Abstract
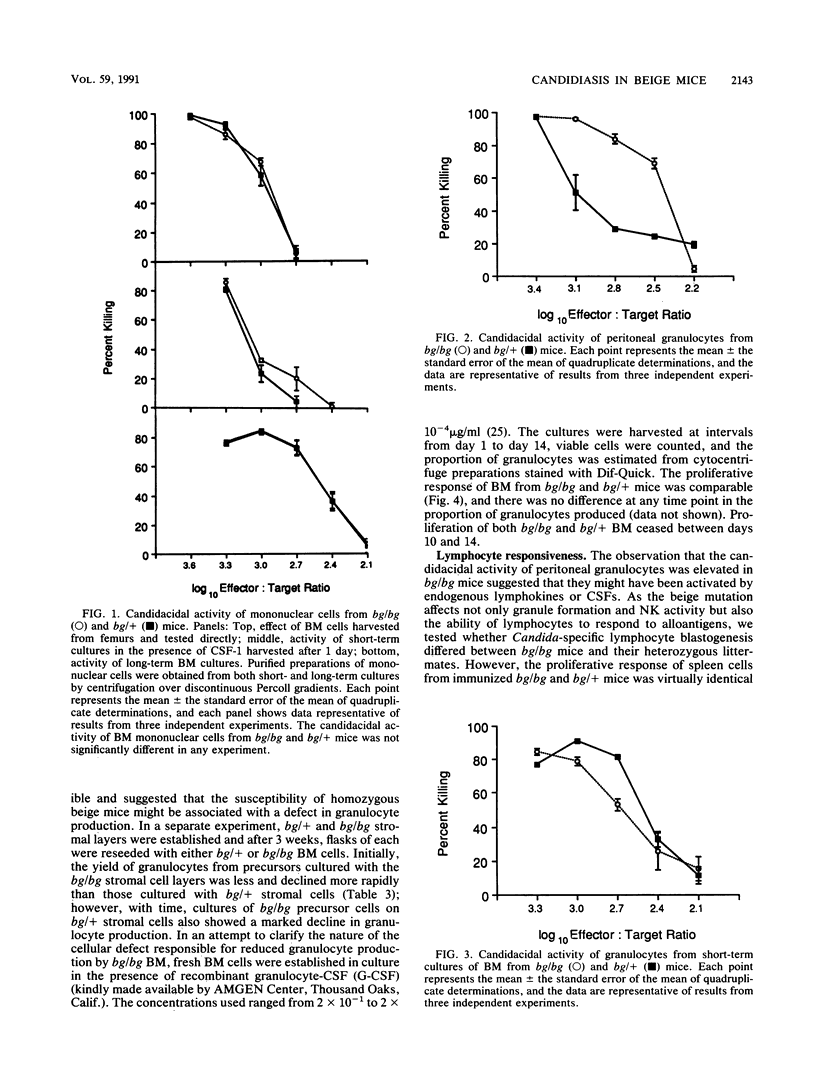
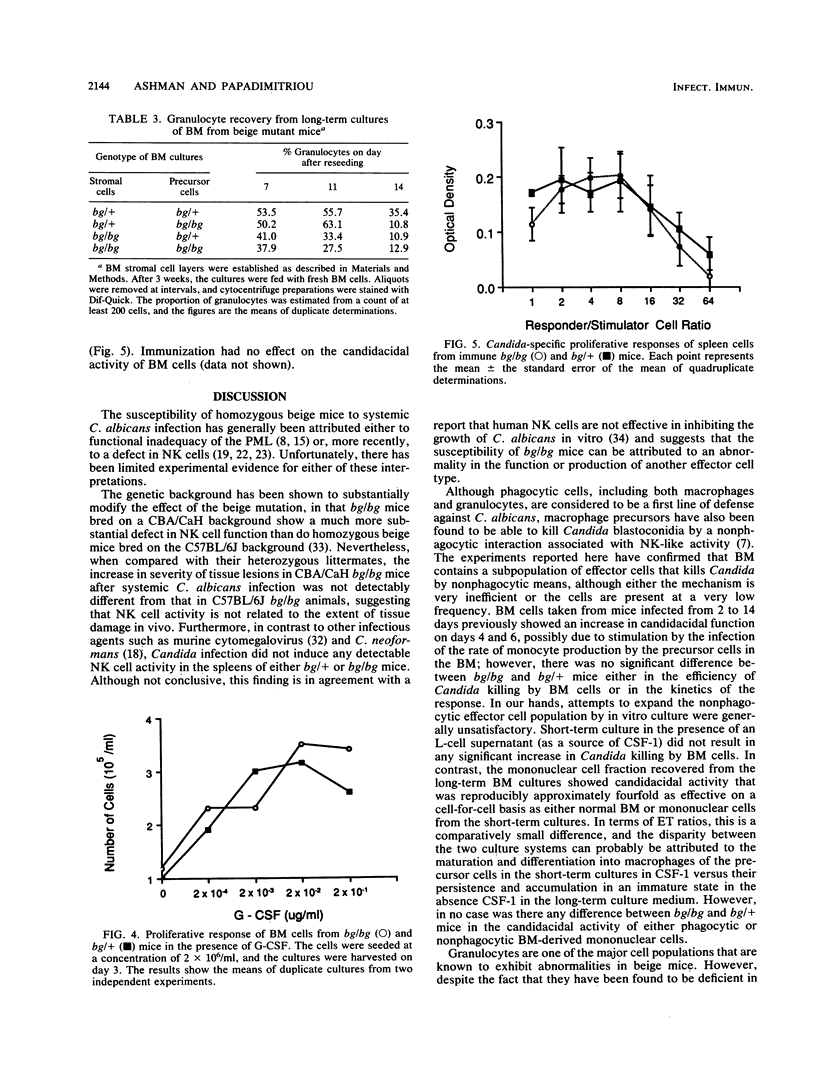
The beige mutation in mice has a pervasive effect on mechanisms of host resistance to infectious agents. Best characterized are defects in granulocyte chemotaxis and phagocytosis, which are associated with increased susceptibility to bacteria, and a deficiency in the levels of natural killer (NK) cells, which has been linked to decreased resistance to both murine cytomegalovirus and the yeast Cryptococcus neoformans. The objective of the present experiments was to explore the cellular basis of the enhanced susceptibility of beige mice to systemic infection with the yeast Candida albicans. In contrast to murine cytomegalovirus and C. neoformans, infection with C. albicans did not induce any detectable NK cell activity in the spleen of bg/bg or bg/+ mice. Unfractionated bone marrow (BM) displayed some candidacidal activity, mediated by both phagocytic and nonphagocytic cells; however, there was no difference between homozygous and heterozygous mice in the effector function of normal BM cells or mononuclear cells derived from either short- or long-term BM cultures. On the other hand, peritoneal granulocytes from bg/bg mice were significantly more effective than those from bg/+ mice in killing Candida blastoconidia in vitro. A similar comparison of granulocytes from short-term BM cultures showed that the activities of cells from bg/bg and bg/+ mice were equivalent, indicating that the granulocytes derived from the peritoneal cavity of bg/bg mice had probably been exposed to some form of nonspecific stimulation in vivo. Somewhat surprisingly, long-term BM cultures did not support the continual growth of bg/bg granulocytes, and it is possible that the beige mutation may be associated with a lesion in the differentiation pathway that leads to the production of granulocytes. Taken together, the data indicate that, in beige mice, granulocytes rather than NK cells are a major determinant of natural resistance to C. albicans infections.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashman R. B. A colorimetric method for measuring the candidacidal activity of leucocytes. J Immunol Methods. 1986 Nov 20;94(1-2):209–214. doi: 10.1016/0022-1759(86)90235-8. [DOI] [PubMed] [Google Scholar]
- Ashman R. B. Mouse candidiasis. II. Host responses are T-cell dependent and regulated by genes in the major histocompatibility complex. Immunogenetics. 1987;25(3):200–203. doi: 10.1007/BF00344035. [DOI] [PubMed] [Google Scholar]
- Ashman R. B., Papadimitriou J. M. Murine candidiasis. Pathogenesis and host responses in genetically distinct inbred mice. Immunol Cell Biol. 1987 Apr;65(Pt 2):163–171. doi: 10.1038/icb.1987.18. [DOI] [PubMed] [Google Scholar]
- Ashman R. B., Papadimitriou J. M. Murine candidiasis: strain dependence of host responses after immunization. Immunol Cell Biol. 1988 Jan;66(Pt 3):231–237. doi: 10.1038/icb.1988.29. [DOI] [PubMed] [Google Scholar]
- Ashman R. B., Papadimitriou J. M., Ott A. K., Warmington J. R. Antigens and immune responses in Candida albicans infection. Immunol Cell Biol. 1990 Feb;68(Pt 1):1–13. doi: 10.1038/icb.1990.1. [DOI] [PubMed] [Google Scholar]
- Ashman R. B., Papadimitriou J. M. What's new in the mechanisms of host resistance to Candida albicans infection? Pathol Res Pract. 1990 Aug;186(4):527–534. doi: 10.1016/S0344-0338(11)80477-2. [DOI] [PubMed] [Google Scholar]
- Baccarini M., Bistoni F., Lohmann-Matthes M. L. In vitro natural cell-mediated cytotoxicity against Candida albicans: macrophage precursors as effector cells. J Immunol. 1985 Apr;134(4):2658–2665. [PubMed] [Google Scholar]
- Baghian A., Lee K. W. Systemic candidosis in beige mice. J Med Vet Mycol. 1989;27(1):51–55. doi: 10.1080/02681218980000071. [DOI] [PubMed] [Google Scholar]
- Campbell P. A. The neutrophil, a professional killer of bacteria, may be controlled by T cells. Clin Exp Immunol. 1990 Feb;79(2):141–143. doi: 10.1111/j.1365-2249.1990.tb05169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci E., Romani L., Vecchiarelli A., Puccetti P., Bistoni F. Role of L3T4+ lymphocytes in protective immunity to systemic Candida albicans infection in mice. Infect Immun. 1989 Nov;57(11):3581–3587. doi: 10.1128/iai.57.11.3581-3587.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., Waller R. Activated macrophages in congenitally athymic "nude mice" and in lethally irradiate mice. J Immunol. 1975 Sep;115(3):844–847. [PubMed] [Google Scholar]
- Cutler J. E., Poor A. H. Effect of mouse phagocytes on Candida albicans in in vivo chambers. Infect Immun. 1981 Mar;31(3):1110–1116. doi: 10.1128/iai.31.3.1110-1116.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker T., Lohmann-Matthes M. L., Baccarini M. Heterogeneous activity of immature and mature cells of the murine monocyte-macrophage lineage derived from different anatomical districts against yeast-phase Candida albicans. Infect Immun. 1986 Nov;54(2):477–486. doi: 10.1128/iai.54.2.477-486.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. E., Jr, Rotrosen D., Fontaine J. W., Haudenschild C. C., Diamond R. D. Neutrophil-mediated protection of cultured human vascular endothelial cells from damage by growing Candida albicans hyphae. Blood. 1987 May;69(5):1450–1457. [PubMed] [Google Scholar]
- Elin R. J., Edelin J. B., Wolff S. M. Infection and immunoglobulin concentrations in Chediak-Higashi mice. Infect Immun. 1974 Jul;10(1):88–91. doi: 10.1128/iai.10.1.88-91.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin J. I., Bujak J. S., Patten E., Wolff S. M. Granulocyte function in the Chediak-Higashi syndrome of mice. Blood. 1974 Feb;43(2):201–206. [PubMed] [Google Scholar]
- Hart P. H., Spencer L. K., Kenny P. A., Lopez A. F., McDonald P. J., Finlay-Jones J. J. Functional neutrophils from long-term murine bone marrow cell cultures. J Immunol Methods. 1987 Jun 26;100(1-2):223–233. doi: 10.1016/0022-1759(87)90193-1. [DOI] [PubMed] [Google Scholar]
- Hidore M. R., Murphy J. W. Natural cellular resistance of beige mice against Cryptococcus neoformans. J Immunol. 1986 Dec 1;137(11):3624–3631. [PubMed] [Google Scholar]
- Krause M. W., Schaffner A. Comparison of immunosuppressive effects of cyclosporine A in a murine model of systemic candidiasis and of localized thrushlike lesions. Infect Immun. 1989 Nov;57(11):3472–3478. doi: 10.1128/iai.57.11.3472-3478.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez A. F., Nicola N. A., Burgess A. W., Metcalf D., Battye F. L., Sewell W. A., Vadas M. Activation of granulocyte cytotoxic function by purified mouse colony-stimulating factors. J Immunol. 1983 Dec;131(6):2983–2988. [PubMed] [Google Scholar]
- Lutzner M. A., Lowrie C. T., Jordan H. W. Giant granules in leukocytes of the beige mouse. J Hered. 1967 Nov-Dec;58(6):299–300. doi: 10.1093/oxfordjournals.jhered.a107620. [DOI] [PubMed] [Google Scholar]
- Marquis G., Montplaisir S., Pelletier M., Auger P., Lapp W. S. Genetics of resistance to infection with Candida albicans in mice. Br J Exp Pathol. 1988 Oct;69(5):651–660. [PMC free article] [PubMed] [Google Scholar]
- Marquis G., Montplaisir S., Pelletier M., Mousseau S., Auger P. Strain-dependent differences in susceptibility of mice to experimental candidosis. J Infect Dis. 1986 Nov;154(5):906–909. doi: 10.1093/infdis/154.5.906. [DOI] [PubMed] [Google Scholar]
- Mizushima Y., Morikage T., Yano S. Differences in in vitro proliferative responsiveness to granulocyte colony-stimulating factor and interleukin 2 of bone marrow cells from mice treated with chemotherapeutic drugs. Cancer Res. 1990 Mar 15;50(6):1847–1852. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Müller-Sieburg C. E., Townsend K., Weissman I. L., Rennick D. Proliferation and differentiation of highly enriched mouse hematopoietic stem cells and progenitor cells in response to defined growth factors. J Exp Med. 1988 Jun 1;167(6):1825–1840. doi: 10.1084/jem.167.6.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadimitriou J. M., Ashman R. B. The pathogenesis of acute systemic candidiasis in a susceptible inbred mouse strain. J Pathol. 1986 Dec;150(4):257–265. doi: 10.1002/path.1711500405. [DOI] [PubMed] [Google Scholar]
- Roder J., Duwe A. The beige mutation in the mouse selectively impairs natural killer cell function. Nature. 1979 Mar 29;278(5703):451–453. doi: 10.1038/278451a0. [DOI] [PubMed] [Google Scholar]
- Sasada M., Johnston R. B., Jr Macrophage microbicidal activity. Correlation between phagocytosis-associated oxidative metabolism and the killing of Candida by macrophages. J Exp Med. 1980 Jul 1;152(1):85–98. doi: 10.1084/jem.152.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena R. K., Saxena Q. B., Adler W. H. Defective T-cell response in beige mutant mice. Nature. 1982 Jan 21;295(5846):240–241. doi: 10.1038/295240a0. [DOI] [PubMed] [Google Scholar]
- Shellam G. R., Allan J. E., Papadimitriou J. M., Bancroft G. J. Increased susceptibility to cytomegalovirus infection in beige mutant mice. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5104–5108. doi: 10.1073/pnas.78.8.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellam G. R., Flexman J. P., Farrell H. E., Papadimitriou J. M. The genetic background modulates the effect of the beige gene on susceptibility to cytomegalovirus infection in mice. Scand J Immunol. 1985 Aug;22(2):147–155. doi: 10.1111/j.1365-3083.1985.tb01867.x. [DOI] [PubMed] [Google Scholar]
- Zunino S. J., Hudig D. Interactions between human natural killer (NK) lymphocytes and yeast cells: human NK cells do not kill Candida albicans, although C. albicans blocks NK lysis of K562 cells. Infect Immun. 1988 Mar;56(3):564–569. doi: 10.1128/iai.56.3.564-569.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


