Abstract
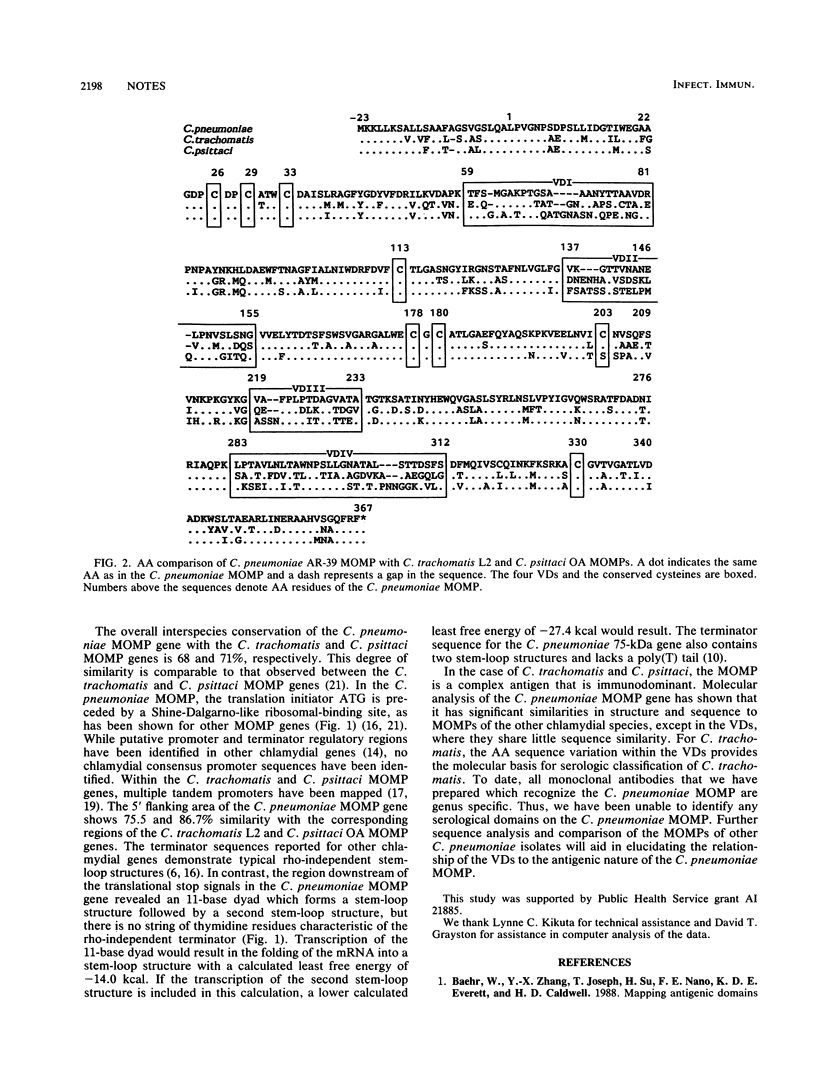
Compared with the major outer membrane proteins (MOMPs) of the other chlamydial species, the Chlamydia pneumoniae MOMP appears to be less antigenically complex, and as determined by immunoblot analysis, it does not appear to be the immunodominant antigen recognized during infection. Nucleotide sequence analysis of the C. pneumoniae MOMP gene (ompA) revealed that it consisted of a 1,167-base open reading frame with an inferred 39,344-dalton mature protein of 366 amino acids plus a 23-amino-acid leader sequence. A ribosomal-binding site was located in the 5' upstream region, and two stop codons followed by an 11-base dyad forming a stable stem-loop structure were identified. This sequence shares 68 and 71% DNA sequence homology to the Chlamydia trachomatis serovar L2 and Chlamydia psittaci ovine abortion agent MOMP genes, respectively. Interspecies alignment identified regions, corresponding to the variable domains, which share little sequence similarity with the other chlamydial MOMPs. All seven cysteines conserved in the C. trachomatis and C. psittaci MOMPs, which are involved in the formation of disulfide cross-linkages, are found in the C. pneumoniae MOMP.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehr W., Zhang Y. X., Joseph T., Su H., Nano F. E., Everett K. D., Caldwell H. D. Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4000–4004. doi: 10.1073/pnas.85.11.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavoil P., Ohlin A., Schachter J. Role of disulfide bonding in outer membrane structure and permeability in Chlamydia trachomatis. Infect Immun. 1984 May;44(2):479–485. doi: 10.1128/iai.44.2.479-485.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L. A., Kuo C. C., Grayston J. T. Structural and antigenic analysis of Chlamydia pneumoniae. Infect Immun. 1990 Jan;58(1):93–97. doi: 10.1128/iai.58.1.93-97.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L. A., Kuo C. C., Wang S. P., Grayston J. T. Serological response to Chlamydia pneumoniae infection. J Clin Microbiol. 1990 Jun;28(6):1261–1264. doi: 10.1128/jcm.28.6.1261-1264.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlan J. W., Clarke I. N., Ward M. E. Epitope mapping with solid-phase peptides: identification of type-, subspecies-, species- and genus-reactive antibody binding domains on the major outer membrane protein of Chlamydia trachomatis. Mol Microbiol. 1988 Sep;2(5):673–679. doi: 10.1111/j.1365-2958.1988.tb00076.x. [DOI] [PubMed] [Google Scholar]
- Danilition S. L., Maclean I. W., Peeling R., Winston S., Brunham R. C. The 75-kilodalton protein of Chlamydia trachomatis: a member of the heat shock protein 70 family? Infect Immun. 1990 Jan;58(1):189–196. doi: 10.1128/iai.58.1.189-196.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayston J. T., Campbell L. A., Kuo C. C., Mordhorst C. H., Saikku P., Thom D. H., Wang S. P. A new respiratory tract pathogen: Chlamydia pneumoniae strain TWAR. J Infect Dis. 1990 Apr;161(4):618–625. doi: 10.1093/infdis/161.4.618. [DOI] [PubMed] [Google Scholar]
- Hackstadt T., Todd W. J., Caldwell H. D. Disulfide-mediated interactions of the chlamydial major outer membrane protein: role in the differentiation of chlamydiae? J Bacteriol. 1985 Jan;161(1):25–31. doi: 10.1128/jb.161.1.25-31.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornak J. M., Kuo C. C., Campbell L. A. Sequence analysis of the gene encoding the Chlamydia pneumoniae DnaK protein homolog. Infect Immun. 1991 Feb;59(2):721–725. doi: 10.1128/iai.59.2.721-725.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Chen H. H., Wang S. P., Grayston J. T. Identification of a new group of Chlamydia psittaci strains called TWAR. J Clin Microbiol. 1986 Dec;24(6):1034–1037. doi: 10.1128/jcm.24.6.1034-1037.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardinia L. M., Engel J. N., Ganem D. Chlamydial gene encoding a 70-kilodalton antigen in Escherichia coli: analysis of expression signals and identification of the gene product. J Bacteriol. 1989 Jan;171(1):335–341. doi: 10.1128/jb.171.1.335-341.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Kuo C. C., Newport G., Agabian N. Molecular cloning and expression of Chlamydia trachomatis major outer membrane protein antigens in Escherichia coli. Infect Immun. 1985 Mar;47(3):713–718. doi: 10.1128/iai.47.3.713-718.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Sanchez-Pescador R., Wagar E. A., Inouye C., Urdea M. S. Diversity of Chlamydia trachomatis major outer membrane protein genes. J Bacteriol. 1987 Sep;169(9):3879–3885. doi: 10.1128/jb.169.9.3879-3885.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Wagar E. A., Edman U. Developmental regulation of tandem promoters for the major outer membrane protein gene of Chlamydia trachomatis. J Bacteriol. 1988 Feb;170(2):744–750. doi: 10.1128/jb.170.2.744-750.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Wagar E. A., Schoolnik G. K. High-resolution mapping of serovar-specific and common antigenic determinants of the major outer membrane protein of Chlamydia trachomatis. J Exp Med. 1988 Mar 1;167(3):817–831. doi: 10.1084/jem.167.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y., Zhang Y. X., Manning D. S., Caldwell H. D. Multiple tandem promoters of the major outer membrane protein gene (omp1) of Chlamydia psittaci. Infect Immun. 1990 Sep;58(9):2850–2855. doi: 10.1128/iai.58.9.2850-2855.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y., Zhang Y. X., Watkins N. G., Caldwell H. D. Nucleotide and deduced amino acid sequences for the four variable domains of the major outer membrane proteins of the 15 Chlamydia trachomatis serovars. Infect Immun. 1989 Apr;57(4):1040–1049. doi: 10.1128/iai.57.4.1040-1049.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X., Morrison S. G., Caldwell H. D., Baehr W. Cloning and sequence analysis of the major outer membrane protein genes of two Chlamydia psittaci strains. Infect Immun. 1989 May;57(5):1621–1625. doi: 10.1128/iai.57.5.1621-1625.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X., Stewart S. J., Caldwell H. D. Protective monoclonal antibodies to Chlamydia trachomatis serovar- and serogroup-specific major outer membrane protein determinants. Infect Immun. 1989 Feb;57(2):636–638. doi: 10.1128/iai.57.2.636-638.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


