Abstract
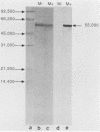
A synthetic 48-bp oligonucleotide specifying the N-terminal 15 amino acids of M protein of Streptococcus pyogenes type 5 (plus a CTA codon, to terminate translation of genes with the insert in reverse orientation) was inserted by blunt-end ligation at the site of the 48-bp EcoRV deletion in the Salmonella flagellin gene in plasmid pLS408 (S. M. C. Newton, C. O. Jacob, and B. A. D. Stocker, Science 244: 70-72, 1989). The resulting plasmid was transferred from Escherichia coli via a restriction-negative Salmonella typhimurium strain into an aromatic-compound-dependent, flagellin-negative live-vaccine strain of Salmonella dublin to produce strain SL7127, which was motile. Expression of the inserted epitope in flagellin and its exposure at the flagellar filament surface were shown by immunoblotting and by the reaction of flagellate bacteria (immobilization, immunogold labeling) with antibody raised by injection of the corresponding synthetic peptide, S-M5(1-15). Rabbits immunized by injection of the live-vaccine strain with flagella composed of the chimeric flagellin or by injection of concentrated flagella from such bacteria developed antibodies reactive in an enzyme-linked immunosorbent assay with peptide S-M5(1-15) and with the large peptic-digest peptide pepM5. These antibodies were opsonic for type 5 streptococci. Mice that were given parenteral live SL7127 (six doses, each 1 x 10(6) to 2 x 10(6), over 8 weeks) developed titers of ca. 12,800 for the M5-specific peptides and opsonizing activity for type 5 streptococci but not for type 24 streptococci. Sera from mice similarly immunized with a control live vaccine strain without an insert in the flagellin gene did not react with the M5-specific antigens. All of the five mice given the control strain, without an insert, died after challenge with type 5 streptococci or type 24 streptococci; by contrast, four of the five mice given strain SL7127, with an insert, survived the M5 challenge, but none of the five challenged with the type 24 strain survived. Therefore, our study shows that an M protein epitope can be expressed in the context of an unrelated protein and maintain its immunogenicity. Furthermore, we demonstrate that mice can be protected against a Streptococcus pyogenes type 5 challenge by immunization with a Salmonella live vaccine with flagella made of flagellin with an insert carrying a protective epitope of M5 protein but without the cross-reactive epitopes of the complete protein.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H., Seyer J. M., Dale J. B., Simpson W. A., Kang A. H. Type-specific protective immunity evoked by synthetic peptide of Streptococcus pyogenes M protein. Nature. 1981 Jul 30;292(5822):457–459. doi: 10.1038/292457a0. [DOI] [PubMed] [Google Scholar]
- Beachey E. H., Seyer J. M., Kang A. H. Primary structure of protective antigens of type 24 streptococcal M protein. J Biol Chem. 1980 Jul 10;255(13):6284–6289. [PubMed] [Google Scholar]
- Beachey E. H., Stollerman G. H., Chiang E. Y., Chiang T. M., Seyer J. M., Kang A. H. Purification and properties of M protein extracted from group A streptococci with pepsin: covalent structure of the amino terminal region of type 24 M antigen. J Exp Med. 1977 Jun 1;145(6):1469–1483. doi: 10.1084/jem.145.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Stollerman G. H., Johnson R. H., Bisno A. L., Ofek I. Immunogenicity in animals and man of a structurally defined polypeptide of streptococcal M protein. Trans Assoc Am Physicians. 1979;92:346–354. [PubMed] [Google Scholar]
- Beachey E. H., Tartar A., Seyer J. M., Chedid L. Epitope-specific protective immunogenicity of chemically synthesized 13-, 18-, and 23-residue peptide fragments of streptococcal M protein. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2203–2207. doi: 10.1073/pnas.81.7.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullas L. R., Ryu J. I. Salmonella typhimurium LT2 strains which are r- m+ for all three chromosomally located systems of DNA restriction and modification. J Bacteriol. 1983 Oct;156(1):471–474. doi: 10.1128/jb.156.1.471-474.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. B., Beachey E. H. Epitopes of streptococcal M proteins shared with cardiac myosin. J Exp Med. 1985 Aug 1;162(2):583–591. doi: 10.1084/jem.162.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. B., Beachey E. H. Protective antigenic determinant of streptococcal M protein shared with sarcolemmal membrane protein of human heart. J Exp Med. 1982 Oct 1;156(4):1165–1176. doi: 10.1084/jem.156.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. B., Seyer J. M., Beachey E. H. Type-specific immunogenicity of a chemically synthesized peptide fragment of type 5 streptococcal M protein. J Exp Med. 1983 Nov 1;158(5):1727–1732. doi: 10.1084/jem.158.5.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971 Sep;8(9):871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- Joys T. M., Martin J. F. Identification of amino acid changes in serological mutants of the i flagellar antigen of Salmonella typhimurium. Microbios. 1973;7(25):71–73. [PubMed] [Google Scholar]
- KAPLAN M. H. IMMUNOLOGIC RELATION OF STREPTOCOCCAL AND TISSUE ANTIGENS. I. PROPERTIES OF AN ANTIGEN IN CERTAIN STRAINS OF GROUP A STREPTOCOCCI EXHIBITING AN IMMUNOLOGIC CROSS-REACTION WITH HUMAN HEART TISSUE. J Immunol. 1963 Apr;90:595–606. [PubMed] [Google Scholar]
- KAPLAN M. H., MEYESERIAN M. An immunological cross-reaction between group-A streptococcal cells and human heart tissue. Lancet. 1962 Apr 7;1(7232):706–710. doi: 10.1016/s0140-6736(62)91653-7. [DOI] [PubMed] [Google Scholar]
- Kaplan M. H. Cross-reaction of group A streptococci and heart tissue: varying serologic specificity of cross-reactive antisera and relation to carrier-hapten specificity. Transplant Proc. 1969 Dec;1(4):976–980. [PubMed] [Google Scholar]
- LANCEFIELD R. C. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962 Sep;89:307–313. [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyampert I. M., Vvedenskaya O. I., Danilova T. A. Study on streptococcus group A antigens common with heart tissue elements. Immunology. 1966 Oct;11(4):313–320. [PMC free article] [PubMed] [Google Scholar]
- Newton S. M., Jacob C. O., Stocker B. A. Immune response to cholera toxin epitope inserted in Salmonella flagellin. Science. 1989 Apr 7;244(4900):70–72. doi: 10.1126/science.2468182. [DOI] [PubMed] [Google Scholar]
- Phillips G. N., Jr, Flicker P. F., Cohen C., Manjula B. N., Fischetti V. A. Streptococcal M protein: alpha-helical coiled-coil structure and arrangement on the cell surface. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4689–4693. doi: 10.1073/pnas.78.8.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier T. P., Kehoe M. A., Beachey E. H. Protective immunity evoked by oral administration of attenuated aroA Salmonella typhimurium expressing cloned streptococcal M protein. J Exp Med. 1988 Jul 1;168(1):25–32. doi: 10.1084/jem.168.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigwart D. F., Stocker B. A., Clements J. D. Effect of a purA mutation on efficacy of Salmonella live-vaccine vectors. Infect Immun. 1989 Jun;57(6):1858–1861. doi: 10.1128/iai.57.6.1858-1861.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Hsu K. C., Gotschlich E. C. Electron microscopic studies on streptococci. I. M antigen. J Exp Med. 1969 Nov 1;130(5):1063–1091. doi: 10.1084/jem.130.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitnack E., Beachey E. H. Antiopsonic activity of fibrinogen bound to M protein on the surface of group A streptococci. J Clin Invest. 1982 Apr;69(4):1042–1045. doi: 10.1172/JCI110508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitnack E., Beachey E. H. Inhibition of complement-mediated opsonization and phagocytosis of Streptococcus pyogenes by D fragments of fibrinogen and fibrin bound to cell surface M protein. J Exp Med. 1985 Dec 1;162(6):1983–1997. doi: 10.1084/jem.162.6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. Y., Newton S., Judd A., Stocker B., Robinson W. S. Expression of immunogenic epitopes of hepatitis B surface antigen with hybrid flagellin proteins by a vaccine strain of Salmonella. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4726–4730. doi: 10.1073/pnas.86.12.4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabriskie J. B., Freimer E. H. An immunological relationship between the group. A streptococcus and mammalian muscle. J Exp Med. 1966 Oct 1;124(4):661–678. doi: 10.1084/jem.124.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabriskie J. B., Hsu K. C., Seegal B. C. Heart-reactive antibody associated with rheumatic fever: characterization and diagnostic significance. Clin Exp Immunol. 1970 Aug;7(2):147–159. [PMC free article] [PubMed] [Google Scholar]
- van de Rijn I., Zabriskie J. B., McCarty M. Group A streptococcal antigens cross-reactive with myocardium. Purification of heart-reactive antibody and isolation and characterization of the streptococcal antigen. J Exp Med. 1977 Aug 1;146(2):579–599. doi: 10.1084/jem.146.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]