Abstract
The voltage sensor domain (VSD) and the ligand sensor (cytoplasmic domain) of BK channels synergistically control channel activities, thereby integrating electrical and chemical signals for cell function. Studies show that intracellular Mg2+ mediates the interaction between these sensory domains to activate the channel through an electrostatic interaction with the VSD. Here we report that Mg2+ binds to a site that consists of amino acid side-chains from both the VSD (Asp99 and Asn172) and the cytoplasmic domain (Glu374 and Glu399). For each Mg2+ binding site the residues in the VSD and those in the cytoplasmic domain come from neighboring subunits. These results suggest that the VSD and the cytoplasmic domains from different subunits may interact during channel gating, and the packing of VSD or the RCK1 domain to the pore in BK channels differ from that in Kv1.2 or MthK channels.
INTRODUCTION
Many ion channels are assembled from modular elements1, in which a sensory module to specific stimuli, such as voltage, ligand binding, post-translational modification, and accessory protein association, usually covalently links to the ion conduction pore and thereby regulates its opening and closing. The direct interaction between the channel pore and various sensory modules in a number of ion channels, such as the membrane-spanning voltage sensor of Kv channels2, the cytoplasmic Ca2+ sensor of MthK channels3, and the extracellular ACh binding domain of ACh receptors4, has been proposed as a key step for channel activation. However, how stimuli alter the interaction among different sensors to promote channel activation is still not clear. Here we study the Slo1 large conductance, voltage- and Ca2+-activated K+ (BK) channel to address this question, because the BK channel has a distinct voltage sensor domain (VSD) and a cytoplasmic ligand-binding domain to separately sense membrane voltage and intracellular ligands5-13, and previous studies have shown that the two sensory domains interact during channel gating14.
BK channels are activated by voltage, intracellular Ca2+ and Mg2+ (Fig. 1 a, b) 5-7, and participate in various physiological functions, such as muscle contraction, neural transmission and hearing7,15-18. Both sequence homology and experimental evidence suggest that the structure of the VSD in BK channels may resemble that of other Kv channels19, while the cytoplasmic domain of BK channels may adopt a similar structure as that of the MthK channel3,8,12,14,20,21. Previous studies on Mg2+-dependent activation of BK channels have revealed structural details that are important for BK channel function. Particularly, two acidic amino acids (Glu374 and Glu399) in the cytoplasmic RCK1 domain of BK channel may contribute to Mg2+ coordination12-14,22; removal of the side chain carboxylate groups from these two residues completely abolishes Mg2+ sensing. These residues in the cytoplasmic domain are located close to the C-terminus of the transmembrane segment S4, enabling the bound Mg2+ to engage in an electrostatic interaction with the voltage-sensing residue Arg213 at the C-terminus of S414 (Fig. 1c).
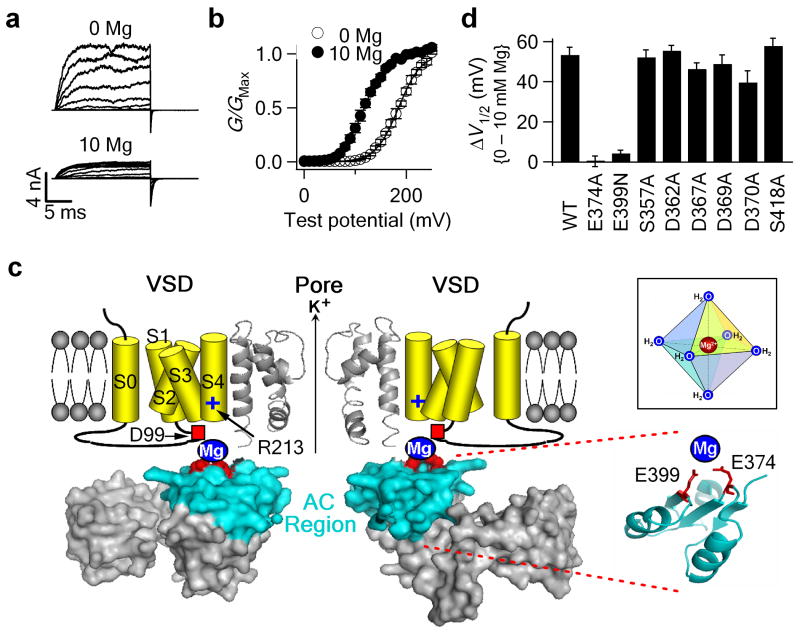
Figure 1.
Mg2+ coordinates in the cytoplasmic domain of the mSlo1 channel. (a,b) Representative macroscopic current traces (a) and mean G-V relationship (b) for WT channels in 0 and 10 mM [Mg2+]i. Testing potentials were from -20 to 240 mV with 20-mV increments. Both holding and repolarizing potentials were -80 mV. The smooth curves in (b) represent Boltzmann fits. (c) BK channel model. The pore domain and the cytoplasmic RCK1 domain is based on the MthK crystal structure (PDB ID: 1LNQ)3. Transmembrane S0-S4 segments are depicted as cartoons. Only two opposite subunits are shown for clarity. Two putative Mg2+ binding residues (Glu374 and Glu399, red spheres) are located in the AC region (cyan). (Inset) Mg2+ binding site is predominantly formed by six oxygen-containing ligands with an octahedral geometry. (d) Shifts of the G-V relation caused by 10 mM [Mg2+]i for the Ala mutations of the oxygen-containing residues in the AC region. When the putative Mg2+ binding residues (Glu374 and Glu399) were destroyed, 10 mM [Mg2+]i still shifts G-V relation to more negative voltages by about 14 mV due to Mg2+ binding to a very low affinity Mg2+ site24. This effect can be mathematically subtracted to obtain the contribution of the Glu374/Glu399 site to Mg2+ sensing. Thus, in all figures in this study, mean G-V relationships show the observed G-V shifts induced by 10 mM [Mg2+]i; while the G-V shifts in all bar graphs represent the contribution of the Glu374/Glu399 site after 14.0 mV subtraction. Error bars represent s.e.m.
To further understand the mechanism of Mg2+-dependent activation of BK channels and explore the structural basis of the interaction between the VSD and the cytoplasmic domains of mouse Slo1 (mSlo1), we studied the composition of the Mg2+ binding site. As a “hard” (closed-shell) divalent cation, Mg2+ is dominantly coordinated by six “hard” oxygen atoms from the side chains of oxygen-containing residues, main chain carbonyl groups in proteins, or water molecules (Fig. 1c, inset)23. Besides two putative Mg2+ coordinates contributed by the carboxylate groups from Glu374 and Glu399 (Fig. 1d), four additional oxygen ligands are required for Mg2+ binding. In this study, we identified two more amino acid side chains, Asp99 and Asn172, in the voltage sensor domain that may also contribute to Mg2+ coordination. Interestingly, our results indicate that Asp99 and Asn172 from the VSD of one subunit may form the Mg2+ binding site with Glu374 and Glu399 from the cytoplasmic domain of a neighboring subunit. Such an inter-domain and inter-subunits formation of the Mg2+ binding site reveals a particular structural alignment of the VSD and the cytoplasmic domain and indicates that the two domains from different subunits may interact during BK channel gating.
RESULTS
Ala-scan in the cytoplasmic domain of BK channels
Figure 1c shows the structure of the cytoplasmic RCK1 domain of MthK, which has been proposed as the structural model for the cytoplasmic domain of BK channels3. The putative Mg2+ coordinates Glu374 and Glu399 are located at the N-terminus of the RCK1 domain (the AC region, colored cyan in Fig. 1c). Since Glu374 and Glu399 are located at the top surface of the cytoplasmic domain, if any side chain from the cytoplasmic domain other than these two residues also contributes to Mg2+ coordination, it should be located in the AC region. To identify such a residue, we made an Ala-scanning of all the residues with oxygen-containing side chains in the AC region. If any of these residues contributes to Mg2+ coordination, the substitution of its side chain with the methyl group of Ala would abolish or substantially reduce the Mg2+-induced shift of the G-V relation (Fig.1b). However, except for the mutations of Glu374 and Glu39912, we did not find any Ala mutation that markedly reduced Mg2+ sensing (Fig. 1d, other mutational results have been published previously12). Therefore, Glu374 and Glu399 are the only two putative Mg2+ coordinates in the cytoplasmic AC region. Here and in all other figures, the bars represent observed G-V shifts induced by 10 mM [Mg2+]i subtracting 14.0 mV due to Mg2+ binding to another low affinity Mg2+ site24.
D99A prevents Mg2+ binding
We then examined if the transmembrane (TM) domain contains side chains that may contribute to Mg2+ coordination. Since mSlo1 (mouse) and dSlo (drosophila) channels show similar Mg2+ sensitivity, 45 oxygen-containing residues in the TM domain conserved between these two species and potentially facing cytosol were mutated into amino acids that contain no side chain oxygen (Fig. 2a, red and blue colors). Among these mutations, only D99A, which is located in the C-terminus of the S0-S1 linker (Fig. 1c, 2a), completely abolishes the G-V shift induced by 10 mM [Mg2+]i; two other mutations, N172A and Y336A, substantially reduce the G-V shift (Fig. 2b-d).
Figure 2.
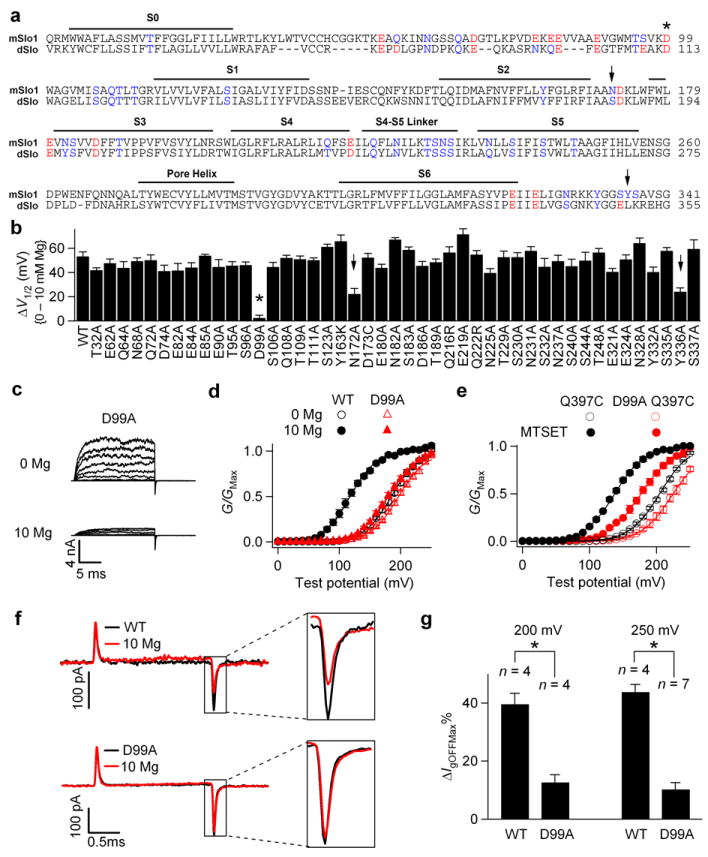
D99A abolishes Mg2+ sensitivity by preventing Mg2+ binding. (a) Sequence alignment of the membrane-spanning (TM) domain in mSlo1 and dSlo channels. Mutations of the oxygen-containing residues that are conserved between mSlo1 and dSlo and potentially face cytosol (red and blue colors) were tested. mSlo1: (mouse, GI: 347143); dSlo: (drosophila, GI: 115311626). (b) Shifts of the G-V relationship induced by 10 mM [Mg2+]i for WT and the mutations. (c) Macroscopic current traces of D99A in 0 and 10 mM [Mg2+]i. Testing potentials were from -20 to 240 mV with 20-mV increments. Both holding and repolarizing potentials were -80 mV. (d) G-V relationship for WT and D99A. (e) Mean G-V relationship of Q397C and D99A Q397C channels before (hollow) and after (filled) 200 μM MTSET treatment. For all the experiments with MTSET treatment in this study, C430A was used as background to remove the endogenous MTSET effect on channel activation14,36. (f) Gating current (Ig) traces for WT (upper panel) and D99A mutant (lower panel) channels with (red) and without (black) 10 mM [Mg2+]i in response to a 2-ms, 250 mV depolarizing pulse. Ig traces of the same patch were first recorded in the absence of Mg2+, and then 10 mM [Mg2+]i. (g) Effects of 10 mM [Mg2+]i on the reduction of peak OFF gating currents (IgOFFMax) in response to 2-ms, 200 mV or 250 mV depolarizing pulses.ΔIgOFFMax%= (IgOFFMax(0Mg)-IgOFFMax(10Mg))/IgOFFMax(0Mg). * indicates p≤0.001.
Recent studies showed that Mg2+ activates the channel by an electrostatic interaction with Arg213 in S4 after binding to the channel14. Therefore, mutation D99A could eliminate Mg2+ sensitivity by either a direct destruction of the Mg2+ binding site to prevent Mg2+ binding or an alteration of the conformation to prevent the interaction between the bound Mg2+ and Arg213. To distinguish these two possibilities, we examined whether D99A also abolishes the effect of a positive charge covalently added to the vicinity of the Mg2+ binding site. Previous studies show that Gln397 is located close to the Mg2+ binding site12,22. A positive charge covalently added to position 397 by modifying Q397C with MTSET(+) shifts G-V relation to more negative voltages (Fig. 2e) because the charge also interacts with Arg213 electrostatically to activate the channel, mimicking the effects of Mg2+ on the WT channel14. If D99A alters the conformation of the channel to prevent the interaction between the bound Mg2+ and Arg213, it should also abolish the effects of MTSET(+) modification of Q397C. However, as shown in Figure 2e, in the presence of D99A, the modification of Cys397 by MTSET(+) still shifted G-V relation by -51.7±1.6 mV, comparable with the shift in the absence of D99A (-66.5±1.8 mV). These results indicate that D99A does not markedly alter the electrostatic interaction between the VSD and the positive charge around the Mg2+ binding site.
Next, we measured the effect of D99A on gating currents with and without 10 mM [Mg2+]i. The off-gating current (IgOFF) is derived from the return of the voltage sensor from the active state to the resting state when most channels are open, while the on-gating current (IgON) is the result of the movement of the voltage sensor from the resting state to the active state when channels are closed14,25-27. Our recent studies demonstrated that Mg2+ affects WT IgOFF through an electrostatic interaction with Arg213 in S4 primarily when the channel is open14. For WT channels, 10 mM [Mg2+]i reduces the amplitude and the decay rate of the IgOFF but does not noticeably affect the IgON (Fig. 2f and Supplementary Fig. 1a). However, D99A almost abolishes the effect of Mg2+ on IgOFF (Fig. 2f, g), indicating that S4 can no longer sense Mg2+. Since D99A does not affect the ability of S4 to sense a positive charge covalently added to the vicinity of the Mg2+ binding site (Fig. 2e), this result indicates that Mg2+ can no longer bind to the site to interact with S4. Similar results were obtained with the mutation E399N, which destroys Mg2+ binding at the Glu374/Glu399 site (Supplementary Fig. 1). Taken together, mutation D99A destroys Mg2+ binding to the Glu374/Glu399 site.
Asp99 is part of the Mg2+ binding site
Why does D99A abolish Mg2+ binding? The simplest hypothesis is that the carboxylate group on the side chain of Asp99 provides another Mg2+ coordinate. For this to be true, we should expect that 1) Asp99 is close to residues Glu374/Glu399 in the cytoplasmic domain so that they can be part of the same Mg2+ binding site; and 2) the oxygen on the side chain of Asp99 is required for Mg2+ binding.
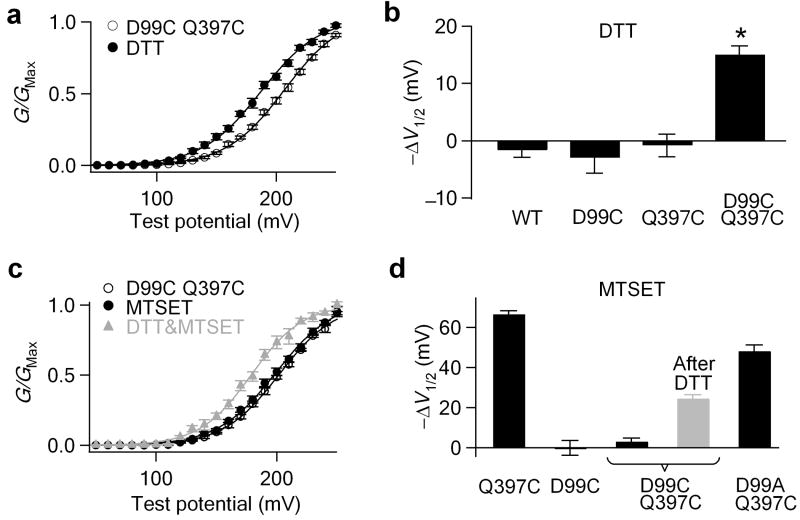
To examine the spatial relationship between Asp99 and Glu374/Glu399, we made a double mutation D99C Q397C and examined whether a disulfide bond could form between these two Cys residues. Since the Cβ- Cβ distance between two Cys residues in a disulfide bond is between 2.9 to 4.6 Å28 and Gln397 is close to Glu39912,14,22, a disulfide bond between Cys99 and Cys397 would indicate that Asp99 and Glu374/Glu399 are located within the dimension of a Mg2+ binding site. Figure 3a and b show that the treatment of D99C Q397C channels with 10 mM DTT induced a shift of -15.0±1.6 mV in the G-V relationship. This shift is significant compared with the DTT effect on WT, D99C and Q397C channels (p<0.001), suggesting a spontaneous disulfide bond formation between Cys99 and Cys397, which is subsequently reduced by DTT.
Figure 3.
Asp99 is spatially close to the cytoplasmic part of the Mg2+ binding site. (a, b)Shifts of the G-V relationship induced by 10 mM DTT in 0 [Ca2+]i and [Mg2+]i. * indicates p<0.001. (c) Mean G-V relationship of D99C Q397C mutant channels before and after 200 μM MTSET or sequential DTT-MTSET (grey) treatment in 0 [Mg2+]i. (d) G-V shifts induced by MTSET treatments, suggesting the disulfide formation between Cys99 and Cys397.
To further demonstrate the existence of this disulfide bond, we treated D99C Q397C mutant channels with 200 μM MTSET(+). MTSET(+) treatment of Q397C channels induces a -66.5±1.8 mV leftward shifts of the G-V relationship in the absence of Mg2+ (Fig. 2e)14. A disulfide bond between Cys99 and Cys397 would protect the thiol group on residue 397, which would be then no longer available for MTSET(+) to modify, resulting in no MTSET(+)-induced G-V shift. Consistent with this prediction, MTSET(+) did not show any effect on the G-V relationship of the D99C Q397C channel (Fig. 3c, d). The absence of the MTSET(+) effect is not due to the mutational effect of Asp99, because D99A Q397C channels still can sense MTSET(+), while single mutant D99C is insensitive to MTSET(+) (Fig. 2e and 3d). The sequential treatment with 10 mM DTT and followed by 200 μM MTSET(+) resulted in a partial recovery of the MTSET(+) effect (-24.5±2.0 mV, Fig. 3c, d), further indicating the existence of the disulfide bond between Cys99 and Cys397. This partial recovery of the MTSET(+) effect suggests that the disulfide bond between Cys99 and Cys397 is broken by 10 mM DTT only in a fraction of the channels, possibly due to the fast reformation of the disulfide bond. Such a phenomenon suggests that Cys99 and Cys397 are located very close to allow a fast, spontaneous formation of the disulfide bond. A similar phenomenon has been reported in the study of the cyclic-nucleotide gated channel29. Therefore, these experiments indicate that Asp99 in the S0-S1 loop is located spatially close to Gln397, and the Glu374/Glu399 site, in the cytoplasmic domain.
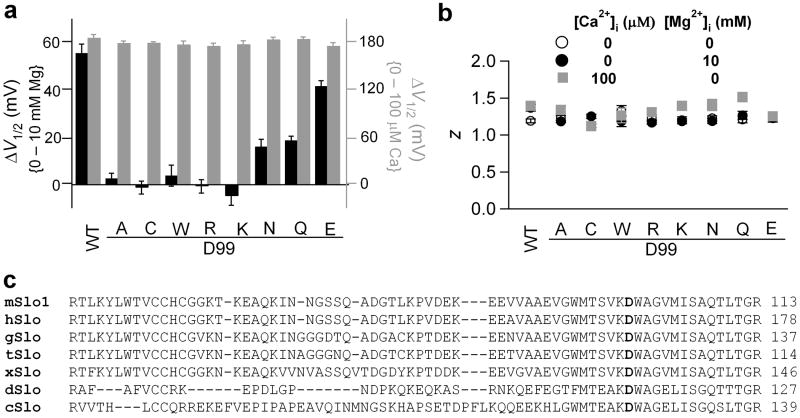
To test the role of side chain oxygen of residue 99 on Mg2+ sensing, we mutated Asp99 to amino acids with various side chains (Fig. 4a). Mg2+ sensing, but not voltage or Ca2+ sensing, is highly correlated with the side chain properties of residue 99 (Fig. 4a, b). Similar to D99A, all the mutations that remove side chain oxygen (D99C, D99W, D99R and D99K) completely abolished Mg2+ sensitivity, while the mutations that preserve oxygen with carbonyl or carboxylate groups (D99Q, D99N and D99E) retained partial Mg2+ sensitivity. Among these mutations, D99E channels showed higher Mg2+ sensitivity than D99Q and D99N channels. This is consistent with the higher preference of carboxylate group over carbonyl group on Mg2+ binding23. To the contrary, none of the mutations noticeably altered the G-V shifts induced by 100 μM [Ca2+]i (Fig. 4a) or the equivalent gating charge z (Fig. 4b), indicating that the mutational effect of residue 99 is specific to Mg2+ sensing of the BK channel. Therefore, the oxygen on its side chain is essential for Mg2+ binding. Based on the spatial proximity, the mutational effects on Mg2+ sensing and Mg2+ binding, as well as the fact that Asp99 is conserved among BK channels from different species (Fig. 4c), we conclude that Asp99 from the VSD and Glu374/Glu399 from the cytoplasmic domain form a putative inter-domain Mg2+ binding site.
Figure 4.
Side chain carboxylate or carbonyl group of residue 99 is required for Mg2+ coordination. (a) G-V shifts induced by 10 mM [Mg2+]i (black) and by 100 μM [Ca2+]i (grey) for Asp99 mutations. (b) Equivalent gating charge z of Asp99 mutations. (c) Sequence alignment of the S0-S1 linker from various spices: mouse (mSlo1, GI: 347143), human (hSLO, GI:46396283), chicken (gSlo, GI:46396408), turtle (tSlo, GI:82224841), frog (xSlo, GI:46396489), drosophila (dSlo, GI: 115311626), nematode (cSlo, GI:46396994).
Asn172 also participates in Mg2+ binding
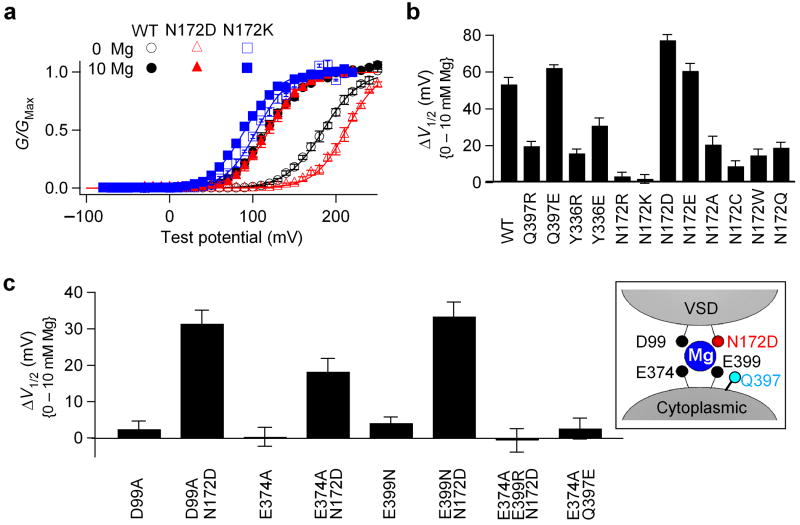
In addition to D99A, two other mutations, N172A in the S2-S3 loop and Y336A in the linker connecting S6 to the cytoplasmic domain, substantially reduced Mg2+ sensing of the channel (Fig. 2b). To investigate whether these two residues contribute to Mg2+ coordination, we first mutated them to basic residues (positively charged) (Fig. 5a, b). We have shown that mutating a putative Mg2+ ligand, Glu374, Glu39922, or Asp99 (Fig. 4a), to basic residues completely abolishes Mg2+ sensitivity of the channel due to the destruction of Mg2+ coordination. On the other hand, if a residue (for example, Gln397) is in the vicinity of the Mg2+ binding site but is not a Mg2+ coordinate, positively charged mutations of this residue (ie. Q397R/K) reduce but cannot abolish Mg2+ sensitivity; while negatively charged mutations (ie. Q397D/E) increase Mg2+ sensitivity 22 (Fig. 5b) . The change of Mg2+ sensitivity by these mutations might be due to the conformational change of the Mg2+ binding site and/or the electrostatic interactions between Mg2+ and the charges. However, when we tested charged mutations of Tyr336, we found that both Y336R and Y336E reduced Mg2+ sensitivity. But none of them abolished or enhanced Mg2+ sensitivity (Fig. 5b). These phenomena indicate that Tyr336 may not be part of the Mg2+ binding site, nor close enough to the binding site to affect Mg2+ binding through electrostatic interactions. Rather, the reduction of Mg2+ sensitivity is likely due to allosteric effects.
Figure 5.
Asn172 may contribute to Mg2+ coordination. (a) Mean G-V relationship of WT, N172D and N172K mutant channels in 0 and 10 mM [Mg2+]i. (b) Shifts of the G-V relationship caused by 10 mM [Mg2+]i for WT and Gln397, Tyr336, and Asn172 mutations. (c) Shifts of the G-V relationship caused by 10 mM [Mg2+]i. Inset, N172D may substitute other putative Mg2+ coordinates to enable Mg2+ binding.
In contrast, N172R and N172K completely abolish Mg2+ sensing (Fig. 5a, b), while N172D and N172E increase Mg2+ sensitivity. Other mutations of Asn172 reduce Mg2+ sensitivity (Fig. 5a, b). Does Asn172 contribute to Mg2+ coordination, or alternatively, is it simply close to the Mg2+ binding site such that adding a positive charge on its side chain completely excludes Mg2+ binding, while adding a negative charge attracts Mg2+ to bind (Fig. 5c, inset)? To answer this question, we tested whether N172D can rescue Mg2+ sensitivity that is abolished by the mutations of the putative Mg2+ binding residues (D99A, E374A or E399N) (Fig. 5c). If Asn172 is part of the Mg2+ binding site, the carboxylate group of N172D may be able to substitute the loss of one carboxylate group induced by single mutations D99A, E374A or E399N, to coordinate Mg2+. Consistent with this hypothesis, the double mutant channels containing N172D show substantially larger Mg2+ sensitivity than these single mutant channels (Fig. 5c), indicating that N172D can substitute these residues and rescue Mg2+ binding. However, N172D cannot rescue any Mg2+ sensitivity from the combinatorial effect of E374A and E399R, which removes two carboxylate groups from the original Mg2+ binding site, suggesting that N172D can only compensate for one lost carboxylate group. Contrary to the effect of N172D, adding a carboxylate group on residue 397 can not rescue any Mg2+ sensitivity when the side chain carboxylate group of Glu374 was removed (Fig. 5c, E374A Q397E). Thus, Asn172 plays a different role than Gln397 on Mg2+ binding. These results suggest that the increase of Mg2+ sensitivity by N172D/E or the elimination of the Mg2+ sensitivity by N172R/K cannot be simply attributed to the electrostatic interaction between Mg2+ and the charge on residue 172. Instead, the carboxylate or carbonyl group on the side chain of residue 172 may contribute to Mg2+ coordination.
Mg2+ binding sites are formed between neighboring subunits
It has been proposed that the structures of the VSD and the cytoplasmic RCK1 domain of BK channels resemble that of the VSD of Kv1.2 channels19 and the RCK domain of MthK channels3,12,14,22, respectively. However, it is not clear how these sensory domains align with each other in BK channels. In Figure 6a we combine the structures of Kv1.2 (PDB ID: 2A79)30 and MthK (PDB ID: 1LNQ)3 by aligning the selectivity filter of the two channels, which provides an opportunity to examine the packing of different structure domains in the BK channel.
Figure 6.
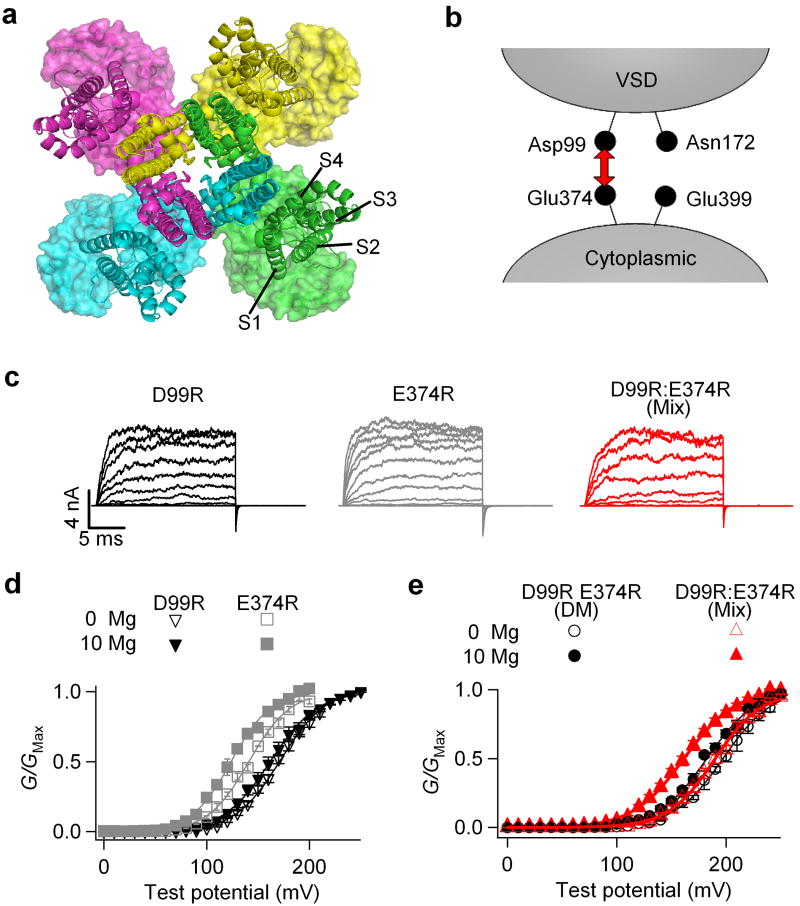
Asp99 and Glu374 in a Mg2+ binding site are not from the same subunit. (a)Superposition of Kv1.2 (ribbons, PDB ID: 2A79)30 and MthK (surface, PDB ID: 1LNQ)3 channel structures by aligning their selectivity filter regions. For clarity, the T1 domains of the Kv1.2 structure and the RCK2 domains of the MthK structure were not shown. Different colors represent four subunits. (b) Experimental design for mixing D99R and E374R mutations. (c) Representative current traces of D99R, E374R and mixed D99R:E374R (1:1) channels. (d) Mean G-V relationship of D99R and E374R channels. The small shift in G-V relations is largely due to Mg2+ binding to another low affinity Mg2+ site24 (e) Mean G-V relationship of the D99R E374R double mutant (DM) and D99R:E374R mixed (1:1) channels.
Figure 6a shows that, if the VSD and the RCK1 domain of BK channels pack against the pore domain similarly as that of Kv1.2 and MthK, the VSD would be located just above the RCK1 domain from the same subunit. Such a model would predict that all four Mg2+ coordinating residues in each Mg2+ binding site, Asp99, Asn172, Glu374 and Glu399, should come from the same Slo1 protein, i.e., the BK channel forms intra-subunit Mg2+ binding sites. To examine this prediction, we mixed single mutations D99R and E374R in 1:1 ratio (Fig. 6b), and tested their Mg2+ sensitivity (Fig. 6c-e). These mixed single mutants form heterotetrameric channels when expressed in Xenopus oocytes with various stoichiometry (Supplementary Fig. 2a). Since each of the single mutation in a Mg2+ binding site is sufficient to abolish Mg2+ binding (Fig. 6c, d), if intra-subunit Mg2+ binding sites are formed, all four sites of a channel should be completely destroyed no matter what the stoichiometry is for the heterotetrameric channels (Supplementary Fig. 2a). Therefore, no Mg2+ sensitivity of any mixed channels should be observed. However, when we mixed D99R and E374R in 1:1 ratio, the channels exhibited a -14.8±1.2 mV (n=9), Mg2+-induced G-V shift (Fig. 6c, e). This residual Mg2+ sensitivity is significantly different (p<0.001) from D99R single mutant (n=13), E374R single mutant (n=11), or D99R E374R double mutant channels (n=7), all of which completely abolish Mg2+ sensing (Fig. 7a).
Figure 7.
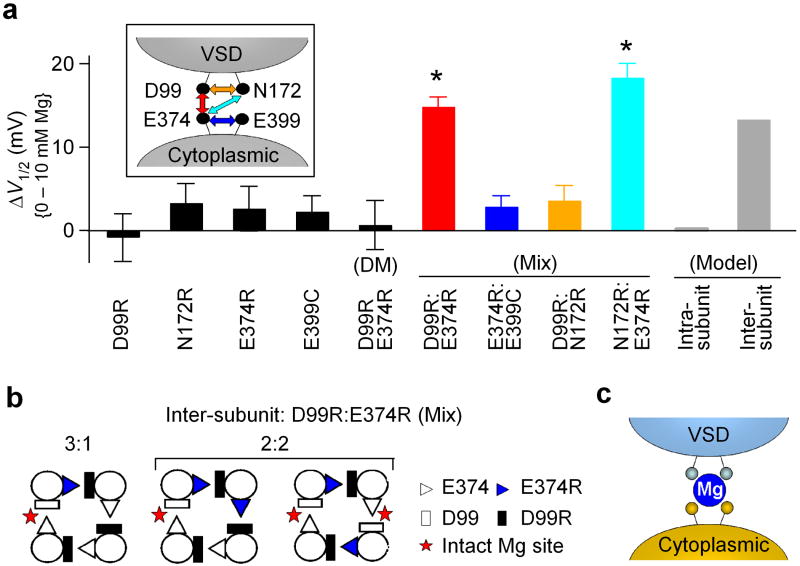
Asp99/Asn172 and Glu374/Glu399 may come from neighboring subunits to form a Mg2+ binding site. (a) Shifts of the G-V relationship caused by 10 mM [Mg2+]i for various single and combinations of mutations. Different mixed mutations are color coded as the arrows shown in the inset. All the mixtures have a mRNA ratio of 1:1. Model prediction was calculated based on a binomial distribution (See Methods). * indicates p<0.001. (b) Intact Mg2+ binding sites can form from mixed mutations based on inter-subunit formation of the Mg2+ binding sites. (c) Cartoon illustrating inter-domain and inter-subunit formation of the Mg2+ binding site.
To explain this phenomenon, we postulated an inter-subunit Mg2+ binding site model, in which Asp99 from the VSD and Glu374 from the cytoplasmic domain are from neighboring subunits (Fig. 7b and Supplementary Fig. 2b). According to this model, some D99R:E374R heterotetrameric channels may contain one or two intact Mg2+ binding site(s). If each binding site is assumed to make an equal contribution to channel activation independently, the model predicts a -13.3 mV shift (25% of that of the WT channel) in the G-V relation of the mixed channels in the presence of 10 mM [Mg2+]i (Supplementary Fig. 2b and METHODS). Our experimental observation is consistent with this model prediction (Fig. 7a). Current amplitudes of D99R channels, E374R channels and D99R:E374R mixed channels were comparable in these experiments (Fig. 6c), indicating that the expression efficiency of D99R and E374R are similar, and hence the mix of D99R and E374R is at a 1:1 ratio.
Similarly, for the 1:1 mixture of N172R:E374R (Fig. 7a, inset), 10 mM [Mg2+]i also induced a G-V shift of -18.3±1.7 mV (Fig. 7a, n=6), suggesting that in the Mg2+ binding site, Asn172 and Glu374 also come from neighboring subunits. Contrary to the mix of mutations that are in different domains, the mix of mutations that are in the same domain, i.e., D99R:N172R (n=12) in the VSD or E374R:E399C (n=12) in the cytoplasmic domain (Fig. 7a, inset), did not rescue any Mg2+ sensitivity (Fig. 7a). This result rules out the possibility that the rescued Mg2+ sensitivity is due to some non-specified artifacts that might have been derived from mixing different mutations. Based on all these experiments, we conclude that Asp99 and Asn172 from the VSD and Glu374 and Glu399 from the cytoplasmic domain form an inter-domain and inter-subunit Mg2+ binding site (Fig. 7c).
DISCUSSION
Our results illustrate that Mg2+ is coordinated at the interface between the VSD and the cytoplasmic domain to activate Slo1 BK channels. Side chains from Asp99/Asn172 in the VSD and Glu374/Glu399 in the cytoplasmic domain form the putative Mg2+ binding site. This coordination scheme positions Mg2+ close to the S4 voltage sensor and enables an electrostatic interaction between the bound Mg2+ with Arg213 in S4 to affect the movements of the VSD, thereby activating the channel14. Interestingly, the mixture experiments shown in Figure 6 and 7 suggest that the putative Mg2+ binding site of BK channels is formed between neighboring subunits. Thus, the VSD and the cytoplasmic domain from different subunits would interact during channel gating.
This special inter-domain and inter-subunit arrangement of the putative Mg2+ binding site provides valuable structural and functional information about BK channels. First, the two sensory domains, the VSD and the cytoplasmic domain, are close to each other and may interact intimately during channel gating. According to the octahedral geometry of Mg2+ binding, the Mg2+ ligands must be positioned within 5 to 6 Å from each other when Mg2+ binds to the site23 (Fig. 1c, inset). Thus, the top surface of the cytoplasmic domain may make close contact with the intracellular face of the VSD. Our recent study on the electrostatic interaction between Arg213 in S4 and the positive charge on residue 397 suggests that these two positive charges are about 9 Å apart14, supporting our current finding on the Mg2+ binding site and the proximity between these two sensory domains. The disulfide bond between Cys99 and Cys397 (Fig. 3) and the ability of N172D to substitute mutations of other Mg2+ ligands to bind Mg2+ (Fig. 5c) further suggest that these two sensory domains interact closely at their interface. However, it is not clear whether there are other places besides the Mg2+ binding site where these two sensory domains interact with each other to regulate channel gating. Second, the formation of inter-subunit Mg2+ binding sites suggests that the VSD of one subunit is packed right on top of the cytoplasmic domain from the neighboring subunit (Fig. 7). Such a structural arrangement does not agree with the prediction of the quaternary structure model based on the superposition of the Kv1.2 and MthK structures (Fig. 6a). This result indicates that although the VSD and the cytoplasmic RCK1 domain of BK channels may adopt a similar tertiary structure as that of Kv1.2 and MthK channels3,8,12,14,19-21, the packing of VSD or the cytoplasmic domain relative to the pore domain may differ from that of Kv1.2 or MthK. Such a difference in the quaternary structure may be the result of the unique S0 transmembrane segment31 and the long S0-S1 loop in the TM domain (Fig. 2a) besides the common VSD (S1-S4) that is found in other Kv channels, as well as the intimate interactions between the VSD and the cytoplasmic domain of BK channels. Since the packing of the voltage sensor and the cytoplasmic ligand sensor relative to the pore domain may influence the energetic coupling between these sensors and the activation gate, it may contribute to the unique functional properties of the BK channel, such as the allosteric mechanism of voltage- and Ca2+-dependent gating26,32.
Based on a quantum chemistry calculation23 of the free energy of successive water-exchange reactions in Mg2+ complexes, Dudev and Lim concluded that neutral carbonyl group(s) (in the case of the mSlo1 BK channel, Asn172) can coordinate Mg2+ when Mg2+ is already bound to up to three negatively charged carboxylate groups (Asp99, Glu374 and Glu399 in the mSlo1 BK channel). Under this composition of a Mg2+ binding site, Mg2+ cannot exchange all its first-shell water molecules for protein ligands. In our study, we did not find any other residue than Asp99, Asn172, Glu374, and Glu399 that contributes a potential ligand to Mg2+ coordination. Our observation is consistent with this theoretical prediction. Therefore, another two coordinates of the Mg2+ site may come from water molecules. It is also possible that additional water molecule(s) may directly coordinate Mg2+ such that some of the side chain(s) among Asp99, Asn172, Glu374, and Glu399 may serve as second shell ligand(s) to stabilize these water molecules in the first coordination shell33.
According to thermodynamic principles, Mg2+ binds to the channel with a higher affinity in the open state of the channel than in the closed state, thus facilitating channel opening24,34,35. The identification of the inter-domain formation of the Mg2+ binding site in this study provides a possible mechanism to explain the increase of Mg2+ binding affinity during channel opening. Recent studies on Mg2+-dependent activation indicate that the electrostatic interaction between Mg2+ and the VSD depends on channels opening, suggesting that a relative movement between the cytoplasmic domain and the VSD may occur during channel gating14,27. This relative movement may induce rearrangement of the relative orientation of Asp99, Asn172, Glu374 and Glu399, thereby resulting in optimal coordination and higher Mg2+ binding affinity in the open state.
METHODS
Mutagenesis and expression
All channel constructs were made from the mbr5 clone of the mouse Slo1 BK (mSlo1) by using PCR with Pfu polymerase (Stratagene, La Jolla, CA). The PCR-amplified regions of all mutants were verified by sequencing. RNA was transcribed in vitro with T3 polymerase (Ambion, Austin, TX). We injected 0.05-50 ng (for macroscopic currents) or 150-250 ng (for gating currents) of RNA into each Xenopus laevis oocyte 2-6 days before recording. For the mixture experiments, the mRNAs of two single mutants were mixed with 1:1 ratio; the same amount of mRNA of each single mutant was also injected as a control to monitor the expression rate.
Electrophysiology
Ionic currents were recorded with inside-out patches using Axopatch 200-B patch-clamp amplifier (Axon Instruments, Union City, CA) and Pulse acquisition software (Pulse; Heka Electronik, Lambrecht/Pfalz, Germany)12. The pipette solution contains (mM): 140 potassium methanesulfonic acid, 20 HEPES, 2 KCl, 2 MgCl2, pH 7.2. The basal internal solution contains (mM): 140 potassium methanesulfonic acid, 20 HEPES, 2 KCl, pH 7.2. MgCl2 was added to the internal solution (with 5 mM EGTA) to give the 10 mM free [Mg2+]i. A sewer pipe flow system was used to perfuse the internal solution on the cytoplasmic face of the patch.
Gating currents were recorded with inside-out patches25. The pipette solution contains (in mM): 127 tetraethylammonium (TEA) hydroxide, 125 methanesulfonic acid, 2 HCl, 2 MgCl2, 20 HEPES, pH 7.2. The internal solution contains (in mM): 141 N-methyl-D-glucamine (NMDG), 135 methanesulfonic acid, 6 HCl, 20 HEPES, 5 EGTA, pH 7.2. Gating currents of the same patch were first recorded in the absence of Mg2+, and then 2.5 M MgCl2 stock solution was added to the distal place of the bath stage (to avoid the disturbance of the seal of the patch), followed by a 6 min equilibration period to reach the target 10 mM before recording.
Voltage commands were filtered at 20 kHz with an eight-pole Bessel filter (Frequency Devices, Haverhill, MA) to prevent the saturation of fast capacitive transients25. Data were sampled at 100 kHz with an 18 bit A/D converter (ITC-18; Instrutech, Port Washington, NY) and filtered at 10 kHz with Axopatch’s internal filter. Capacitive transients and leak currents were subtracted using a P/5 protocol with a holding potential of -120 mV. All chemicals were obtained from Sigma-Aldrich Corp. (St Louis, MO, USA) unless otherwise noted.
Chemical Modification
10 mM dithiothreitol (DTT) was freshly made before each experiment. The inside-out patches were recorded first, followed by a 10-min treatment, and then were recorded again to see the DTT effect. [2-(trimethylammonium)ethyl] methanethiosulfonate bromide (MTSET) was purchased from Toronto Research Chemicals. An aliquot of 100 mM MTSET stock solution was thawed and diluted 500-fold into the basal internal solution immediately before use. In ionic current recordings, currents were recorded after 2.5 min of MTSET treatment and 0.5 min of washing of the intracellular side of patches.
Data Analysis and the model for inter-domain Mg2+ binding site
Relative conductance was determined by measuring macroscopic tail current amplitudes at -80 mV. The conductance-voltage (G-V) relations of the WT and mutant channels were fitted with the Boltzmann equation:
| (1) |
where, z is the number of equivalent gating charges, V1/2 is the voltage for channel in half activation, e is the elementary charge, k is Boltzmann’s constant and T is the absolute temperature. Each G-V curve was obtained from n = 5-24 patches. Error bars represent the standard error of mean in all figures.
Supplementary Figure 2b illustrates all the combinations of D99R:E374R mixture in 1:1 ratio with a binomial distribution, assuming that the Mg2+ binding site is formed by Asp99 and Glu374 from neighboring subunits. Each BK channel expressed from this mix contains n subunits of E374R (n ≤ 4) and (4-n) subunits of D99R. Thus, the probability of n = 0, 1, 2, 3, and 4 will be 0.0625, 0.25, 0.375, 0.25 and 0.0625, respectively. For n = 0 or 4, the channel is formed by either E374R or D99R so that there is no chance to form any intact binding site. For n = 1 or 3, there are four different arrangements of the channels, each of which is formed by one E374R and three D99R or vice versa, so that there is always one intact binding site. For n = 2, there are six different arrangements of the channels, each of which is formed by two E374R and two D99R. Among these six arrangements, four contain one intact binding site and two contain two intact binding sites. Therefore, the probability of forming a channel with 0, 1 and 2 intact binding sites is 0.125, 0.75 and 0.125, respectively. If each intact Mg2+ binding site makes an equal contribution to channel activation independently (25% of total Mg2+ sensing), the remaining Mg2+ sensitivity is:
where -53.2 is the Mg2+ sensitivity contributed by the Glu374/Glu399 site of WT channels from 0 to 10 mM [Mg2+]i.
Statistics were performed using SigmaStat 3.5 (Systat Software, Inc., San Jose, CA); Student’s t-test or One-Way ANOVA with an all-pairwise multiple comparison procedure (Tukey test) was performed. A p value of <0.05 was considered significant.
Structural Model
The structure of Mg2+ binding site of the mSlo1 channel was generated based on the crystal structure of the MthK channel by using the PyMol molecular graphics system (http://www.pymol.org).
Supplementary Material
Acknowledgments
We thank Lei Hu for calculations of Mg2+ sensitivity according to the model of inter-subunit Mg2+ binding site. We thank Chris Lingle, Lawrence Salkoff, and Lei Hu for critical discussion. The mSlo1 clone was kindly provided by Lawrence Salkoff (Washington University, St. Louis, MO). Frank Horrigan (Baylor College of Medicine, Houston, TX) kindly provided Y163K mutant mSlo1 cDNA. This work was supported by National Institutes of Health Grant R01-HL70393 and National Science Foundation of China Grant 30528011 (J.C.). J.C. is an Associate Professor of Biomedical Engineering on the Spencer T. Olin Endowment.
Footnotes
AUTHOR CONTRIBUTIONS H.Y., J.S., and J.C. designed the research; H.Y., J.S., G.Z., J.Y. and K.D. performed the experiments; H.Y., G.Z. and J.Y. analyzed the data; H.Y. and J.C. wrote the paper.
An abstract of this work has been presented in the 52nd Annual Meeting of Biophysical Society.
References
- 1.Hille B. Ion channels of excitable membranes. Sinauer; Sunderland, MA: 2001. [Google Scholar]
- 2.Long SB, Campbell EB, Mackinnon R. Voltage sensor of Kv1.2: structural basis of electromechanical coupling. Science. 2005;309:903–8. doi: 10.1126/science.1116270. [DOI] [PubMed] [Google Scholar]
- 3.Jiang Y, Lee A, Chen J, Cadene M, Chait BT, MacKinnon R. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 2002;417:515–22. doi: 10.1038/417515a. [DOI] [PubMed] [Google Scholar]
- 4.Miyazawa A, Fujiyoshi Y, Unwin N. Structure and gating mechanism of the acetylcholine receptor pore. Nature. 2003;423:949–55. doi: 10.1038/nature01748. [DOI] [PubMed] [Google Scholar]
- 5.Latorre R, Brauchi S. Large conductance Ca2+-activated K+ (BK) channel: activation by Ca2+ and voltage. Biol Res. 2006;39:385–401. doi: 10.4067/s0716-97602006000300003. [DOI] [PubMed] [Google Scholar]
- 6.Magleby KL. Gating mechanism of BK (Slo1) channels: so near, yet so far. J Gen Physiol. 2003;121:81–96. doi: 10.1085/jgp.20028721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci. 2006;7:921–931. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- 8.Hou S, Xu R, Heinemann SH, Hoshi T. Reciprocal regulation of the Ca2+ and H+ sensitivity in the SLO1 BK channel conferred by the RCK1 domain. Nat Struct Mol Biol. 2008;15:403–10. doi: 10.1038/nsmb.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou S, Xu R, Heinemann SH, Hoshi T. The RCK1 high-affinity Ca2+ sensor confers carbon monoxide sensitivity to Slo1 BK channels. Proc Natl Acad Sci U S A. 2008;105:4039–43. doi: 10.1073/pnas.0800304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang XD, Xu R, Reynolds MF, Garcia ML, Heinemann SH, Hoshi T. Haem can bind to and inhibit mammalian calcium-dependent Slo1 BK channels. Nature. 2003;425:531–5. doi: 10.1038/nature02003. [DOI] [PubMed] [Google Scholar]
- 11.Zeng XH, Xia XM, Lingle CJ. Divalent cation sensitivity of BK channel activation supports the existence of three distinct binding sites. J Gen Physiol. 2005;125:273–86. doi: 10.1085/jgp.200409239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi J, Krishnamoorthy G, Yang Y, Hu L, Chaturvedi N, Harilal D, Qin J, Cui J. Mechanism of magnesium activation of calcium-activated potassium channels. Nature. 2002;418:876–80. doi: 10.1038/nature00941. [DOI] [PubMed] [Google Scholar]
- 13.Xia XM, Zeng X, Lingle CJ. Multiple regulatory sites in large-conductance calcium-activated potassium channels. Nature. 2002;418:880–4. doi: 10.1038/nature00956. [DOI] [PubMed] [Google Scholar]
- 14.Yang H, Hu L, Shi J, Delaloye K, Horrigan FT, Cui J. Mg2+ mediates interaction between the voltage sensor and cytosolic domain to activate BK channels. Proc Natl Acad Sci U S A. 2007;104:18270–5. doi: 10.1073/pnas.0705873104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 2006;21:69–78. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- 16.Toro L, Wallner M, Meera P, Tanaka Y. Maxi-KCa, a Unique Member of the Voltage-Gated K Channel Superfamily. News Physiol Sci. 1998;13:112–117. doi: 10.1152/physiologyonline.1998.13.3.112. [DOI] [PubMed] [Google Scholar]
- 17.Fettiplace R, Fuchs PA. Mechanisms of hair cell tuning. Annu Rev Physiol. 1999;61:809–34. doi: 10.1146/annurev.physiol.61.1.809. [DOI] [PubMed] [Google Scholar]
- 18.Du W, Bautista JF, Yang H, Diez-Sampedro A, You SA, Wang L, Kotagal P, Luders HO, Shi J, Cui J, Richerson GB, Wang QK. Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat Genet. 2005;37:733–8. doi: 10.1038/ng1585. [DOI] [PubMed] [Google Scholar]
- 19.Ma Z, Lou XJ, Horrigan FT. Role of Charged Residues in the S1-S4 Voltage Sensor of BK Channels. J Gen Physiol. 2006;127:309–328. doi: 10.1085/jgp.200509421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang Y, Pico A, Cadene M, Chait BT, MacKinnon R. Structure of the RCK domain from the E. coli K+ channel and demonstration of its presence in the human BK channel. Neuron. 2001;29:593–601. doi: 10.1016/s0896-6273(01)00236-7. [DOI] [PubMed] [Google Scholar]
- 21.Fodor AA, Aldrich RW. Statistical limits to the identification of ion channel domains by sequence similarity. J Gen Physiol. 2006;127:755–66. doi: 10.1085/jgp.200509419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang H, Hu L, Shi J, Cui J. Tuning Magnesium Sensitivity of BK Channels by Mutations. Biophys J. 2006;91:2892–900. doi: 10.1529/biophysj.106.090159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dudev T, Lim C. Principles governing Mg, Ca, and Zn binding and selectivity in proteins. Chem Rev. 2003;103:773–88. doi: 10.1021/cr020467n. [DOI] [PubMed] [Google Scholar]
- 24.Hu L, Yang H, Shi J, Cui J. Effects of Multiple Metal Binding Sites on Calcium and Magnesium-dependent Activation of BK Channels. J Gen Physiol. 2006;127:35–50. doi: 10.1085/jgp.200509317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horrigan FT, Aldrich RW. Allosteric voltage gating of potassium channels II. Mslo channel gating charge movement in the absence of Ca2+ J Gen Physiol. 1999;114:305–36. doi: 10.1085/jgp.114.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horrigan FT, Aldrich RW. Coupling between voltage sensor activation, Ca2+ binding and channel opening in large conductance (BK) potassium channels. J Gen Physiol. 2002;120:267–305. doi: 10.1085/jgp.20028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horrigan FT, Ma Z. Mg2+ enhances voltage sensor/gate coupling in BK channels. J Gen Physiol. 2008;131:13–32. doi: 10.1085/jgp.200709877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hazes B, Dijkstra BW. Model building of disulfide bonds in proteins with known three-dimensional structure. Protein Eng. 1988;2:119–25. doi: 10.1093/protein/2.2.119. [DOI] [PubMed] [Google Scholar]
- 29.Flynn GE, Zagotta WN. Conformational changes in S6 coupled to the opening of cyclic nucleotide-gated channels. Neuron. 2001;30:689–98. doi: 10.1016/s0896-6273(01)00324-5. [DOI] [PubMed] [Google Scholar]
- 30.Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- 31.Wallner M, Meera P, Toro L. Determinant for beta-subunit regulation in high-conductance voltage-activated and Ca2+-sensitive K+ channels: an additional transmembrane region at the N terminus. Proc Natl Acad Sci U S A. 1996;93:14922–7. doi: 10.1073/pnas.93.25.14922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cox DH, Cui J, Aldrich RW. Allosteric gating of a large conductance Ca-activated K+ channel. J Gen Physiol. 1997;110:257–81. doi: 10.1085/jgp.110.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dudev T, Lin YL, Dudev M, Lim C. First-second shell interactions in metal binding sites in proteins: a PDB survey and DFT/CDM calculations. J Am Chem Soc. 2003;125:3168–80. doi: 10.1021/ja0209722. [DOI] [PubMed] [Google Scholar]
- 34.Shi J, Cui J. Intracellular Mg2+ enhances the function of BK-type Ca2+-activated K+ channels. J Gen Physiol. 2001;118:589–606. doi: 10.1085/jgp.118.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, Solaro CR, Lingle CJ. Allosteric regulation of BK channel gating by Ca2+ and Mg2+ through a nonselective, low affinity divalent cation site. J Gen Physiol. 2001;118:607–36. doi: 10.1085/jgp.118.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang G, Horrigan FT. Cysteine modification alters voltage- and Ca2+-dependent gating of large conductance (BK) potassium channels. J Gen Physiol. 2005;125:213–36. doi: 10.1085/jgp.200409149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.