween-20 (TBST) and 5% non-fat dried milk) for 1 h, followed by incubation in primary anti-α1A-adrenoceptor antibody (1:200), anti-α1B-adrenoceptor antibody (1:200) or anti-α1D-adrenoceptor antibody (1:200) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted in TBST. The membranes were then washed (15 min, 3 × 5 min in TBST) and subsequently incubated with anti-rabbit HRP-conjugated secondary antibody (1:2000; Cell Signaling Technology, Danvers, MA, USA) or bovine anti-goat HRP-conjugated secondary antibody (1:30 000; Santa Cruz Biotechnology) diluted in TBST (1 h). The membranes were then washed (15 min, 3 × 5 min in TBST), followed by chemiluminescent detection with ECL western blotting substrate (Pierce, Rockford, IL, USA) to visualize proteins. Wherever applicable, blots were stripped (Restore Plus western blot stripping buffer, Pierce) according to the manufacturer's protocol and re-probed using different antibodies.
Cloning and sequencing portions of the hamster α1A-and α1D-adrenoceptor genes Adra1a and Adra1d
Sequence and exon structure information for rat Adra1a and Adra1d and rat and hamster Adra1b were obtained from The National Center for Biotechnology Information (NCBI). All three α1-adrenoceptor subtypes have a relatively simple structure with only one intron splice site in the coding region. The sequence of hamster Adra1b has been published earlier (Cotecchia et al., 1988) (accession number: J04084). However no sequences for hamster Adra1a and Adra1d were available. Therefore, we PCR cloned and sequenced hamster Adra1a and Adra1d. About 60 mg of hamster brain was homogenized in 1 mL Trizol (Invitrogen), then total RNA was extracted according to the manufacturer's instructions. Genomic DNA was digested prior to RT with DNAfree (Ambion, Austin, TX, USA). RT was performed using SuperScript III and oligo dT primers according to the manufacturer's protocol. cDNA was amplified using AmpliTaq Gold and the primers listed below. Adra1a forward: AGCGCTGGGCTCTTTCTAC, Adra1a reverse: GCTGTGGTACAGGCAGATTG or GGTGTGGATCTTAATGGTTGG; Adra1d forward: ACTGCCTCCATCCTTAGCCT, Adra1d reverse: GTAGAAGGAGCACACGGAAG or CTGGAGCAGGGGTAGATGAG. Primers were designed by inserting the rat RefSeq (http://www.ncbi.nlm.nih.gov/RefSeq/) sequences into Primer3 (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). PCR clones were sequenced by the Michigan State University Research Technology Support Facility using each of the above primers with the appropriate clone. Overlapping regions of three sequences for each gene with 100% similarity were selected for submission to GenBank (accession numbers: EF688277 and EF688278). The NCBI Blast 2 sequence tool was used to compare EF688277 and EF688278 with NCBI RefSeq sequences for Adra1a and Adra1d, respectively. Similarity was greater than 90% and included the desired intron splice site for subsequent primer design for real-time PCR. The available hamster sequence for Adra1b (accession number: J04084) yielded similar results. The more closely related mouse and rat sequences were compared with 95% similarity for Adra1a and 94% similarity for Adra1d.
Collection of cells for RT-PCR
After enzymatic dissociation, cells were collected using a micropipette coupled to a screw-driven syringe mounted on an inverted microscope. For each sample, 50 VSMCs were aspirated individually based on their distinct morphology (Jackson et al., 1997). Endothelial tubes (Cohen and Jackson, 2005) containing approximately 50 cells were collected similarly, taking care not to include tubes with any obviously attached smooth muscle cells.
Reverse transcription
Reverse transcription was performed using a modification of Invitrogen's Cells Direct protocol concluding in two 19 μL reactions per sample. Six 3 μL aliquots from each set were available for PCR, thus each subsequent PCR reaction contained an equivalent of about four cells. Cell samples, in approximately 0.5 μL of buffer, were ejected into a solution containing 7.5 μL lysis buffer (Invitrogen), 0.75 μL lysis enhancer (Invitrogen) and 0.1 μL control RNA (alien QRT-PCR inhibitor alert; Strategene, LaJolla, CA, USA). Samples were immediately flash frozen in liquid nitrogen and then stored at −80 °C for less than 1 week.
On the day of RT, samples were thawed, centrifuged at 4 °C (30 s, 1000 g), then placed in a thermocycler for a 10 min incubation at 70 °C. Samples were cooled back to 4 °C between each subsequent incubation step. Next, 2.4 μL of DNase I (0.25 U μL−1, all concentrations listed are final) and 10 × buffer were added and incubated for 5 min at 25 °C. DNase I was inactivated by heating to 70 °C for 10 min following the addition of EDTA (5 mM), oligo dT (2.5 μM) and dNTP (0.5 mM each). Samples were centrifuged (30 s, 1000 g), and then half the volume was transferred to fresh PCR tubes before adding either an RT master mix containing SuperScript III reverse transcriptase (2 U μL−1, RT samples), or one containing water in place of reverse transcriptase (no RT controls). Additional reaction components included SuperScript III 5 × RT buffer and dithiothreitol (5 mM), MgCl2 (5 mM in addition to that in the 5 × buffer to bind EDTA) and RNase I (2 U μL−1). Samples were incubated for 60 min at 50 °C, and then reverse transcriptase was inactivated by incubating for 5 min at 85 °C. RNase H (0.1 U μL−1) was added to each sample, and then incubated for 20 min at 37 °C. cDNA samples were stored at −20 °C prior to PCR.
Polymerase chain reaction
PCR was carried out in two rounds using semi-nested primers for each gene analysed (see Table 1). All PCR reagents were ABI Sybr Green core reagents, with the exception of ABI AmpliTaq Gold 10 × PCR buffer used for the first round. The first round of PCR was performed using an Eppendorf Mastercycler and the second round using an ABI 7500 real-time PCR instrument with Sybr Green detection for absolute quantification and melting curve analysis. Additionally, PCR products from the second round were run on an agarose gel to verify product size. All PCR reactions were 30 μL in volume with 10% being either RT/NRT sample or first-round PCR sample.
Table 1.
Primers used for reverse transcription-PCR
| Gene Symbol | Common | Inner | Outer | Predicted size of inner product (bp) |
|---|---|---|---|---|
| Adra1a | AGGGCTTGAAATCAGGGAAG | AGACTCTGGGCATCGTGGT | GGGTGAACAGTGCCAAGAA | 112 |
| Adra1b | TTCATCGCTCTCCCACTTG | AGCCCAGCCAGAATACCAC | CGCTTGAACTCCTTGCTG | 81 |
| Adra1d | TAGATGAGCGGGTTCACACA | GGTTCCCCTTCTTCTTCGTC | GAGAAGAAGGCTGCCAAGAC | 123 |
| Acta2 | CGGACAATCTCACGCTCA | CCGAGATCTCACCGACTACC | TGGACTCTGGAGATGGTGTT | 83 |
| Nos3 | TTGGCGTACAGAACTCAGGA | TCAGCCAGGTGGAACAGAC | GGCAAGTTGGGATCAGGT | 63 |
The first round of PCR using primers for Adra1a, Adra1b or Adra1d was carried out for 35 cycles. For α-smooth muscle actin (Acta2) or the EC isoform of NOS (e-NOS, Nos3), the first round of PCR was carried out for 25 cycles. The second round of all reactions was carried out for 40 cycles. The final concentrations of the primers were 30 nM for the first round of PCR and 150 nM for the second round. The final concentrations for all other PCR reagents were the same for both rounds: MgCl2 (25 mM), dNTP (1 mM total) and AmpliTaq (0.033 U μL−1).
Agarose gel electrophoresis
An aliquot of each PCR sample was run for one hour at 95 V on a 2% agarose gel stained with Sybr Gold, and then visualized under u.v. illumination. Molecular weights were estimated by comparison with a ladder containing 100–1000 bp markers in increments of 100 bp.
Whole-arteriole RNA isolation and RT
Whole arterioles were removed from three or four cremaster muscles per sample. RNA was extracted with Trizol reagent and resuspended in 8.5 μL of RNA storage solution (Ambion). RT-PCR was performed as described for cell-specific RT-PCR outlined above.
Statistics and data analysis
All data are presented as means±s.e. Data were analysed with paired students t-test or ANOVA as appropriate. If ANOVA indicated a significant difference, a Student-Newman-Keuls post hoc analysis was performed. Significance was set at P<0.05. In concentration response experiments, the effective concentrations that produced 50% of the maximum response (EC50) were estimated and compared between groups using Prism 4 for Macintosh (GraphPad Software, San Diego CA, USA; http://www.graphpad.com) to fit a logistic equation to the data. Receptor nomenclature conforms to the guidelines described by Alexander et al. (2008).
Drugs and chemicals
All drugs and chemicals were purchased from Sigma with the following exceptions: Fura 2-AM was purchased from Invitrogen, bovine serum albumin from USB (Cleveland, OH, USA) and elastase from Calbiochem (LaJolla, CA, USA).
Results
α1-Adrenoceptor agonists increase EC [Ca2+]in in arterioles
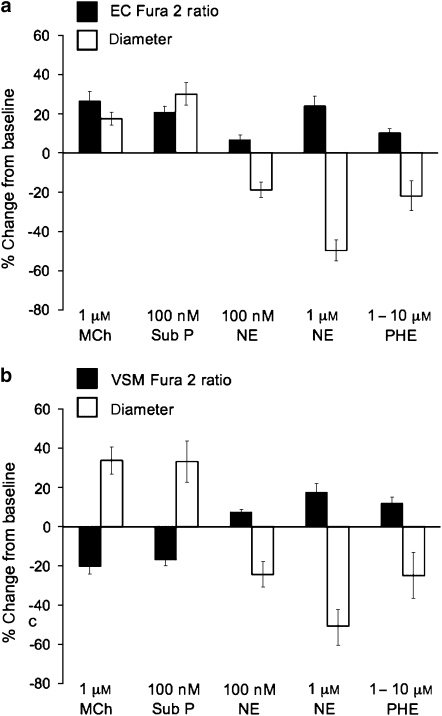
In confirmation of previous studies (Dora et al., 1997; Schuster et al., 2001; Tuttle and Falcone, 2001), we observed that both phenylephrine (Figures 1 and 2a) and noradrenaline (Figure 2a) constricted hamster cremasteric arterioles and increased EC [Ca2+] as indicated by significant increases in the Fura 2 ratio in pressurized arterioles. To verify the cell selectivity of our Fura-2-loading procedure, we also assessed the effects of the endothelium-dependent agonists methacholine (1 μM) and substance P (100 nM) on Fura 2 signals. As seen in Figures 1 and 2a, both dilators predictably increased the endothelial Fura 2 ratio and dilated the arterioles. In contrast, when VSMCs were selectively loaded with Fura 2 (Figure 2b), both methacholine and substance P led to significant decreases in the smooth muscle Fura 2 ratio along with the expected dilation, confirming the cell selectivity of our Fura 2 loading procedures. The magnitude of the methacholine dilation in vessels with Fura 2-loaded ECs (Figure 2a) appeared smaller than that observed when VSMCs were loaded with Fura 2 (Figure 2b; P<0.05 by two-sample t-test). We suppose this resulted from inclusion of two (of nine) vessels in the group shown in Figure 2a that displayed very small dilations to methacholine (5–7%), and the small sample size of the group in Figure 2b (n=4). If the two outliers were removed from the comparison, this apparent difference was eliminated (P>0.05) suggesting that it did not result from a systematic difference between the two groups.
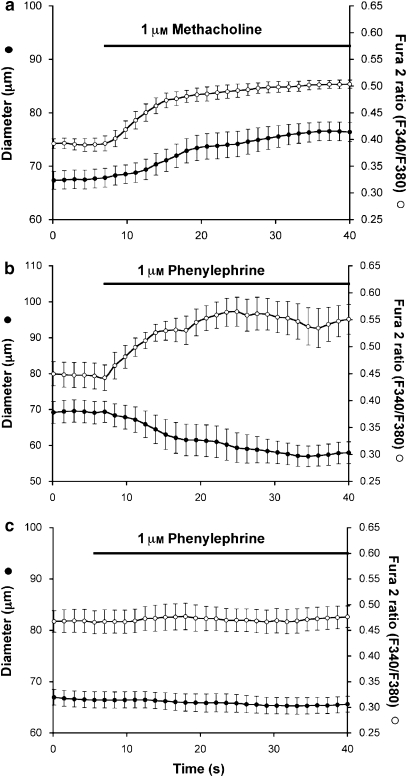
Figure 1.
Endothelial cell (EC) calcium signals in cannulated arterioles. Data are means±s.e. (n=4) diameter (left y axis) or Fura 2 ratio (right y axis) as indicated. (a) Response to the endothelium-dependent, muscarinic agonist methacholine; data consistent with selective EC loading of Fura 2. (b) Response to the α1-adrenoceptor agonist phenylephrine, confirming an increase in EC [Ca2+]in associated with phenylephrine-induced vasoconstriction. (c) Inhibition of phenylephrine-induced responses by 30 min pre-incubation with the α1-adrenoceptor antagonist prazosin (30 nM), demonstrating the essential role of α1-adrenoceptors in phenylephrine-induced constriction and associated EC Ca2+-transients.
Figure 2.
Endothelial and smooth muscle cell Ca2+ responses to endothelium-dependent agonists and α1-adrenoceptor agonists. Data are mean peak changes±s.e. in diameter or Fura 2 ratio expressed as a percent of the baseline values. (a) Endothelial cell (EC) Fura 2 signals and arteriolar diameter in response to the endothelium-dependent agonists methacholine (MCh, 1 μM, n=9) and substance P (Sub P, 100 nM, n=6), or the α1-adrenoceptor agonists noradrenaline (NE, 100 nM, n=6 or 1 μM, n=10, as indicated) and phenylephrine (PHE, 1 μM, n=6). For each agonist, the peak change in Fura 2 ratio or diameter were significantly greater than 0 (P<0.05). (b) Responses to the same agonists as in (a) but with the smooth muscle cells (VSM) loaded with Fura 2 (n=4 per group).
Pharmacological characterization of α1-adrenoceptors in hamster cremasteric arterioles
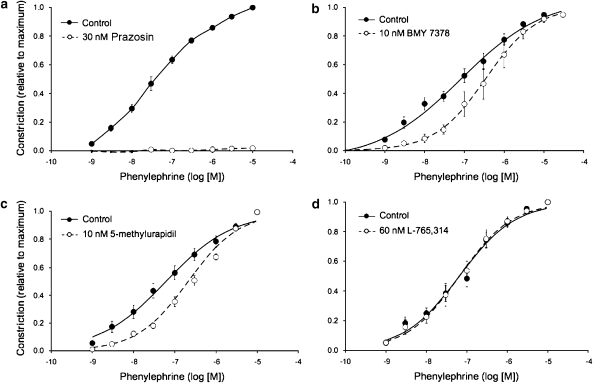
We found that phenylephrine-induced constriction of cremasteric arterioles (Figures 1c and 3a) and the resulting increase in EC [Ca2+]in were abolished by the α1-adrenergic antagonist prazosin (30 nM). These data indicate that activation of the α1-adrenoceptors is sufficient to produce both activation of VSMCs and rise in EC [Ca2+]in. Phenylephrine-induced vasoconstriction was also significantly inhibited by the α1D-adrenoceptor subtype-selective inhibitor BMY 7378 (10 nM) (Figure 3b and Table 2) and the α1A-adrenoceptor subtype-selective inhibitor 5-methylurapidil (10 nM) (Figure 3c and Table 2). Although there was a tendency for an increase in the slope of the concentration–response relationship in vessels treated with BMY 7378, this did not reach statistical significance (Table 2, P>0.05). The slope of the concentration–response relationship for 5-methylurapidil was significantly increased (Table 2, P<0.05). In contrast to our findings with BMY 7378 and 5-methylurapidil, the α1B-adrenoceptor subtype-selective inhibitor L-765 314 (60 nM) (Figure 3d and Table 2) had no significant effect on phenylephrine-induced constriction.
Figure 3.
Pharmacology of phenylephrine-induced constriction of hamster cremasteric arterioles. Data are means±s.e. (n as given below). Lines in (b–d) represent the best fit of the logistic equation to the data sets (parameters given in Table 2). (a) Abolition of phenylephrine-induced vasoconstriction by the α1-adrenoceptor subtype nonspecific antagonist prazosin (n=5, P<0.05), consistent with the data shown in Figure 1. (b) Inhibition of phenylephrine-induced vasoconstriction by the α1D-adrenoceptor antagonist BMY 7378 (n=7, P<0.05). (c) Inhibition of phenylephrine-induced vasoconstriction by the α1A-adrenoceptor antagonist 5-methylurapidil (n=7, P<0.5). (d) Lack of effect of the α1B-adrenoceptor antagonist L-765314 on phenylephrine-induced vasoconstriction (n=5, P>0.05).
Expression of α1-adrenoceptor subtype protein in cremasteric arterioles
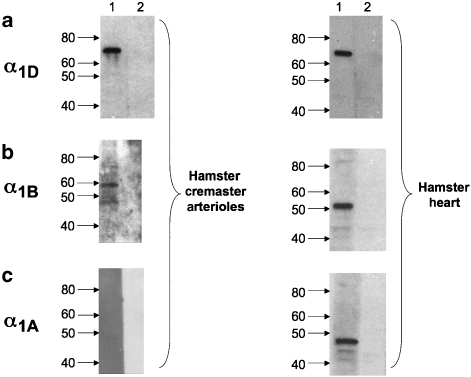
Western blots of arteriole whole homogenates indicated strong expression of α1D-adrenoceptors (Figure 4a). Several immunoreactive bands were observed using antibodies selective for α1B-adrenoceptors (Figure 4b). However, note that these bands did not fall at the same molecular weight as the immunoreactive signal observed in hamster heart homogenates, a positive control. No immunoreactive band was detected using antibodies for α1A-adrenoceptors (Figure 4c), even when lanes were loaded with 100 μg of total arteriolar protein (data not shown). In contrast, we were able to detect a clean immunoreactive signal of the appropriate molecular weight in the total protein obtained from hamster heart (Figure 4c) and in as little as 10 μg hamster brain (data not shown).
Figure 4.
Expression of α1-adrenoceptor proteins in hamster cremasteric arterioles. Representative western blots (see text for details) are shown for the three α1-adrenoceptor subtypes in whole-arteriole homogenates and homogenates of hamster heart (positive control), as indicated. Images are representative of at least three experiments from different samples of arterioles and heart. (a) Detection of a 70 kDa band in 10 μg protein from arteriolar (left) or heart (right) homogenates. (b) 48 and 57 kDa bands in 41.5 μg protein from arteriolar homogenates (left) and 51 kDa band in 41.5 μg protein from the heart (right panel). (c) No immunoreactive band was detected for α1A-adrenoceptor in 41.5 μg from arterioles (left panel), despite detection of an appropriate 48 kDa band in 41.5 μg protein from the heart (right panel).
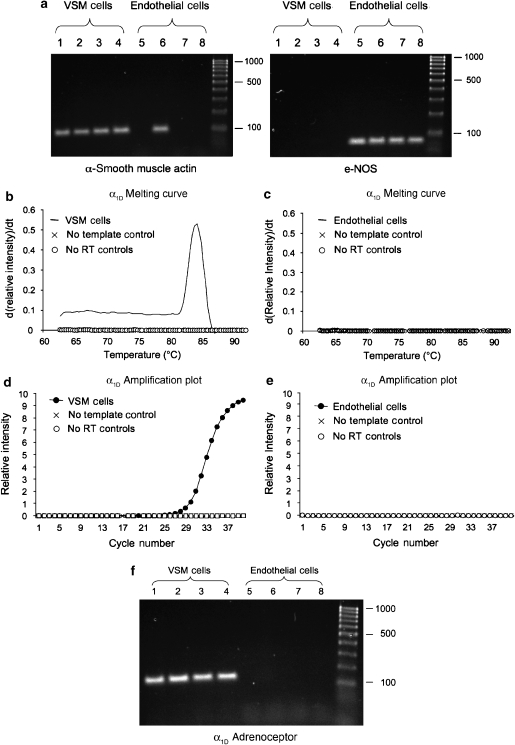
Expression of α1-adrenoceptor subtype transcripts in smooth muscle cells and ECs
We used RT-PCR applied to enzymatically isolated VSMCs and ECs to define cell-specific expression of α1-adrenoceptor subtypes in cremasteric arterioles. Figure 5a shows representative results using primers for α-smooth muscle actin and e-NOS as cell-specific markers. All 16 smooth muscle samples were positive for α-smooth muscle actin and negative for e-NOS (0 out of 16) (Figure 5a). We found it difficult to obtain EC tubes that were not contaminated with a few smooth muscle cells. Thus, a minority of EC samples (4 out of 15) were positive for α-smooth muscle actin transcripts as shown in Figure 5a. All but one EC sample was positive for e-NOS (13 out of 14).
Figure 5.
Cell-specific RT-PCR using enzymatically isolated smooth muscle and endothelial cells. (a) A representative agarose gel for RT-PCR products from four different samples of 50 smooth muscle (VSM) and endothelial cells, as indicated, using α-smooth muscle actin (VSM-specific) and e-NOS (endothelial cell-specific) as representative cell-specific markers. Note that all VSM samples were positive for α-smooth muscle actin, but negative for e-NOS. Although all endothelial cell samples were positive for e-NOS, there was some contamination of the endothelial cell samples with smooth muscle, as indicated by the presence of one α-smooth muscle actin-positive endothelial cell sample in this experiment. However, the majority of endothelial cell samples (11 out of 15) were not contaminated. (b) A typical primer-melting curve for a smooth muscle cell (VSM) sample along with appropriate control indicating specific amplification of α1D-adrenoceptor message. (c) A typical result from an endothelial cell sample indicating no amplification (that is, the lack of presence of message in these cells). (d) A typical real-time PCR result in a VSM sample along with appropriate controls. (e) Typical negative result for real-time PCR for endothelial cell samples. (f) Representative agarose gels from four samples of smooth muscle (VSM) and endothelial cells, as indicated. Note that all four VSM samples were positive for α1D-adrenoceptor transcripts, whereas no products were amplified in the four endothelial cell samples shown.
Figures 5b–f display examples of our results for expression of transcripts for α1D-adrenoceptors. We found that the majority of smooth muscle cell samples (12 out of 15) were positive for α1D-adrenoceptor message. In contrast, none of the EC samples (0 out of 14) were positive for these transcripts. Message for α1A-adrenoceptors was detected in all 15 smooth muscle cell samples. As with the α1D-adrenoceptor, EC samples (6 out of 6) were negative for α1A-adrenoceptor transcripts. Message for α1B-adrenoceptors was detected in neither smooth muscle (0 out of 16) nor EC (0 out of 16) samples, although the primers used consistently amplified transcripts from hamster brain (data not shown).
Analysis of transcript expression in whole arterioles showed that all samples (11 out of 11) were positive for α1A- and α1D-adrenoceptor message, whereas 7 of 11 samples were positive for α1B-adrenoceptors.
Lack of functional α1-adrenoceptors in isolated ECs
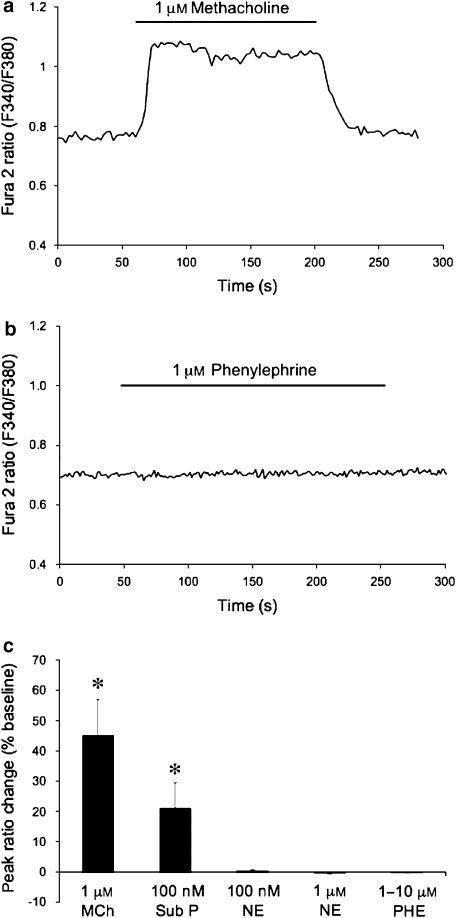
The transcript profiles noted above indicate that arteriolar ECs do not express α1-adrenoceptors. To functionally confirm this observation we assessed the effects of phenylephrine and noradrenaline on EC tubes isolated from hamster cremasteric arterioles. Figures 6a and b display typical results. As we have reported previously (Cohen and Jackson, 2005), enzymatically isolated EC tubes loaded with Fura 2 consistently respond to the endothelial agonists methacholine (1 μM; Figures 6a and c) or substance P (100 nM; Figure 6c) with a typical calcium transient. However, even at concentrations as high as 10 μM, neither phenylephrine (Figure 6c) nor noradrenaline (data not shown) had any effect on EC [Ca2+]in. Figure 6c displays a summary of these experiments.
Figure 6.
Ca2+-transients in isolated endothelial cells. (a and b) Fura 2 ratios from the same endothelial cell tube exposed to the endothelium-dependent agonist methacholine, or the α1-adrenergic agonist phenylephrine, as indicated. (c) Summary data for agonist-induced Ca2+-transients in endothelial cell tubes. Data are means±s.e. Peak Fura 2 ratios expressed as percentage change from baseline for methacholine (MCh, 1 μM, n=10), substance P (Sub P, 100 nM, n=8), noradrenaline (NE, 100 nM, n=5; 1 μM, n=8) and phenylephrine (PHE, 1–10 μM, n=10). Both MCh and Sub P consistently elicited Ca2+-transients (*P<0.05), whereas neither NE nor PHE at any concentration between 100 nM and 10 μM had any significant effect (P>0.05).
Discussion and conclusions
This study confirms that in intact arterioles, α1-adrenoceptor-mediated vasoconstriction is associated with an increase in EC [Ca2+]in. Furthermore, our data support the hypothesis that this α1-adrenoceptor-mediated rise in EC [Ca2+]in results from movement of a signal, either Ca2+, as originally postulated by Dora et al. (1997), and/or IP3, as suggested by others (Lamboley et al., 2005; Isakson et al., 2007), from smooth muscle cells to ECs, presumably via myoendothelial gap junctions (Sandow and Hill, 2000). Our data do not support the idea that α1-adrenoceptors located on ECs mediate this response. We found no evidence for expression of transcripts for α1-adrenoceptors in freshly isolated ECs, despite positive results for an equivalent number of smooth muscle cells isolated using identical methods. Moreover, we saw no evidence of α1-adrenoceptor-mediated Ca2+ signalling in freshly isolated EC tubes, confirming earlier studies in mesenteric ECs (Dora et al., 2000). This lack of EC response to α1-adrenergic agonists occurred despite consistent, robust Ca2+-transients induced by methacholine and substance P. We also found that smooth muscle cells, isolated by the same protocol, contract (Jackson et al., 1997; Cohen and Jackson, 2003) and display robust Ca2+-transients (Cohen and Jackson, 2003) when exposed to α1-adrenoceptor agonists. These data suggest that the lack of response of isolated ECs to α1-adrenoceptor agonists cannot be explained simply based on methodology. Therefore, we reject the hypothesis that α1-adrenergic agonists act directly on ECs to stimulate Ca2+-transients in intact arterioles.
We observed that the non-subtype selective α1-adrenoceptor antagonist prazosin potently inhibited phenylephrine-induced responses in cremasteric arterioles (Figures 1c and 2a). The near-complete inhibition of responses to phenylephrine, at concentrations up to 10 μM, suggests a very high affinity of this antagonist in cremasteric arterioles. Consistent with our findings of a high prazosin affinity, other investigators have reported pKB values for prazosin as high as 9.9 in rat aorta (Hussain and Marshall, 1997) and pKi in the range of 10.1–10.4 in cell systems expressing cloned α1D-adrenoceptors (Schwinn et al., 1995).
We propose that α1D-adrenoceptors predominantly mediate α1-adrenergic agonist-induced vasoconstriction in hamster cremasteric arterioles, and, by inference, the associated increase in EC [Ca2+]in. We show for the first time that α1D-adrenoceptor protein is in high abundance in hamster cremasteric arterioles, and that VSMCs, but not ECs from these vessels, contain mRNA for this adrenoceptor subtype. The pharmacological profile of phenylephrine-induced constriction of cremasteric arterioles further supports our conclusion for a major role for α1D-adrenoceptors. We found that BMY 7378 inhibited phenylephrine-induced constriction with a pKB of approximately 8.53. This pKB is well within the range of values reported in the literature for effects of BMY 7378 in rat aorta (Table 2), a tissue that predominantly expresses α1D-adrenoceptors (Marti et al., 2005). Our estimated pKB for BMY 7378 is also within the range of pKi values for this antagonist estimated in binding studies using cloned α1D-adrenoceptors in expression systems (Table 2). Furthermore, our estimated pKB=8.53 for BMY 7378 falls outside the range of values obtained by others in the studies of rat tail artery (Table 2), a tissue that predominantly expresses α1A-adrenoceptors (Marti et al., 2005), or values of pKi for BMY 7378 estimated using expression systems and cloned α1A-adrenoceptors (Table 2). Coupled with our finding of expression of mRNA for α1D-adrenoceptors in cremasteric VSMCs, and protein for α1D-adrenoceptors in whole-vessel homogenates, these data support the hypothesis that a significant portion of the response of hamster cremaster muscle arterioles to phenylephrine is mediated by α1D-adrenoceptors.
We also observed inhibition of phenylephrine-induced constriction with 5-methylurapidil, an α1A-adrenoceptor-selective antagonist. Comparison of our estimated pKB for this antagonist (8.39) with data in the literature from both functional and binding studies (Table 2) suggests that a portion of the response to phenylephrine may involve activation of α1A-adrenoceptors as well. This idea is supported by our consistent finding of α1A-adrenoceptor mRNA in cremasteric VSMCs (see Results). However, we were unable to detect protein for α1A-adrenoceptors in whole-arteriole homogenates, despite positive results in both hamster heart and brain. These data suggest a low level of expression of this adrenoceptor isoform in cremasteric arterioles. Given the close correspondence of our estimated pKB for BMY 7378 with data from both native tissue and cloned α1D-adrenoceptors (Table 2), the broad range of effects of 5-methylurapidil noted in the literature (Table 2), the high level of α1D-adrenoceptor protein expression and the apparent low level of expression of α1A-adrenoceptor protein in cremasteric arterioles, we suggest that α1D-adrenoceptors are dominant in this system. If both α1A- and α1D-adrenoceptors contributed equally, or α1A-adrenoceptors dominated, we would have expected the pKB for BMY 7378 to be substantially lower than what we found. For example, in rat iliac artery, where α1A- and α1D-adrenoceptor expression is approximately equal, the estimated pKB for BMY 7378 is 7.61 (Marti et al., 2005) (compare with values for rat aorta and rat tail artery shown in Table 2). Future studies will be required to resolve the precise contribution of α1A- and α1D-adrenoceptors in skeletal muscle arterioles.
Our findings for a major role for α1D-adrenoceptors in hamster cremasteric arterioles differ from the results obtained in rat mesenteric resistance arteries (Daly et al., 2002; Zacharia et al., 2004), human skeletal muscle (Jarajapu et al., 2001a) and cutaneous (Jarajapu et al., 2001b) resistance arteries where α1A-adrenoceptors appear to dominate. However, our results concur with the observations of Leech and Faber (1996) in first-order rat cremasteric arterioles that α1D-adrenoceptors appear to play a major role. Rat femoral arteries also appear to use α1D-adrenoceptors (Hrometz et al., 1999). Thus, there appear to be regional differences in α1-adrenoceptors expression and function with both α1D-adrenoceptors (Hosoda et al., 2005) and α1A-adrenoceptors (Rokosh and Simpson, 2002) contributing to the regulation of peripheral vascular resistance.
We can unequivocally eliminate a role for α1B-adrenoceptors in phenylephrine-induced changes in the arteriolar tone. The selective α1B-adrenergic antagonist L-765314, at a concentration (60 nM) that should occupy 90% of available α1B-adrenoceptors, had no effect on the concentration–response relationship to phenylephrine (Figure 3). Also, we detected no transcripts for α1B-adrenoceptors in cremasteric arteriolar smooth muscle cells or ECs. We did find immunoreactive bands for α1B-adrenoceptor protein in whole vessel homogenates using an antibody for this receptor. In addition, mRNA isolated from whole arterioles (smooth muscle, endothelial and other cells) was positive in 7 of 11 experiments. We interpret these data to indicate that cells, other than smooth muscle or endothelial, that are present in intact arterioles (fibroblasts, mast cells, immune cells, nerve cells, and so on) may express α1B-adrenoceptors, but that VSMCs and ECs in hamster cremaster muscle arterioles are devoid of these receptors. Thus, α1-adrenergic agonist-induced responses do not rely on α1B-adrenoceptors in hamster cremasteric arterioles. A similar conclusion has been drawn by Leech and Faber (1996) in rat cremasteric arterioles and from more recent studies using α1B-adrenoceptor knockout mice (Daly et al., 2002; Hosoda et al., 2005).
In summary, our data indicate that α1-adrenergic agonists interact with α1D-adrenoceptors, and we propose to a lesser extent with α1A-adrenoceptors, located on VSMCs, to mediate both vasoconstriction and the concomitant increase in EC [Ca2+]in. Our findings support the hypothesis that a signal from the smooth muscle cells is communicated to the ECs to cause the endothelial Ca2+-transient. We can only speculate on the nature of this signal and the pathway that it traverses. At face value, previous studies suggesting that Ca2+ (Dora et al., 1997) and/or IP3 (Lamboley et al., 2005; Isakson et al., 2007) appear reasonable. However, the observations of Tuttle and Falcone (2001) that only α1-adrenergic agonists (phenylephrine and noradrenaline) but not prostaglandin F2α or the α2-adrenergic agonist UK-14304 result in EC Ca2+-transients suggest that we do not yet know all the ‘rules' that govern signalling between smooth muscle cells and the underlying endothelium.
Acknowledgments
These studies were supported by Public Health Services Grant HL 32469 to William F Jackson and an American Heart Association Postdoctoral Fellowship to Kenneth D Cohen.
Abbreviations
- [Ca2+]in
concentration of intracellular Ca2+
- EC
endothelial cell
- VSMC
vascular smooth muscle cell
Conflict of interest
The authors state no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels, 3rd edn. Br J Pharmacol. 2008;153 Suppl 2:S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brekke JF, Jackson WF, Segal SS. Arteriolar smooth muscle Ca2+ dynamics during blood flow control in hamster cheek pouch. J Appl Physiol. 2006;101:307–315. doi: 10.1152/japplphysiol.01634.2005. [DOI] [PubMed] [Google Scholar]
- Burns WR, Cohen KD, Jackson WF. K+-induced dilation of hamster cremasteric arterioles involves both the Na+/K+-ATPase and inward-rectifier K+ channels. Microcirculation. 2004;11:279–293. doi: 10.1080/10739680490425985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll WA, Sippy KB, Esbenshade TA, Buckner SA, Hancock AA, Meyer MD. Two novel and potent 3-[(o-methoxyphenyl)piperazinylethyl]-5-phenylthieno[2,3-d]pyrimidine-2,4-diones selective for the α1D receptor. Bioorg Med Chem Lett. 2001;11:1119–1121. doi: 10.1016/s0960-894x(01)00159-7. [DOI] [PubMed] [Google Scholar]
- Cohen KD, Jackson WF. Hypoxia inhibits contraction but not calcium channel currents or changes in intracellular calcium in arteriolar muscle cells. Microcirculation. 2003;10:133–141. doi: 10.1038/sj/mn.7800178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen KD, Jackson WF. Membrane hyperpolarization is not required for sustained muscarinic agonist-induced increases in intracellular Ca2+ in arteriolar endothelial cells. Microcirculation. 2005;12:169–182. doi: 10.1080/10739680590904973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotecchia S, Schwinn DA, Randall RR, Lefkowitz RJ, Caron MG, Kobilka BK. Molecular cloning and expression of the cDNA for the hamster alpha 1-adrenergic receptor. Proc Natl Acad Sci USA. 1988;85:7159–7163. doi: 10.1073/pnas.85.19.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly CJ, Deighan C, McGee A, Mennie D, Ali Z, McBride M, et al. A knockout approach indicates a minor vasoconstrictor role for vascular alpha1B-adrenoceptors in mouse. Physiol Genomics. 2002;9:85–91. doi: 10.1152/physiolgenomics.00065.2001. [DOI] [PubMed] [Google Scholar]
- Deng XF, Chemtob S, Varma DR. Characterization of alpha 1 D-adrenoceptor subtype in rat myocardium, aorta and other tissues. Br J Pharmacol. 1996;119:269–276. doi: 10.1111/j.1476-5381.1996.tb15981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dora KA, Doyle MP, Duling BR. Elevation of intracellular calcium in smooth muscle causes endothelial cell generation of NO in arterioles. Proc Natl Acad Sci USA. 1997;94:6529–6534. doi: 10.1073/pnas.94.12.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dora KA, Hinton JM, Walker SD, Garland CJ. An indirect influence of phenylephrine on the release of endothelium-derived vasodilators in rat small mesenteric artery. Br J Pharmacol. 2000;129:381–387. doi: 10.1038/sj.bjp.0703052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone JC, Kuo L, Meininger GA. Endothelial cell calcium increases during flow-induced dilation in isolated arterioles. Am J Physiol Heart Circ Physiol. 1993;264:H653–H659. doi: 10.1152/ajpheart.1993.264.2.H653. [DOI] [PubMed] [Google Scholar]
- Filippi S, Parenti A, Donnini S, Granger HJ, Fazzini A, Ledda F. alpha(1D)-adrenoceptors cause endothelium-dependent vasodilatation in the rat mesenteric vascular bed. J Pharmacol Exp Ther. 2001;296:869–875. [PubMed] [Google Scholar]
- Ford AP, Daniels DV, Chang DJ, Gever JR, Jasper JR, Lesnick JD, et al. Pharmacological pleiotropism of the human recombinant alpha1A-adrenoceptor: implications for alpha1-adrenoceptor classification. Br J Pharmacol. 1997;121:1127–1135. doi: 10.1038/sj.bjp.0701207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisbert R, Madrero Y, Sabino V, Noguera MA, Ivorra MD, D'Ocon P. Functional characterization of alpha 1-adrenoceptor subtypes in vascular tissues using different experimental approaches: a comparative study. Br J Pharmacol. 2003;138:359–368. doi: 10.1038/sj.bjp.0705033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz AS, King HK, Ward SD, True TA, Rimele TJ, Saussy DL., Jr BMY 7378 is a selective antagonist of the D subtype of alpha 1-adrenoceptors. Eur J Pharmacol. 1995;272:R5–R6. doi: 10.1016/0014-2999(94)00751-r. [DOI] [PubMed] [Google Scholar]
- Gross G, Hanft G, Rugevics C. 5-Methyl-urapidil discriminates between subtypes of the alpha 1-adrenoceptor. Eur J Pharmacol. 1988;151:333–335. doi: 10.1016/0014-2999(88)90819-9. [DOI] [PubMed] [Google Scholar]
- Heijnen CJ, Rouppe van der Voort C, van de Pol M, Kavelaars A. Cytokines regulate alpha(1)-adrenergic receptor mRNA expression in human monocytic cells and endothelial cells. J Neuroimmunol. 2002;125:66–72. doi: 10.1016/s0165-5728(02)00034-6. [DOI] [PubMed] [Google Scholar]
- Hosoda C, Koshimizu TA, Tanoue A, Nasa Y, Oikawa R, Tomabechi T, et al. Two alpha1-adrenergic receptor subtypes regulating the vasopressor response have differential roles in blood pressure regulation. Mol Pharmacol. 2005;67:912–922. doi: 10.1124/mol.104.007500. [DOI] [PubMed] [Google Scholar]
- Hrometz SL, Edelmann SE, McCune DF, Olges JR, Hadley RW, Perez DM, et al. Expression of multiple alpha1-adrenoceptors on vascular smooth muscle: correlation with the regulation of contraction. J Pharmacol Exp Ther. 1999;290:452–463. [PubMed] [Google Scholar]
- Hussain MB, Marshall I. Characterization of alpha1-adrenoceptor subtypes mediating contractions to phenylephrine in rat thoracic aorta, mesenteric artery and pulmonary artery. Br J Pharmacol. 1997;122:849–858. doi: 10.1038/sj.bjp.0701461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakson BE, Ramos SI, Duling BR. Ca2+ and inositol 1,4,5-trisphosphate-mediated signaling across the myoendothelial junction. Circ Res. 2007;100:246–254. doi: 10.1161/01.RES.0000257744.23795.93. [DOI] [PubMed] [Google Scholar]
- Jackson WF, Huebner JM, Rusch NJ. Enzymatic isolation and characterization of single vascular smooth muscle cells from cremasteric arterioles. Microcirculation. 1997;4:35–50. doi: 10.3109/10739689709148316. [DOI] [PubMed] [Google Scholar]
- Jarajapu YP, Coats P, McGrath JC, Hillier C, MacDonald A. Functional characterization of alpha(1)-adrenoceptor subtypes in human skeletal muscle resistance arteries. Br J Pharmacol. 2001a;133:679–686. doi: 10.1038/sj.bjp.0704130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarajapu YP, Johnston F, Berry C, Renwick A, McGrath JC, MacDonald A, et al. Functional characterization of alpha1-adrenoceptor subtypes in human subcutaneous resistance arteries. J Pharmacol Exp Ther. 2001b;299:729–734. [PubMed] [Google Scholar]
- Kenny BA, Chalmers DH, Philpott PC, Naylor AM. Characterization of an alpha 1D-adrenoceptor mediating the contractile response of rat aorta to noradrenaline. Br J Pharmacol. 1995;115:981–986. doi: 10.1111/j.1476-5381.1995.tb15907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachnit WG, Tran AM, Clarke DE, Ford AP. Pharmacological characterization of an alpha 1A-adrenoceptor mediating contractile responses to noradrenaline in isolated caudal artery of rat. Br J Pharmacol. 1997;120:819–826. doi: 10.1038/sj.bjp.0700983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamboley M, Pittet P, Koenigsberger M, Sauser R, Beny J-L, Meister J-J. Evidence for signaling via gap junctions from smooth muscle to endothelial cells in rat mesenteric arteries: possible implication of a second messenger. Cell Calcium. 2005;37:311. doi: 10.1016/j.ceca.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Leech CJ, Faber JE. Different alpha-adrenoceptor subtypes mediate constriction of arterioles and venules. Am J Physiol. 1996;270:H710–H722. doi: 10.1152/ajpheart.1996.270.2.H710. [DOI] [PubMed] [Google Scholar]
- Marrelli SP. Selective measurement of endothelial or smooth muscle [Ca(2+)](i) in pressurized/perfused cerebral arteries with fura-2. J Neurosci Methods. 2000;97:145–155. doi: 10.1016/s0165-0270(00)00176-x. [DOI] [PubMed] [Google Scholar]
- Marti D, Miquel R, Ziani K, Gisbert R, Ivorra MD, Anselmi E, et al. Correlation between mRNA levels and functional role of alpha1-adrenoceptor subtypes in arteries: evidence of alpha1L as a functional isoform of the alpha1A-adrenoceptor. Am J Physiol Heart Circ Physiol. 2005;289:H1923–H1932. doi: 10.1152/ajpheart.00288.2005. [DOI] [PubMed] [Google Scholar]
- Patane MA, Scott AL, Broten TP, Chang RS, Ransom RW, DiSalvo J, et al. 4-Amino-2-[4-[1-(benzyloxycarbonyl)-2(S)-[[(1,1-dimethylethyl)amino]carbonyl]-piperazinyl]-6,7-dimethoxyquinazoline (L-765314): a potent and selective alpha1b adrenergic receptor antagonist. J Med Chem. 1998;41:1205–1208. doi: 10.1021/jm980053f. [DOI] [PubMed] [Google Scholar]
- Rokosh DG, Simpson PC. Knockout of the alpha 1A/C-adrenergic receptor subtype: the alpha 1A/C is expressed in resistance arteries and is required to maintain arterial blood pressure. Proc Natl Acad Sci USA. 2002;99:9474–9479. doi: 10.1073/pnas.132552699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res. 2000;86:341–346. doi: 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- Schuster A, Oishi H, Beny JL, Stergiopulos N, Meister JJ. Simultaneous arterial calcium dynamics and diameter measurements: application to myoendothelial communication. Am J Physiol Heart Circ Physiol. 2001;280:H1088–H1096. doi: 10.1152/ajpheart.2001.280.3.H1088. [DOI] [PubMed] [Google Scholar]
- Schwinn DA, Johnston GI, Page SO, Mosley MJ, Wilson KH, Worman NP, et al. Cloning and pharmacological characterization of human alpha-1 adrenergic receptors: sequence corrections and direct comparison with other species homologues. J Pharmacol Exp Ther. 1995;272:134–142. [PubMed] [Google Scholar]
- Tuttle JL, Falcone JC. Nitric oxide release during alpha1-adrenoceptor-mediated constriction of arterioles. Am J Physiol Heart Circ Physiol. 2001;281:H873–H881. doi: 10.1152/ajpheart.2001.281.2.H873. [DOI] [PubMed] [Google Scholar]
- Vinci MC, Bellik L, Filippi S, Ledda F, Parenti A. Trophic effects induced by alpha1D-adrenoceptors on endothelial cells are potentiated by hypoxia. Am J Physiol Heart Circ Physiol. 2007;293:H2140–H2147. doi: 10.1152/ajpheart.00390.2007. [DOI] [PubMed] [Google Scholar]
- Vinet R, Rojas F, Luxoro M, Vargas F, Cortes M. Catecholamines-evoked cytosolic Ca2+ rise in endothelial cells from bovine adrenal medulla. Mol Cell Biochem. 2000;203:53–58. doi: 10.1023/a:1007089415685. [DOI] [PubMed] [Google Scholar]
- Yashiro Y, Duling BR. Integrated Ca(2+) signaling between smooth muscle and endothelium of resistance vessels. Circ Res. 2000;87:1048–1054. doi: 10.1161/01.res.87.11.1048. [DOI] [PubMed] [Google Scholar]
- Yoshio R, Taniguchi T, Itoh H, Muramatsu I. Affinity of serotonin receptor antagonists and agonists to recombinant and native alpha1-adrenoceptor subtypes. Jpn J Pharmacol. 2001;86:189–195. doi: 10.1254/jjp.86.189. [DOI] [PubMed] [Google Scholar]
- Zacharia J, Hillier C, MacDonald A. Alpha1-adrenoceptor subtypes involved in vasoconstrictor responses to exogenous and neurally released noradrenaline in rat femoral resistance arteries. Br J Pharmacol. 2004;141:915–924. doi: 10.1038/sj.bjp.0705690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Zhang Y, Han C. Characterization of subtype of alpha1-adrenoceptor mediating vasoconstriction in perfused rat hind limb. Eur J Pharmacol. 1997;329:55–61. doi: 10.1016/s0014-2999(97)10104-2. [DOI] [PubMed] [Google Scholar]