Abstract
Objective
Prospective studies have reported a positive association of coagulation factors with risk of coronary heart disease (CHD). It is unclear whether these coagulation factors interact.
Methods and Results
Using a prospective case-cohort design, we analyzed by Cox proportional hazard regression interactions between soluble thrombomodulin (sTM) and fibrinogen, factor VIII (FVIII), FVII, or plasminogen activator inhibitor-1 (PAI-1) in 410 CHD cases and 721 non-cases from the Atherosclerosis Risk in Communities (ARIC). There was a significant interaction between sTM and fibrinogen (p=0.027). We next assessed risk ratios (RR) by combined tertile analysis. Combined analysis revealed that being in the upper sTM tertile counteracted the CHD risk imposed by higher fibrinogen whereas being in the lower sTM tertile amplified the CHD risk of higher fibrinogen. sTM and fibrinogen mutually influenced CHD incidence in a concentration-dependent manner. When analyzed as single factors by tertiles, FVIII, FVII and PAI-1 were not associated with CHD. However, when analyzed together with sTM, FVIII and PAI-1 were both positively associated with CHD for those in the lower sTM tertile.
Conclusion
There is a complex interaction between sTM and prothrombotic coagulation factors. Combined analysis improves CHD risk assessment.
Keywords: soluble thrombomodulin, fibrinogen, factor VIII, PAI-1, coronary heart disease
INTRODUCTION
Thrombosis that develops at the ruptured atheromatous plaque surface is considered to cause acute coronary syndromes. The plaque rupture exposes collagen, von Willebrand factor (vWF) and tissue factor to circulating platelets and coagulation proteins. Platelets adhere to the damaged vascular wall, form aggregates and provide an active surface for rapid coagulation reactions.1 A number of coagulation factors, including factor VIII (FVIII) and fibrinogen, are required for the cascade of biochemical reactions leading to fibrin formation. The platelet aggregates are eventually enmeshed in fibrin fibriles, forming an expanding thrombus that occludes the coronary artery, causing myocardial ischemia and infarction. Normal endothelium generates several active molecules to prevent and dissolve platelet aggregate and fibrin formation. One of the important molecules, thrombomodulin (TM), serves as a scaffold for thrombin to activate protein C which, in the presence of protein S, degrades FVa and FVIIIa, thereby restraining the coagulation reactions and restricting fibrin formation. Reduction in TM and other protective molecules as a consequence of endothelial denudation and dysfunction at the atheromatous plaque results in uncontrolled thrombus formation. In addition to being an anticoagulant, TM also participates in the regulation of fibrinolysis.2,3 TM can mediate both anti- and profibrinolytic effects depending on its concentration. At relative low TM concentrations, fibrinolysis is downregulated by stimulation of antifibrinolytic TAFI (thrombin-activable fibrinolysis inhibitor) activation, whereas the profibrinolytic effects become more pronounced at relative high concentrations of TM by the generation of the activated Protein C (APC), which inhibits TAFI activation.4 In addition, APC can stimulate fibrinolysis by forming a complex with antifibrinolytic plasminogen activator inhibitor 1 (PAI-1), leading to its inactivation. PAI-1 is one of the key inhibitors of the fibrinolytic system.5
There have been long-term interests in determining the association of quantitative and functional changes in the circulating platelet and coagulation components of thrombosis with coronary heart disease (CHD). Despite numerous reports on the association of plasma thrombotic factors with myocardial infarction, only a small number of factors are validated by prospective studies to be independently associated with and considered as independent risk factors for CHD. Among them, plasma fibrinogen levels have been shown by several prospective studies to be independently associated with CHD.6–9 Plasma soluble TM (sTM) shedded from endothelial TM is inversely associated with risk of CHD.10 Several coagulation and fibrinolytic factors including FVIII, FVII and PAI-1 were reported to be associated with CHD in some studies but not in others9, 11, 12 and their associations with CHD are considered to be weak. We postulated that as thrombus formation is governed by an interaction between prothrombotic factors and antithrombotic molecules, analysis of association with an individual factor, be it prothrombotic or antithrombotic, has a relatively low sensitivity which will be enhanced by combined analysis of prothrombotic and anticoagulant factors. Results from previous Atherosclerosis Risk in Communities (ARIC) analyses indicated that soluble intercellular adhesion molecule-1 (sICAM-1), a marker of endothelial inflammation, and sTM levels are associated with risk of CHD in opposite direction. Applying combined analysis of sTM and sICAM-1, the ARIC study provided novel information regarding the interaction of sTM and sICAM-1 in predicting CHD risk, showing that the upper tertile sICAM had a significant increase in the risk of a CHD event only when sTM was in the lowest tertile.13 To better understand the interaction between antithrombotic and prothrombotic plasma factors, we performed analysis of CHD risk in the ARIC prospective case-cohort study using a combinatorial approach.
METHODS
Study Population
The ARIC study recruited a population-based cohort of men and women 45 to 64 years of age from four U.S. communities in 1987 through 1989.14 A total of 15,792 participants completed a home interview and baseline clinic examination. The participants were re-examined on a 3-year cycle: 1990 to 1992 (93% return rate); 1993 to 1995 (86% return rate); and 1996 to 1998 (80% return rate). These participants have been prospectively followed for development of CHD since enrollment. ARIC followed the cohort and ascertained CHD events using standardized methods described previously.15 We defined CHD incident cases as (1) a definite or probable myocardial infarction (MI); (2) a silent MI; (3) a definite CHD death; or (4) a coronary revascularization.16
For the nested case-cohort studies, a random sample of the entire cohort was selected to serve as reference by a procedure previously described.17 There were eight strata for sampling the cohort and recreating the original frequency distribution of the strata in the entire cohort. Excluded from the analysis were participants who were neither white nor black, had prevalent CHD at baseline, or had a history of stroke or transient ischemic attacks. For this analysis, we included CHD cases that occurred between the initial visit (1987–1989) and December 31, 2000. The mean follow-up period was 11.4 years. After exclusions, the final sample contained 410 cases and 721 in the reference cohort designated as non-cases.
Baseline measurements
The methods of baseline measurements during the first ARIC clinic visit have been described previously.9 Blood collection and processing were performed according to standardized procedures.18,19 Plasma and serum samples were stored at −80°C until assay. Fibrinogen, FVIII coagulant activity (FVIIIc), and FVII coagulant activity (FVIIc), were measured on citrated plasma prepared from the entire cohort during the initial clinical visit, according to procedures described previously.9 Briefly, fibrinogen was measured by Clauss method with reagents from General Diagnostics (Organon Technika Co., Morris Plains, NJ); FVIIc and FVIIIc were assessed with relevant human factor deficient plasmas (George King Biomedical Inc., Overland Park, Kansas). The level of factors was determined from a standard curve constructed from freeze-dried reference plasma (Pacific Hemostasis, Houston, Texas), and was expressed as percentage of reference plasma. PAI-1antigen, sTM, and CRP were measured using ELISA kits (Imubind PAI-1, American Diagnostica, Asserachrom TM, Diagnostica Stago, and CRP-United Biotech Magiwel, CA, respectively).10,12,20 The intra-assay and intra-individual coefficients of variation (CVs) of each assay were systematically evaluated.20,21
Data analysis
We used a case-cohort analysis.22,23 ANCOVA was used to compute age-, race- and sex-adjusted mean values of sTM, fibrinogen, FVIIc, FVIIIc, and PAI-1 for CHD cases and non-cases after appropriate weighting for the stratified sampling design. We also used ANCOVA to compute age-, race- and sex-adjusted mean or percentage values of study variables according to the upper, middle and lower tertiles of sTM and other parameters in the cohort sample after appropriate weighting for the stratified sampling design. We computed risk ratios (RR) and 95% confidence intervals (CI) for the time to the development of CHD in relation to sTM and coagulation parameters using a Cox proportional hazard regression analysis as previously described.13 Multiplicative interaction of sTM with fibrinogen, FVIIc, FVIIIc or PAI-1 was determined by Cox proportional hazard regression. We trichotomized the hemostatic variables to determine the RR for the upper tertile sTM/lower tertile coagulation parameters (reference group) versus eight other combined tertile groups. Tertiles were chosen to show dose-response in three categories of sTM by three categories of another coagulation factor (nine total categories), without having too many categories with small numbers in each that could make interpretation difficult. We examined if the baseline characteristics were significantly different between the reference and comparison groups, with a Bonferroni correction to adjust for the effect of multiple group comparisons. A p-value less than 0.0062 (=0.05/8) was considered statistically significant.
RESULTS
We analyzed by tertile the association of each parameter with CHD risk. As shown in Table 1, sTM was associated inversely with CHD incidence (RR=0.43 for middle tertile and 0.27 for upper tertile). By contrast, fibrinogen was positively associated with CHD incidence with a RR of 2.19 for the upper versus lower tertile. Neither FVIIIc nor PAI-1in the middle or upper tertiles significantly increased CHD risk when compared to the lower tertiles.
Table 1.
Risk ratios of CHD according to tertiles of sTM, fibrinogen, factor VIIIc and PAI-1ag
| Parameter | RR (95% CI)§ | ||
|---|---|---|---|
| Lower tertile | Middle tertile | Upper tertile | |
| sTM* | 1 | 0.43 (0.24–0.75) | 0.27 (0.16–0.48) |
| Fibrinogen* | 1 | 1.04 (0.58–1.84) | 2.19 (1.32–3.64) |
| Factor VIIIc* | 1 | 1.30 (0.74–2.26) | 0.67 (0.39–1.14) |
| PAI-1* | 1 | 0.68 (0.38–1.23) | 1.09 (0.60–1.98) |
Risk ratio and 95% confidence interval (CI) for the time to CHD development after adjusting for age, sex, race, hypertension, diabetes mellitus, total cholesterol, HDL, cigarette smoking and alcohol intake
sTM: lower tertile < 28.3 ng/ml, and upper tertile ≥ 43.2 ng/ml; Fibrinogen: lower tertile < 269 mg/dl, and upper tertile ≥ 316 mg/dl; Factor VIIIc: lower tertile < 114%, and upper tertile ≥ 143%; PAI-1ag: lower tertile ≤ 10.7 ng/ml, and upper tertile ≥ 23.4 ng/ml.
We next analyzed the interaction between sTM and fibrinogen by Cox proportional hazard regression. For comparison, we also analyzed the interaction between sTM and FVIIIc, FVIIc or PAI-1. There was a significant interaction between sTM and fibrinogen (p=0.027) and between sTM and FVIIIc (p=0.029), but not with PAI-1 or FVIIc.
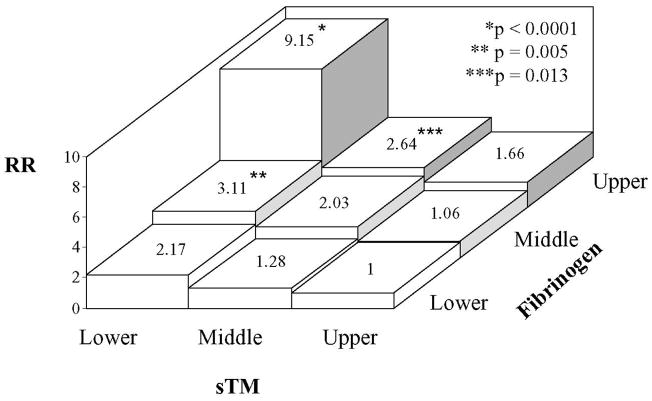
To evaluate the influence of the sTM-fibrinogen interaction on CHD risk assessment, we divided the cases and cohort reference into nine groups based on combined sTM and fibrinogen tertiles (Table 2). We chose the upper sTM/lower fibrinogen tertile as the reference group as this group theoretically has the lowest CHD risk. The basic group characteristics were generally comparable with some notable differences indicated in Table 2. The adjusted risk ratio of CHD for Groups 2–9 vs. Group 1 is shown in Fig. 1. The risk ratio for Group 9 (lower sTM/upper fibrinogen tertile) was highly elevated (RR=9.15, 95% CI 4.04–20.72). As Group 9 has a higher percentage of women, we attempted to repeat the analysis in women. However, the female cases were few and the sample size was too small to obtain a valid analysis. Group 9 had a low level of triglycerides and we made additional adjustment by body mass index (BMI), which did not alter the RR of any of the eight groups (see Supplemental Fig. 1). Since inflammation may be an important confounding factor, we recomputed the RR adjusted for C-reactive protein (CRP). The RR for Group 9 (lower sTM/upper fibrinogen tertile) was 11.9 after adjustment, which was not significantly different from that without CRP adjustment. The RR of other groups was not changed (see Supplemental Fig. 2). As shown in Fig. 1, the risk ratios were also increased for Group 6 (lower sTM/middle fibrinogen tertile): RR=3.11, 95% CI 1.40–6.89, and Group 8 (middle sTM/upper fibrinogen): RR=2.64, 95% CI 1.23–5.69. Interestingly, the CHD risk ratio for group 7 (upper sTM/upper fibrinogen) was not significantly increased (RR=1.66, 95% CI 0.75–3.64) despite involving an upper fibrinogen tertile. The data suggest that the increased CHD risk of high fibrinogen levels was neutralized by high levels of sTM. On the other hand, the risk of elevated fibrinogen was augmented by low levels of sTM.
Table 2.
Baseline characteristics of groups of participants according to combined sTM and fibrinogen tertiles
| Group 1* (UT/LF)§ (n=87) | Group 2 (MT/LF) (n=90) | Group 3 (LT/LF) (n=86) | Group 4 (UT/MF) (n=81) | Group 5 (MT/MF) (n=89) | Group 6 (LT/MF) (n=89) | Group 7 (UT/UF) (n=99) | Group 8 (MT/UF) (n=83) | Group 9 (LT/UF) (n=87) | |
|---|---|---|---|---|---|---|---|---|---|
| Age, yrs | 52.3 | 52.3 | 51.7 | 55.9 | 53.8 | 53.7 | 57.5+ | 54.0 | 54.0 |
| Men, % | 60.0 | 47.3 | 29.9 | 45.0 | 50.2 | 35.4 | 45.6 | 43.9 | 20.7 |
| Afr. American, % | 1.7 | 11.6 | 27.6+ | 21.7 | 35.2+ | 29.4+ | 29.1 | 36.1+ | 32.7+ |
| SBP, mmHg | 122.5 | 117.5 | 119.5 | 120.7 | 118.4 | 121.2 | 126.7 | 123.0 | 116.4 |
| TC, mg/dl | 217.9 | 219.9 | 219.9 | 212.7 | 208.1 | 209.7 | 213.7 | 212.2 | 206.4 |
| HDL-C, mg/dl | 55.6 | 55.5 | 59.4 | 51.5 | 51.7 | 58.3 | 49.8 | 48.8 | 51.1 |
| TG, mg/dl | 130.4 | 110.7 | 118.8 | 139.5 | 128.9 | 116.2 | 142.1 | 119.1 | 102.6 |
| Cigarette-years | 104.4 | 267.8 | 303.0 | 295.7 | 254.4 | 271.9 | 370.7 | 403.6 | 434.4+ |
| Ethanol intake, gr/wk | 19.6 | 76.5 | 84.5+ | 25.9 | 21.3 | 42.9 | 15.9 | 42.8 | 37.9 |
| WBC, × 103/μL | 5.5 | 5.5 | 5.3 | 5.8 | 6.5 | 6.1 | 6.8+ | 6.9+ | 6.9+ |
| VWF, % | 107.2 | 112.4 | 105.9 | 118.1 | 118.5 | 116.8 | 145.9+ | 138.2+ | 130.8 |
| Hypertension, % | 40.4 | 19.0 | 41.9 | 32.6 | 27.5 | 19.8 | 32.9 | 37.6 | 11.3 |
| Diabetes, % | 3.1 | 1.8 | 0.5 | 1.3 | 2.0 | 2.7 | 6.2 | 4.8 | 3.8 |
Reference group.
UT, MT and LT, denote upper, middle and lower sTM tertiles, respectively; UF, MF and LF, upper, middle and lower fibrinogen tertiles, respectively.
Statistically significant (p<0.0062) compared with Group 1
SBP denotes systolic blood pressure; TC, total cholesterol; HDL-C, high density lipoprotein cholesterol; TG, triglyceride.
Diabetes denotes fasting glucose greater than or equal to 140 mg/dL or nonfasting glucose greater or equal to 200 mg/dL or physician diagnosed diabetes or pharmacologic treatment for diabetes. Hypertension defined as systolic BP ≥ 140 mmHg or diastolic BP ≥ 90 mmHg or current use of antihypertensive medications.
Fig. 1.
Risk ratio (RR) of CHD for participants with various combinations of sTM and fibrinogen tertiles vs. upper tertile sTM/lower tertile fibrinogen as the reference group. Adjustment was made for age, sex, race and cardiovascular risk factors listed in Table 1. p values refer to comparison with the reference group.
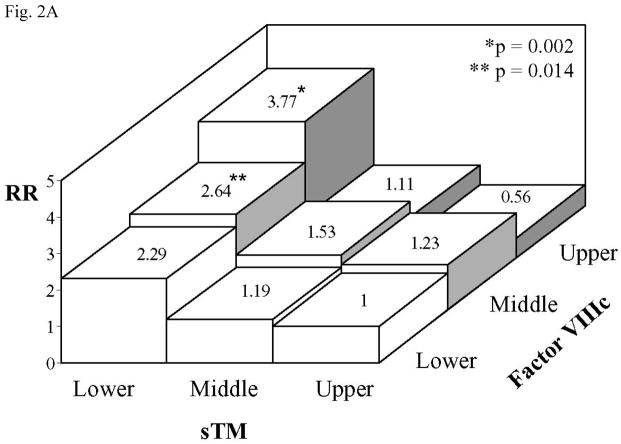
Combined sTM/FVIIIc analysis was similarly performed by dividing the sTM and FVIIIc tertiles into a reference group (Group 1: upper sTM/lower FVIIIc) and 8 study groups (Table 3 and Fig 2A). Although analysis of FVIIIc tertile individually did not show an association with CHD (Table 1), combined FVIIIc/sTM analysis revealed a significant increase in the risk of CHD for the upper FVIIIc tertile in the presence of lower sTM tertile (Group 7 vs. Group 1: RR=3.77, 95% CI 1.65–8.02). The RRs of CHD for Group 8 (middle sTM/upper FVIIIc) and Group 7 (upper sTM/upper FVIIIc) were not significantly different from the reference group (Fig. 2A). Interestingly, being in the middle FVIIIc tertile combined with lower sTM tertile (Group 6) carried a significantly elevated RR (2.64, 95% CI 1.22–5.71) compared with the reference group (Fig. 2A).
Table 3.
Baseline characteristics of groups of participants according to combined sTM and factor VIIIc tertiles
| Group 1* (UT/LE)§ (n=81) | Group 2 (MT/LE) (n=89) | Group 3 (LT/LE) (n=101) | Group 4 (UT/ME) (n=76) | Group 5 (MT/ME) (n=81) | Group 6 (LT/ME) (n=88) | Group 7 (UT/UE) (n=110) | Group 8 (MT/UE) (n=89) | Group 9 (LT/UE) (n=76) | |
|---|---|---|---|---|---|---|---|---|---|
| Age, yrs | 52.5 | 52.2 | 51.8 | 55.3 | 52.1 | 55.0 | 57.4+ | 55.4 | 54.0 |
| Men, % | 56.8 | 63.2 | 35.5 | 52.5 | 34.6+ | 22.6 | 44.2 | 44.3 | 23.0 |
| Afr. American, % | 0.1 | 13.3+ | 24.4+ | 19.4+ | 40.9+ | 27.4+ | 30.5+ | 30.9+ | 42.7+ |
| SBP, mmHg | 121.6 | 120.3 | 117.6 | 119.3 | 115.7 | 120.8 | 127.5+ | 122.3 | 120.1 |
| TC, mg/dl | 215.8 | 224.7 | 215.3 | 209.4 | 205.3 | 214.7 | 218.0 | 211.1 | 199.9 |
| HDL-C, mg/dl | 54.6 | 55.1 | 57.2 | 54.5 | 47.4 | 57.1 | 49.4 | 52.5 | 53.8 |
| TG, mg/dl | 130.2 | 129.1 | 111.9 | 120.2 | 122.6 | 116.1 | 153.1 | 111.1 | 108.9 |
| Fibrinogen, mg/dl | 280.9 | 286.4 | 280.4 | 297.8 | 303.9 | 299.7 | 321.3+ | 335.1 | 319.5+ |
| Cigarette- years | 286.0 | 286.9 | 395.8 | 195.6 | 278.5 | 314.1 | 257.4 | 362.0 | 230.8 |
| Ethanol intake, gr/wk | 17.1 | 52.7 | 55.7 | 38.0 | 21.3 | 61.0 | 14.6 | 60.1 | 41.0 |
| WBC, ×103/μL | 6.0 | 5.8 | 5.8 | 5.9 | 6.6 | 6.3 | 6.1 | 6.6 | 6.3 |
| VWF, % | 92.4 | 82.7 | 92.0 | 106.8 | 117.0+ | 116.7+ | 160.4+ | 161.7+ | 164.0+ |
| Hypertension, % | 25.8 | 19.9 | 23.9 | 25.9 | 32.1 | 24.6 | 48.9 | 34.1 | 22.2 |
| Diabetes, % | 1.2 | 2.2 | 1.3 | 1.0+ | 2.8 | 3.7 | 7.5 | 4.2 | 3.0 |
Reference group.
UT, MT and LT denote upper, middle and lower sTM tertiles, respectively; UE, ME and LE, upper, middle and lower FVIIIc tertiles, respectively.
Statistically significant (p<0.0062) compared with Group 1.
Diabetes denotes fasting glucose greater than or equal to 140 mg/dL or nonfasting glucose greater or equal to 200 mg/dL or physician diagnosed diabetes or pharmacologic treatment for diabetes. Hypertension defined as systolic BP ≥ 140 mmHg or diastolic BP ≥ 90 mmHg or current use of antihypertensive medications.
Fig. 2.
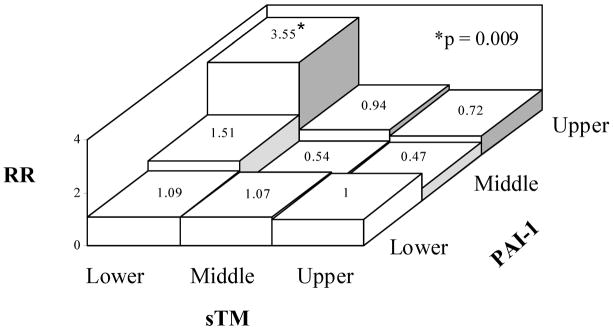
Risk ratio (RR) of CHD for participants with various combinations of A. sTM and FVIIIc tertiles vs. upper tertile sTM/lower tertile FVIIIc as the reference group and B. sTM and PAI-1 tertiles vs. upper tertile sTM/lower tertile PAI-1 as the reference group. Adjustment was made as described in Fig. 1. p values refer to comparison with the reference group.
Although initially there was no significant interaction between sTM and PAI-1, and analysis of PAI-1 tertiles individually did not show an association with CHD (Table 1), because of the antifibrinolytic properties of both molecules, we performed a combined sTM/PAI-1 analysis. We divided the sTM and PAI-1 tertiles into a reference group (Group 1: upper sTM/lower PAI-1) and 8 study groups (Fig 2B). This analysis revealed a significant increase in the risk of CHD for the upper PAI-1 tertile in the presence of sTM in the lower tertile (RR=3.55, 95% CI 1.37–9.20). The RRs of CHD for Group 8 (middle sTM/upper PAI-1) and Group 7 (upper sTM/upper PAI-1) were not significantly different from the reference group (Fig. 2B).
DISCUSSION
Results from this study show significant interactions of sTM with fibrinogen, FVIII and possibly PAI-1 in association with CHD risk. An important finding from the sTM/fibrinogen combined analysis is the modification of fibrinogen CHD risk by sTM. Fibrinogen was not a CHD risk factor within the upper tertile of sTM, whereas being in the lower sTM tertile augmented the CHD risk of high fibrinogen. In the middle sTM tertile, fibrinogen showed a dose-dependent positive association with CHD incidence. Thus, combined sTM/fibrinogen analysis provides new insights into CHD risk and interaction between these two hemostatic factors. Dose-dependent risk associations suggest a possible functional interaction between sTM and fibrinogen. Thrombomodulin binds thrombin and alters thrombin conformation to render it catalytically active in cleaving protein C and thereby degrading activated FVa and FVIIIa.24 Thus, at high sTM levels, coagulation reactions may be restrained due to limited FVa and FVIIIa levels resulting in reduced fibrin formation even in the presence of high levels of fibrinogen. Conversely, at low sTM levels, fibrin formation may become excessive even in the presence of an average level of fibrinogen.
The upper fibrinogen/lower sTM tertile group exhibits interesting characteristics including more cigarette smoking and a higher WBC count than the reference (lower fibrinogen/upper sTM) group, suggesting that inflammation and/or oxidative damage related effects may be confounding factors. Yet, adjustment for CRP, a systemic inflammatory marker associated with CHD risk, did not alter RR values. There is a strong relationship between cigarette smoking and hemostatic parameters to increase the risk of cardiovascular events.25 Oxidative damage to the endothelium surface occurs in chronic smokers and may result in an acquired APC deficiency.26 It has been reported that the oxidation of the functionally important Met 388 residue in the TM molecule, can down regulate its gene transcription and slow the rate at which thrombin-thrombomodulin activates Protein C.27 Oxidation is elevated in smokers and in other conditions with increased oxidative stress to the body. These results together with our previous findings that sTM interacts with sICAM-113 suggest that sTM may be affected by local endothelial inflammation and/or oxidation possibly conferred by white blood cells and smoking.
Combined sTM/FVIIIc analysis yielded similar CHD risk modifying effects. FVIIIc alone in the upper tertile did not increase risk for CHD. However, when sTM was in the lowest tertile, FVIIIc in the upper and even middle tertile had increased CHD risk in a concentration-dependent manner. By contrast, in the upper or middle sTM tertiles, elevated FVIIIc did not carry an increased CHD risk. It is surprising to note that the upper VIIIc/upper sTM group had a lower risk (RR=0.56) than the reference group, although the difference in RR did not reach statistical significance. This result is consistent with our previous report of 258 incident CHD cases after six years of follow-up, in which we first observed the association of sTM with CHD risk was modified by factor VIIIc.10 Taken together, these results suggest a biphasic influence of greater FVIIIc on the association of sTM with CHD risk. The reason for the paradoxical effect of FVIIIc on sTM is unclear and requires further investigation. The similarity in risk association between FVIII and fibrinogen may also be due to genetic clustering as previously reported.28
The fibrinolytic system plays an important role in the dissolution and removal of fibrin deposition from the circulation and PAI-1 is one of the key inhibitors of the fibrinolytic system.5 An important finding from the sTM/PAI-1 combined analysis is the potential modification of PAI-1 CHD risk by sTM. PAI-1 alone did not increase risk for CHD. However, when sTM was in the lowest tertile, PAI-1 in the upper tertile increased CHD risk (RR 3.55). By contrast, in the upper or middle sTM tertiles, elevated PAI-1 did not carry significantly increased CHD risk. Our results are in line with the concentration-dependent regulatory effect of TM.3 Lower TM, having an antifibrinolytic effect (through TAFI activation), potentiates the prothrombotic/antifibrinolytic status of higher PAI-1 concentrations. In addition, activated TAFI itself most likely increases the rate of PAI-1 mediated inhibition of the fibrinolysis.29 On the other hand, at a high TM concentration, APC is likely generated which counteracts the prothrombotic PAI-1 (and TAFI) activities.
There are limitations in this study. First, the sample size of each subgroup in combined analysis was relatively small, which affects the precision of the associations. Second, functional implications derived from these epidemiologic data are presumptive because we cannot verify that plasma sTM represents the endothelial TM level. This assumption may be further confounded by factors such as inflammation or infection and oxidative damage from different sources, which enhance endothelial TM degradation and/or dysfunction25,26,30
In summary, our findings indicate that sTM exhibits a complex modifying potential of CHD risk imposed by prothrombotic and anti-fibrinolytic factors. These results underscore the advantage of combined analysis of CHD risk by including sTM and prothrombotic factors (for example, fibrinogen, FVIII and PAI-1). Such analysis offers a possible approach for identifying high risk individuals for antithrombotic and/or anti-inflammatory therapy.
Supplementary Material
Acknowledgments
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021 and N01-HC-55022). The authors thank the staff and participants of the ARIC study for their important contributions. We thank Ms. Susan Mitterling for preparing this manuscript and editorial assistance.
References
- 1.Wu KK, Matijevic-Aleksic N. Molecular aspects of thrombosis and antithrombotic drugs. Crit Rev Clin Lab Sci. 2005;42:249–277. doi: 10.1080/10408360590951171. [DOI] [PubMed] [Google Scholar]
- 2.Bajzar L, Nesheim M, Morser J, Tracy PB. Both cellular and soluble forms of thrombomodulin inhibit fibrinolysis by potentiating the activation of thrombin-activable fibrinolysis inhibitor. J Biol Chem. 1998;273:2792–2798. doi: 10.1074/jbc.273.5.2792. [DOI] [PubMed] [Google Scholar]
- 3.Wu KK, Matijevic-Aleksic N. Thrombomodulin: a linker of coagulation and fibrinolysis and predictor of risk of arterial thrombosis. Ann Med. 2000;32 (Suppl 1):73–77. [PubMed] [Google Scholar]
- 4.Mosnier LO, Meijers JCM, Bouma BN. Regulation of fibrinolysis in plasma by TAFI and Protein C is dependent on the concentration of thrombomodulin. Thromb Haemost. 2001;85:5–11. [PubMed] [Google Scholar]
- 5.Robbie LA, Bennett B, Croll AM, Brown PA, Booth NA. Proteins of the fibrinolytic system in human thrombi. Thromb Haemost. 1996;75:127–133. [PubMed] [Google Scholar]
- 6.Wilhelmsen L, Svardsudd K, Korsan-Bengtsen K, Larsson B, Welin L, Tibblin G. Fibrinogen as a risk factor for stroke and myocardial infarction. N Engl J Med. 1984;311:501–505. doi: 10.1056/NEJM198408233110804. [DOI] [PubMed] [Google Scholar]
- 7.Meade TW, Mellows S, Brozovic M, Miller GJ, Chakrabarti RR, North WR, Haines AP, Stirling Y, Imeson JD, Thompson SG. Haemostatic function and ischaemic heart disease: principal results of the Northwick Park Heart Study. Lancet. 1986;2:533–537. doi: 10.1016/s0140-6736(86)90111-x. [DOI] [PubMed] [Google Scholar]
- 8.Kannel WB, Wolf PA, Castelli WP, D’Agostino RB. Fibrinogen and risk of cardiovascular disease. The Framingham Study. JAMA. 1987;258:1183–1186. [PubMed] [Google Scholar]
- 9.Folsom AR, Wu KK, Rosamond WD, Sharrett AR, Chambless LE. Prospective study of hemostatic factors and incidence of coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 1997;96:1102–1108. doi: 10.1161/01.cir.96.4.1102. [DOI] [PubMed] [Google Scholar]
- 10.Salomaa V, Matei C, Aleksic N, Sansores-Garcia L, Folsom AR, Juneja H, Chambless LE, Wu KK. Soluble thrombomodulin as a predictor of incident coronary heart disease and symptomless carotid artery atherosclerosis in the Atherosclerosis Risk in Communities (ARIC) Study: a casecohort study. Lancet. 1999;353:1729–1734. doi: 10.1016/s0140-6736(98)09057-6. [DOI] [PubMed] [Google Scholar]
- 11.Thompson SG, Kienast J, Pyke SD, Haverkate F, van de Loo JC. Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. N Engl J Med. 1995;332:635–641. doi: 10.1056/NEJM199503093321003. [DOI] [PubMed] [Google Scholar]
- 12.Folsom AR, Aleksic N, Park E, Salomaa V, Juneja H, Wu KK. Prospective study of fibrinolytic factors and incident coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb Vasc Biol. 2001;21:611–617. doi: 10.1161/01.atv.21.4.611. [DOI] [PubMed] [Google Scholar]
- 13.Wu KK, Aleksic N, Ballantyne CM, Ahn C, Juneja H, Boerwinkle E. Interaction between soluble thrombomodulin and intercellular adhesion molecule-1 in predicting risk of coronary heart disease. Circulation. 2003;107:1729–1732. doi: 10.1161/01.CIR.0000064894.97094.4F. [DOI] [PubMed] [Google Scholar]
- 14.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 15.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 16.Rosamond WD, Chambless LE, Folsom AR, Cooper LS, Conwill DE, Clegg L, Wang CH, Heiss G. Trends in the incidence of myocardial infarction and in mortality due to coronary heart disease, 1987 to 1994. N Engl J Med. 1998;339:861–867. doi: 10.1056/NEJM199809243391301. [DOI] [PubMed] [Google Scholar]
- 17.Aleksic N, Juneja H, Folsom AR, Ahn C, Boerwinkle E, Chambless LE, Wu KK. Platelet PLA2 allele and incidence of coronary heart disease. Results from the ARIC study. Circulation. 2000;102:1901–1905. doi: 10.1161/01.cir.102.16.1901. [DOI] [PubMed] [Google Scholar]
- 18.Papp AC, Hatzakis H, Bracey A, Wu KK. ARIC hemostasis study--I. Development of a blood collection and processing system suitable for multicenter hemostatic studies. Thromb Haemost. 1989;61:15–59. [PubMed] [Google Scholar]
- 19.Wu KK, Papp AC, Patsch W, Rock R, Eckfeldt J, Sharrett R. ARIC hemostasis study--II. Organizational plan and feasibility study. Thromb Haemost. 1990;64:521–525. [PubMed] [Google Scholar]
- 20.Folsom AR, Aleksic N, Catellier D, Juneja HS, Wu KK. C-Reactive protein and incident coronary heart disease in the Atherosclerosis Risk in Cimmunities (ARIC) Study. Am Heart J. 2002;244:233–238. doi: 10.1067/mhj.2002.124054. [DOI] [PubMed] [Google Scholar]
- 21.Wu KK, Folsom AR, Heiss G, Davis CE, Conlan MG, Barnes R. Association of coagulation factors and inhibitors with carotid artery atherosclerosis. Early results of the Atherosclerosis Risk in Communities (ARIC) Study. Ann Epidemiol. 1992;2:471–480. doi: 10.1016/1047-2797(92)90097-a. [DOI] [PubMed] [Google Scholar]
- 22.Prentice RL. A case-cohort design for epidemiological cohort studies and disease prevention risk. Biometrika. 1986;73:1. [Google Scholar]
- 23.Barlow WE. Robust variance estimation for the case-cohort design. Biometrics. 1994;50:1064–1072. [PubMed] [Google Scholar]
- 24.Esmon CT. Thrombomodulin as a model of molecular mechanisms that modulate protease specificity and function at the vessel surface. FASEB J. 1995;9:946–955. doi: 10.1096/fasebj.9.10.7615164. [DOI] [PubMed] [Google Scholar]
- 25.Leone A. Smoking, haemostatic factors, and cardiovascular risk. Curr Pharm Des. 2007;13(16):1661–1667. doi: 10.2174/138161207780831347. [DOI] [PubMed] [Google Scholar]
- 26.Fernández JA, Gruber A, Heeb MJ, Griffin JH. Protein C pathway impairment in nonsymptomatic cigarette smokers. Blood Cells Mol Dis. 2002;29:73–82. doi: 10.1006/bcmd.2002.0542. [DOI] [PubMed] [Google Scholar]
- 27.Stites WE, Froude JW., 2nd Does the oxidation of methionine in thrombomodulin contribute to the hypercoaguable state of smokers and diabetics? Med Hypotheses. 2007;68:811–821. doi: 10.1016/j.mehy.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Hylckama VA, Callas PW, Cushman M, Bertina RM, Rosendaal FR. Inter-relation of coagulation factors and D-dimer levels in healthy individuals. J Thromb Haemost. 2003;1:516–522. doi: 10.1046/j.1538-7836.2003.00080.x. [DOI] [PubMed] [Google Scholar]
- 29.Mutch NJ, Thomas L, Moore NR, Lisiak KM, Booth NA. TAFIa, PAI-1 and α2-antiplasmin: complementary roles in regulating lysis of thrombi and plasma clots. J Thromb Haemost. 2007;5:812–17. doi: 10.1111/j.1538-7836.2007.02430.x. [DOI] [PubMed] [Google Scholar]
- 30.Blann AD, Amiral J, McCollum CN. Prognostic value of increased soluble thrombomodulin and increased soluble E-selectin in ischemic heart disease. Eur J Haematol. 1997;59:115–120. doi: 10.1111/j.1600-0609.1997.tb00735.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.