Abstract
Study Objectives:
In patients with obstructive sleep apnea (OSA), the severity and frequency of respiratory events is increased in the supine body posture compared with the lateral recumbent posture. The mechanism responsible is not clear but may relate to the effect of posture on upper airway shape and size. This study compared the effect of body posture on upper airway shape and size in individuals with OSA with control subjects matched for age, BMI, and gender.
Participants:
11 males with OSA and 11 age- and BMI-matched male control subjects.
Results:
Anatomical optical coherence tomography was used to scan the upper airway of all subjects while awake and breathing quietly, initially when supine, and then in the lateral recumbent posture. A standard head, neck, and tongue position was maintained during scanning. Airway cross-sectional area (CSA) and anteroposterior (A-P) and lateral diameters were obtained in the oropharyngeal and velopharyngeal regions in both postures. A-P to lateral diameter ratios provided an index of regional airway shape. In equivalent postures, the ratio of A-P to lateral diameter in the velopharynx was similar in OSA and control subjects. In both groups, this ratio was significantly less for the supine than for the lateral recumbent posture. CSA was smaller in OSA subjects than in controls but was unaffected by posture.
Conclusions:
The upper airway changes from a more transversely oriented elliptical shape when supine to a more circular shape when in the lateral recumbent posture but without altering CSA. Increased circularity decreases propensity to tube collapse and may account for the postural dependency of OSA.
Citation:
Walsh JH; Leigh MS; Paduch A; Maddison KJ; Armstrong JJ; Sampson DD; Hillman DR; Eastwood PR. Effect of body posture on pharyngeal shape and size in adults with and without obstructive sleep apnea. SLEEP 2008;31(11):1543–1549.
Keywords: Pharyngeal anatomy, pharyngeal size, pharyngeal shape, body posture, anatomical optical coherence tomography
OBSTRUCTIVE SLEEP APNEA (OSA) IS EXACERBATED BY THE SUPINE POSTURE IN THE MAJORITY OF PATIENTS AND APPROXIMATELY 60% OF PATIENTS have positional sleep apnea, defined as a supine apnea hypopnea index (AHI) twice that observed when in the lateral recumbent posture.1,2 Apnea severity (apnea duration, minimum oxygen desaturation, arousal length and frequency) is increased when supine.3 The optimal level of continuous positive airway pressure,4 and the critical closing pressure, an objective measure of airway collapsibility, are higher when a subject is supine than when in the lateral recumbent posture.5,6 Similarly, the pressure required to reestablish airflow is higher in the supine than in the lateral recumbent posture.7 In some individuals, merely avoiding the supine posture during sleep is sufficient to resolve sleep apnea.1
The mechanism responsible for the worsening of sleep disordered breathing in the supine posture is not clear but most likely relates to the effect of gravity on upper airway size or shape. Gravitational effects could act directly on the upper airway by displacing anterior pharyngeal structures and the pharynx,6 or indirectly by displacing the abdominal contents into the thorax and decreasing lung volume,8 and thereby decreasing the tension within the walls of the upper airway9 and increasing its susceptibility to collapse. In the lateral recumbent posture, these compressive gravitational effects are reduced.
It is commonly thought that these effects result in a smaller pharyngeal airway in the supine than in the lateral recumbent posture, making it more vulnerable to collapse.10 However, reports are inconsistent in this regard, with some studies reporting the pharynx to be smaller in the supine than in the lateral recumbent posture6,11,12 and others reporting a similar pharyngeal size in the 2 postures.13–15 It is possible that airway shape may also contribute to its propensity to collapse, as several studies have suggested that orientation of the elliptically shaped upper airway differs between individuals with and without OSA. Specifically, they suggest that in individuals with OSA, the long axis of the ellipse is oriented anteroposteriorally, making the lateral pharyngeal walls more susceptible to collapse, whereas in subjects without OSA, the long axis of the ellipse is oriented transversely.16–19 However, this observation has not been consistent; numerous other studies report airway shape to be similar in apneics and non-apneic controls.14,20–26 The effect of posture on pharyngeal shape is unknown.
The aim of the current study was to address these questions by measuring upper airway shape and size, and the effect on them of change in body position, in awake individuals with and without OSA. We used anatomical optical coherence tomography (aOCT), a quantitative imaging technique particularly suited to repeated measurements in the same individual.27–29
METHODS
Subjects
Eleven male volunteers with a BMI < 30 kg/m2 were recruited from patients with recently diagnosed OSA (AHI > 10/h) on a laboratory-based polysomnogram.30,31 They were not selected on the basis of presence or absence of positional OSA (defined as supine AHI > 2 times lateral AHI and a total AHI of > 12.5/h, having slept ≥ 30 min in each posture).2,32 They had not previously received treatment for OSA, including upper airway surgery, and were otherwise healthy. Eleven healthy BMI- and age-matched male control subjects without a history of habitual snoring were recruited from local service clubs. OSA was excluded (AHI < 10/h) by a full night of laboratory-based polysomnography. The Human Research Ethics Committee of Sir Charles Gairdner Hospital approved the project, and informed written consent was obtained from all participants.
Protocol
Measurements of velopharyngeal and oropharyngeal shape and size were obtained in each subject using anatomical optical coherence tomography (aOCT).27–29 Briefly, aOCT requires a sealed, transparent catheter (3.0 mm outside diameter) to be inserted via the nares to mid-esophageal level. An optical probe is moved systematically within the catheter, which is fixed in position. The distance between the head of the optical probe and the air-tissue interface of the airway wall is determined from reflected light, using a low-coherence optical interferometer. A software program controls the longitudinal translation and rotation (1.25 Hz) of the probe head, enabling collection of quantitative cross-sectional images at regions of interest within the pharynx.
All aOCT scans were performed while the subject was awake; initially supine, then repeated in the lateral recumbent posture. Because head and body position have been shown to influence airway size,12 measurements were obtained with the head and neck in a controlled neutral posture. Specifically, when supine, the head was supported with a Shea headrest (Gyrus ENT, Memphis, TN, USA) and a goniometer was used to position the Frankfort plane (line from infraorbital rim to tragus of the ear) perpendicular to the bed. When lateral recumbent, the body was perpendicular to the axis of the bed and the head supported with pillows and foam pads to eliminate rotation or lateral flexion/extension of the head and/or neck. A goniometer was used to align the Frankfort plane perpendicular to the long axis of the body. The subject was instructed to breathe quietly, not speak, and to maintain a constant head and tongue position during all scans.
Rib cage and abdominal motion were continuously monitored at 1000Hz (Powerlab model 16s; ADInstruments, Sydney, NSW, Australia) by respiratory inductance pneumography (Respitrace, Ambulatory Monitoring, Ardsley, NY, USA).
Airway Imaging
A “pullback” scan was performed in each subject, in each posture, by systematically retracting the aOCT probe from the upper esophagus to the nasal cavity at a constant speed (0.2 mm/sec). Each pullback scan took between 9 and 12 min, during which time approximately 900 images were obtained. Each image displayed airway cross-sectional dimensions for the previous 0.8 sec. Images were time-synchronized with the summed pneumography signal and reconstructed to provide a video with each frame providing a single quantitative cross-sectional image of the pharynx.
Two regions of interest were defined from the reconstructed video: the oropharynx (tip of epiglottis to base of uvula); and the velopharynx (distal portion of the nasopharynx immediately proximal to the base of the uvula). The precise locations of the selected images within each region were determined a priori according to the following: oropharyngeal cross-sectional images were obtained from the mid-oropharynx or, where the uvula was visible in the mid-oropharynx, just distal to the tip of the uvula; velopharyngeal cross-sectional images were obtained approximately 7 mm craniad to the base of the uvula.
Analysis
Oropharyngeal and velopharyngeal images were selected by the same experienced investigator at the point of maximum and minimum cross-sectional area (CSA) during multiple successive respiratory cycles. In instances where images from 3 respiratory cycles were not available for analysis, 2 successive cycles or 1 cycle was used if, by inspection of the video, they were judged to be representative of that region. In instances where a complete airway profile was not visible, images were either excluded from analysis or, if at least 75% of the profile was visible, a straight line connected the visible portions of the airway. In cases where images for successive respiratory cycles at a given location were analyzed, each was performed independently and mean values used for statistical analyses.
No assumptions were made as to the relationship between maximum and minimum CSA and phase of respiration; however, for all measurements, the phase of respiration in which maximum and minimum CSA occurred was documented.
Analyses of aOCT images were performed using ImageJ software (National Institutes of Health, Bethesda, MD). For each image, the mucosa-lumen interface was manually traced by the same experienced investigator and airway CSA calculated. A-P diameter was calculated at the widest point in the parasagittal plane and lateral diameter was measured at the widest point in the coronal plane, perpendicular to the A-P diameter.14,17 The intraclass correlation coefficient for repeat measurements of airway CSA and diameters was 0.99 (P < 0.0001) and is reported in more detail elsewhere.28
Statistical Analysis
Student unpaired t-tests were used to compare anthropometric and polysomnographic measurements between control and OSA groups. Two-way repeated-measures ANOVA (SigmaStat, San Jose, CA, USA) was used to compare differences in regional pharyngeal dimensions and locations, at both maximum and minimum CSA, between OSA and control groups in the supine and lateral recumbent postures. A Holm-Sidak test was applied for all post hoc comparisons. Unless stated, all data are reported as mean ± SD. Significance was assumed at P < 0.05.
RESULTS
Anthropometric and polysomnographic measurements in the 11 OSA and 11 control subjects are presented in Table 1. The 2 groups were well-matched for age and BMI. AHI ranged from 15.0 to 76.8 events/h in the OSA group. In all OSA subjects, AHI was less in the lateral posture than in the supine. Seven OSA subjects met the criteria for positional sleep apnea.2,32 In all but one control subject, AHI was less in the lateral posture than in the supine. Although total AHI was < 10/h in the control subjects, 7 had a supine AHI greater than twice that in the lateral posture.
Table 1.
Anthropometric and Polysomnographic Measurements in OSA and Healthy Control Subjects
| OSA (n = 11) | Control (n = 11) | |
|---|---|---|
| Age (y) | 56 ± 13 | 59 ± 9 |
| BMI (kg/m2) | 27.9 ± 1.0 | 25.9 ± 1.7 |
| AHI (events/h) | 39.6 ± 19.1* | 3.3 ± 2.5 |
| Supine AHI (events/h) | 53.7 ± 22.2* | 9.2 ± 9.2 |
| NREM (events/h) | 55.1 ± 23.4* | 9.2 ± 9.2 |
| REM (events/h) | 52.9 ± 21.6* | 4.1 ± 10.3 |
| Lateral Recumbent AHI (events/h) | 23.8 ± 21.6*† | 1.0 ± 1.0† |
| NREM (events/h) | 25.8 ± 22.0*† | 0.5 ± 0.6† |
| REM (events/h) | 38.0 ± 13.9*† | 2.4 ± 2.8† |
Values are mean ± SD.
P < 0.05 vs control
P < 0.01 vs equivalent when supine; BMI, body mass index; AHI, apnea hypopnea index.
Image Location and Analysis
In some individuals, at some sites, it was not possible to visualize the complete circumference of the airway. In the 22 subjects examined in the present study, axial images with ≥ 75% of the airway circumference (including lateral extents) visible were obtained in 91% and 95% of scans performed in the supine and lateral postures, respectively. Of the images analyzed, 56% were complete profiles, with the remainder requiring modest straight-line extrapolation to connect the visible portions of the airway profile with similar frequency of extrapolation in subjects with and without OSA.
The locations of velopharyngeal and oropharyngeal images relative to anatomical landmarks were similar in the OSA and control subjects and in the lateral and supine posture (ANOVA, P = 0.27). For example, velopharyngeal images in the supine posture were obtained 6.8 ± 1.4 and 6.4 ± 3.4 mm craniad to the base of the uvula in the OSA and control groups, respectively. Oropharyngeal images were 22.0 ± 4.8 and 17.2 ± 3.5 mm caudad to the base of the uvula in the OSA and control groups, respectively.
Effect of Posture on Airway Size and Dimensions
The effects of posture on wakeful velopharyngeal and oropharyngeal shape and size are shown in the images in Figure 1. The tissue-air boundaries in each figure appear fuzzy because aOCT also detects subsurface reflections, but the interface between the airway and airway wall is sharp, allowing accurate surface location. Most notable features from these images are: (i) the smaller velopharynx in the individual with OSA than the matched control subject; and (ii) the marked effect of body posture on airway shape in both individuals in the velopharynx and oropharynx. These changes were representative of the group data, which are presented below.
Figure 1.
Representative aOCT images of the velopharynx and oropharynx from one healthy control subject and one OSA subject in the supine and lateral recumbent postures. All scans were obtained when the airway was at its minimum cross-sectional area during the respiratory cycle. The inner and outer walls of the imaging catheter are visible within each airway. All images have been rotated to align the anterior pharyngeal wall with the top of the page.
Cross-Sectional Area
OSA vs Control
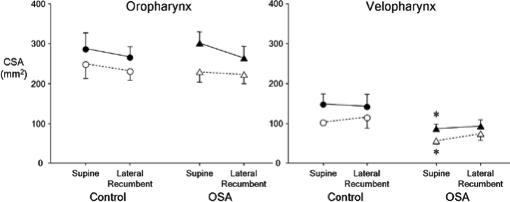
Velopharyngeal maximum and minimum CSA were significantly less in OSA subjects than in control subjects in supine (P < 0.05 and P < 0.01, respectively) but not lateral recumbent posture (P = 0.12 and 0.11, respectively) (Figure 2). Oropharyngeal maximum and minimum CSA were similar in OSA and control groups for each posture (P > 0.2 for all comparisons).
Figure 2.
Maximum (closed symbols) and minimum (open symbols) cross-sectional area (CSA) in the oropharynx (left panel) and velopharynx (right panel) in healthy control (circles) and OSA subjects (triangles) in the supine and lateral recumbent postures. n = 11 per group; mean ± SE; * significantly different from control group; P < 0.05.
Supine vs Lateral
Moving from the supine to lateral recumbent posture had no effect on velopharyngeal CSA (P > 0.2 for all comparisons) or oropharyngeal CSA (P > 0.3 for all comparisons) in either group (Figure 2). Maximum velopharyngeal CSA occurred during expiration in 72% of control subjects and in 67% of OSA subjects, whereas maximum oropharyngeal CSA occurred during expiration in 62% of controls and in 72% of OSA subjects (pooled data from both postures).
Lateral Diameter
OSA vs Control
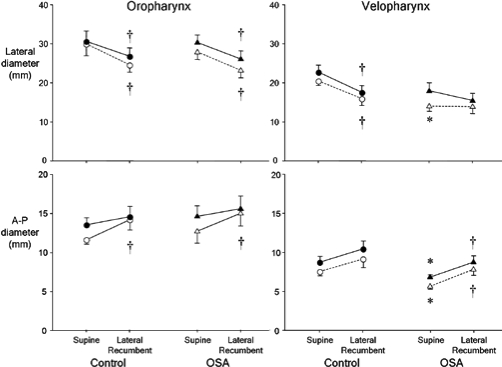
Velopharyngeal lateral diameter at minimum velopharyngeal CSA was less in OSA subjects than controls in supine (P < 0.05), but not lateral recumbent posture (P > 0.4; Figure 3). Lateral velopharyngeal diameter at maximum CSA was similar in both groups in both postures. Oropharyngeal lateral diameter was similar in OSA and in control subjects in both postures when measured at maximum and minimum CSA (Figure 3).
Figure 3.
Lateral (upper) and anteroposterior (A-P) (lower) diameter at maximum (closed symbols) and minimum (open symbols) cross-sectional area (CSA) in the oropharynx (left panel) and velopharynx (right panel) in healthy control (circles) and OSA subjects (triangles) in the supine and lateral recumbent postures. n = 11 per group; mean ± SE; * significantly different from control group; P < 0.05; †significantly different from supine posture; P < 0.05.
Supine vs Lateral
Moving from the supine to the lateral recumbent posture (i) decreased velopharyngeal lateral diameter at both maximum and minimum velopharyngeal CSA in control subjects (P < 0.01 for both) but not in OSA subjects (P > 0.2 for both); and (ii) decreased oropharyngeal lateral diameter in control and OSA groups at both minimum and maximum CSA (P < 0.04 for all comparisons) (Figure 3).
Anteroposterior (A-P) Diameter
OSA vs Control
There was a significant group and posture effect on velopharyngeal A-P diameter at both maximum and minimum CSA. However, because of a large increase in A-P diameter in one control subject when moving from supine to lateral (10.7 to 19.0 mm at maximum CSA) and the associated increase in variability, post hoc analyses did not identify the differences. When this subject was excluded from the analysis, it was revealed that velopharyngeal A-P diameter was smaller in OSA than control subjects in supine, but not in lateral recumbent posture when measured at maximum and minimum CSA (P < 0.05 for both) (Figure 3). Oropharyngeal A-P diameter was similar in OSA and control subjects in both postures when measured at maximum and minimum CSA.
Supine vs Lateral
Moving from the supine to lateral recumbent posture (i) increased the A-P diameter at both maximum and minimum CSA in the OSA and control subjects (P < 0.05 for all comparisons); and (ii) increased oropharyngeal A-P diameter in both the OSA and control groups (P < 0.05 for both) when at minimum CSA, but did not alter oropharyngeal A-P diameter in either group when at maximum CSA (P > 0.2 for both) (Figure 3).
Shape
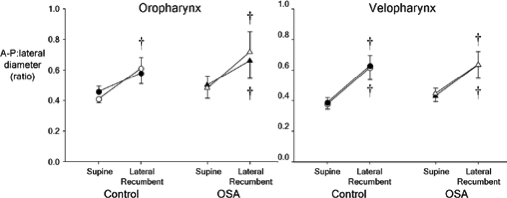
The ratio of A-P to lateral diameter provides an index of the circularity of the airway, with a ratio of 1.0 representing a circle, a ratio <1.0 representing an ellipse with its long axis oriented laterally, and a ratio >1.0 representing an ellipse with its long axis oriented in the A-P dimension.
OSA vs Control
The shape of the airway changed minimally with respiration, as seen by the lack of change in this ratio between minimum and maximum CSA (Figure 4). The ratio at maximum and minimum CSA was similar in the OSA and control groups in both the supine and lateral recumbent postures, indicating a similarly shaped airway in both groups in both body postures.
Figure 4.
Ratio of anteroposterior (A-P):lateral diameter at maximum (closed symbols) and minimum (open symbols) cross-sectional area (CSA) in the oropharynx (left panel) and velopharynx (right panel) in healthy control (circles) and OSA subjects (triangles) in the supine and lateral recumbent postures. n = 11 per group; mean ± SE; † significantly different from supine posture; P < 0.05
Supine vs Lateral
Except for the oropharynx of the OSA group at maximum CSA, this ratio was less than 1.0 and increased with change from the supine to lateral recumbent posture in both groups and in both pharyngeal regions, both at minimum and maximum CSA (P < 0.05 for all comparisons) (Figure 4). In the oropharynx of the OSA group at maximum CSA, the ratio increased, but this change did not reach statistical significance (P = 0.10).
DISCUSSION
The mechanism underlying aggravation of sleep disordered breathing in the supine posture remains to be defined, although gravity-related changes in pharyngeal dimensions have been thought to play a major role. Although consideration has been given to posture-related changes in airway caliber, the effect of posture on pharyngeal shape has not previously been examined.
The present study utilized a novel imaging technique suitable for repetitive, quantitative imaging of the upper airway, aOCT, to demonstrate that moving from the supine to the lateral recumbent posture alters the shape, but not the size of the velopharyngeal and oropharyngeal airways in individuals with and without OSA. Specifically, the airway changes from a transversely oriented elliptical shape when supine to a more circular shape when in the lateral recumbent posture. This change in shape may be an important factor underlying the decreased propensity of the upper airway to collapse when in a lateral recumbent posture. Laplace’s Law states that at equilibrium, the transmural pressure across a concave surface is directly proportional to wall tension and inversely proportional to its radius of curvature. It follows that the transmural pressure gradient required to collapse the airway varies inversely with its radius of curvature. Hence, as the transverse elliptical airway assumes a more circular shape with change to the lateral posture, its propensity to collapse decreases as a function of the reduction in radius of curvature of its anterior and posterior walls. This propensity can be expressed dimensionally as proportional to the ratio of the lengths of the major and minor axes of the elliptical cross-section.
Our finding that, when supine, individuals with OSA and BMI- and age-matched control subjects have a similarly shaped airway (an ellipse with its long axis oriented laterally) in both the velopharyngeal and oropharyngeal regions contrasts with some,16–19 but not all14,20–26 previous reports of pharyngeal shape. The different findings could be attributable to a number of factors, including differences in location and orientation of images, variable head or neck flexion/extension,16 averaging of images over several breaths16 versus breath holding,18 or to the presence of adenotonsillar hypertrophy on the lateral airway walls.19 Our study addresses these potential confounding factors through careful matching of age and BMI in our all-male subjects; control of head posture; and the use of an imaging technique (aOCT) that produces quantitative, breath-by-breath images orthogonal to the airway wall. The accuracy of the orthogonal plane alignment is a central issue that has proved difficult to control with older computed tomography (CT) scanning technologies.33 Failure to obtain orthogonal images would introduce substantial error into assessments of size and shape.
Measurements obtained in the present study indicate a lack of posture influence on upper airway caliber. This finding is also in agreement with some,13–15 but not all,6,11,12 previous imaging studies. Two studies utilizing acoustic reflection found no difference in total airway volume or area at the level of the oropharyngeal junction between supine and lateral recumbent postures in non-snoring, snoring, and sleep apneic subjects during wakefulness.13,15 Similarly, awake CT studies in positional and non-positional OSA patients showed no difference in minimum or mean CSA of the entire airway between the supine and lateral recumbent postures.14 In contrast, Isono et al. used videoendoscopy to show that CSA was larger in the supine than lateral recumbent posture in anesthetized and paralyzed OSA patients at a range of static airway pressures.6 Magnetic resonance imaging (MRI) studies in healthy sedated children11 and awake young adults12 have also shown decreased retroglossal airway volumes and CSA in supine than lateral recumbent posture. The reasons for the discrepancies between studies are not entirely clear, but may relate to differences in gender or age of study participants, conscious state, disease severity, or use of image gating with phase of respiration.
The findings of the present study provide several insights into the positional dependence of OSA. The combined effects of posture, gravity and upper airway anatomy can be considered in terms of the “bony enclosure’”model described by Isono et al.,6 which suggests that non-uniform distribution of soft tissue around the pharyngeal airway may result in regional differences in the extraluminal forces acting on the airway (Figure 6). Based upon an airway with similar lateral and A-P dimensions in both postures (i.e., a circular airway), Isono’s model proposes that the larger soft tissue mass in the anterior pharyngeal airway compartment exerts greater pressure on the anterior airway when in the supine posture than the relatively smaller tissue mass in the lateral compartment exerts on the lateral airway wall when in the lateral recumbent posture. In other words, the increased extraluminal tissue pressure on the anterior pharyngeal airway when supine increases its susceptibility to collapse in this posture.
Our findings suggest airway shape as an alternate or additional influence. The greater radius of curvature of the anterior and posterior walls of the transverse elliptical airway in the supine posture, relative to the circular airway in the lateral recumbent posture, increases its propensity to collapse, as outlined earlier. This may be particularly relevant when airway CSA is reduced, as has been observed in OSA patients in the present study and by others.17,19,22,24,34 A smaller CSA requires less change in airway caliber for collapse to occur and is also associated with more negative intraluminal pressure for a given inspiratory flow, which also increases the tendency to collapse.
Other factors may also contribute to the positional dependency of OSA, such as posture-related changes in lung volume or activity of pharyngeal muscles. Previous studies have demonstrated that functional residual capacity is less in the supine than in the lateral recumbent posture.35–37 A lower lung volume could act to decrease pharyngeal patency and increase pharyngeal collapsibility through a decrease in longitudinal airway tension.9,38–40 It is possible that such volume-related changes in airway stability could occur without accompanying changes in pharyngeal dimensions.41 Changes in upper airway muscle activity may also contribute to posture-related changes in airway shape. Several studies have demonstrated increased genioglossus muscle activity when supine compared to the upright42–44 and lateral recumbent postures.44,45 However, others have reported decreased suprahyoid muscle activity in the supine compared to lateral recumbent posture.46,47 Thus, a consistent relationship between upper airway shape and size and muscle activity is yet to be defined. It is important to note, however, that the findings of the present study were obtained in wakeful subjects. Thus, confirmation of the role of changes in pharyngeal shape and size on the postural worsening of OSA will require studies of sleeping subjects, which represents a natural extension of the present study.
In conclusion, aOCT-derived measurements when supine indicate that healthy control subjects have a larger velopharyngeal airway than subjects with OSA, despite having a similarly shaped airway: elliptical with the long axis in the lateral dimension. Change to the lateral recumbent posture makes the airway more circular in both groups, but does not alter its CSA. This change in shape provides a cogent explanation for the reduced propensity for pharyngeal collapse in the lateral recumbent relative to supine posture.
Figure 5.
Schematic representation of the compartmental tissue arrangement surrounding the pharyngeal airway when in the supine and lateral recumbent postures. BE, bony enclosure; PA, pharyngeal airway; A, anterior soft tissue mass; P, posterior soft tissue mass; L, lateral soft tissue mass. Note (i) the increased circularity of the airway in the lateral recumbent posture, (ii) greater radius of curvature of the anterior and posterior airway walls in the supine posture, and (iii) the relatively greater mass on the anterior pharyngeal airway when supine (shaded region, A) than the mass on the lateral pharyngeal airway when lateral recumbent (shaded region, L). Modified from Isono’s bony enclosure model.6
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Hillman has received research support from ResMed and has consulted for ResMed and Inspiration Medical. Dr. Eastwood has consulted for Inspiration Medical. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Sources of Funding: The study has been supported by the National Health and Medical Research Council Australia (Project Grant No. 403953; Development Grant No. 303319). PRE was supported by a NHMRC Senior Research Fellowship (No. 513704).
ABBREVIATIONS
- OSA
obstructive sleep apnea
- BMI
body mass index
- CSA
cross sectional area
- A-P
anteroposterior
- AHI
apnea hypopnea index
- (aOCT)
anatomical optical coherence tomography
- CT
computed tomography
- MRI
magnetic resonance imaging
REFERENCES
- 1.Cartwright RD. Effect of sleep position on sleep apnea severity. Sleep. 1984;7:110–4. doi: 10.1093/sleep/7.2.110. [DOI] [PubMed] [Google Scholar]
- 2.Oksenberg A. Positional vs nonpositional obstructive sleep apnea patients: anthropomorphic, nocturnal polysomnographic, and multiple sleep latency test data. Chest. 1997;112:629–39. doi: 10.1378/chest.112.3.629. [DOI] [PubMed] [Google Scholar]
- 3.Oksenberg A. Association of body position with severity of apneic events in patients with severe nonpositional obstructive sleep apnea. Chest. 2000;118:1018–24. doi: 10.1378/chest.118.4.1018. [DOI] [PubMed] [Google Scholar]
- 4.Oksenberg A. The sleep supine position has a major effect on optimal nasal continuous positive airway pressure: relationship with rapid eye movements and non-rapid eye movements sleep, body mass index, respiratory disturbance index, and age. Chest. 1999;116:1000–6. doi: 10.1378/chest.116.4.1000. [DOI] [PubMed] [Google Scholar]
- 5.Boudewyns A, et al. Abbreviated method for assessing upper airway function in obstructive sleep apnea. Chest. 2000;118:1031–41. doi: 10.1378/chest.118.4.1031. [DOI] [PubMed] [Google Scholar]
- 6.Isono S. Lateral position decreases collapsibility of the passive pharynx in patients with obstructive sleep apnea. Anesthesiology. 2002;97:780–5. doi: 10.1097/00000542-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Neill AM. Effects of sleep posture on upper airway stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1997;155:199–204. doi: 10.1164/ajrccm.155.1.9001312. [DOI] [PubMed] [Google Scholar]
- 8.Fouke JM. Effect of position and lung volume on upper airway geometry. J Appl Physiol. 1987;63:375–80. doi: 10.1152/jappl.1987.63.1.375. [DOI] [PubMed] [Google Scholar]
- 9.Kairaitis K. Tracheal traction effects on upper airway patency in rabbits: the role of tissue pressure. Sleep. 2007;30:179–86. doi: 10.1093/sleep/30.2.179. [DOI] [PubMed] [Google Scholar]
- 10.Oksenberg A. The effect of body posture on sleep-related breathing disorders: facts and therapeutic implications. Sleep Med Rev. 1998;2:139–62. doi: 10.1016/s1087-0792(98)90018-1. [DOI] [PubMed] [Google Scholar]
- 11.Litman RS, et al. Effect of lateral positioning on upper airway size and morphology in sedated children. Anesthesiology. 2005;103:484–8. doi: 10.1097/00000542-200509000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Ono T. Effects of head and body position on two- and three-dimensional configurations of the upper airway. J Dent Res. 2000;79:1879–84. doi: 10.1177/00220345000790111101. [DOI] [PubMed] [Google Scholar]
- 13.Jan MA. Effect of posture on upper airway dimensions in normal human. Am J Respir Crit Care Med. 1994;149:145–8. doi: 10.1164/ajrccm.149.1.8111573. [DOI] [PubMed] [Google Scholar]
- 14.Pevernagie DA. Effects of body position on the upper airway of patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1995;152:179–85. doi: 10.1164/ajrccm.152.1.7599821. [DOI] [PubMed] [Google Scholar]
- 15.Martin SE. The effect of posture on airway caliber with the sleep-apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1995;152:721–4. doi: 10.1164/ajrccm.152.2.7633733. [DOI] [PubMed] [Google Scholar]
- 16.Rodenstein DO, et al. Pharyngeal shape and dimensions in healthy subjects, snorers, and patients with obstructive sleep apnoea. Thorax. 1990;45:722–7. doi: 10.1136/thx.45.10.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwab RJ. Dynamic upper airway imaging during awake respiration in normal subjects and patients with sleep disordered breathing. Am Rev Respir Dis. 1993;148:1385–400. doi: 10.1164/ajrccm/148.5.1385. [DOI] [PubMed] [Google Scholar]
- 18.Fogel RB, et al. Anatomic and physiologic predictors of apnea severity in morbidly obese subjects. Sleep. 2003;26:150–5. doi: 10.1093/sleep/26.2.150. [DOI] [PubMed] [Google Scholar]
- 19.Arens R, et al. Changes in upper airway size during tidal breathing in children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2005;171:1298–304. doi: 10.1164/rccm.200411-1597OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuna ST. Effect of nasal airway positive pressure on upper airway size and configuration. Am Rev Respir Dis. 1988;138:969–75. doi: 10.1164/ajrccm/138.4.969. [DOI] [PubMed] [Google Scholar]
- 21.Stanford W. Effects of awake tidal breathing, swallowing, nasal breathing, oral breathing and the Muller and Valsalva maneuvers on the dimensions of the upper airway. Evaluation by ultrafast computerized tomography. Chest. 1988;94:149–54. doi: 10.1378/chest.94.1.149. [DOI] [PubMed] [Google Scholar]
- 22.Schwab RJ. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med. 1995;152:1673–89. doi: 10.1164/ajrccm.152.5.7582313. [DOI] [PubMed] [Google Scholar]
- 23.Ryan CF. Mechanical properties of the velopharynx in obese patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1996;154:806–12. doi: 10.1164/ajrccm.154.3.8810623. [DOI] [PubMed] [Google Scholar]
- 24.Ciscar MA, et al. Magnetic resonance imaging of the pharynx in OSA patients and healthy subjects. Eur Respir J. 2001;17:79–86. doi: 10.1183/09031936.01.17100790. [DOI] [PubMed] [Google Scholar]
- 25.Schwab RJ, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168:522–30. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 26.Hsu PP. Clinical predictors in obstructive sleep apnea patients with computer-assisted quantitative videoendoscopic upper airway analysis. Laryngoscope. 2004;114:791–9. doi: 10.1097/00005537-200405000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong JJ, et al. In vivo size and shape measurement of the human upper airway using endoscopic long-range optical coherence tomography. Opt Exp. 2003;11:1817–26. doi: 10.1364/oe.11.001817. [DOI] [PubMed] [Google Scholar]
- 28.Armstrong JJ. Quantitative upper airway imaging with anatomic optical coherence tomography. Am J Respir Crit Care Med. 2006;173:226–33. doi: 10.1164/rccm.200507-1148OC. [DOI] [PubMed] [Google Scholar]
- 29.Leigh MS, et al. Anatomical optical coherence tomography for long-term, portable, quantitative endoscopy. IEEE Trans Biomed Eng. 2008;55:1438–46. doi: 10.1109/TBME.2007.913409. [DOI] [PubMed] [Google Scholar]
- 30.Rechtschaffen A. Washington DC: National Institutes of Health; 1968. A manual of standardized terminology, technique and scoring system for sleep stages of human sleep. [Google Scholar]
- 31.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 32.Cartwright RD. The effects of sleep posture and sleep stage on apnea frequency. Sleep. 1991;14:351–3. doi: 10.1093/sleep/14.4.351. [DOI] [PubMed] [Google Scholar]
- 33.Kumar V. Comparison of conventional and cone beam CT synthesized cephalograms. Dentomaxillofac Radiol. 2007;36:263–9. doi: 10.1259/dmfr/98032356. [DOI] [PubMed] [Google Scholar]
- 34.Haponik EF. Computerized tomography in obstructive sleep apnea. Correlation of airway size with physiology during sleep and wakefulness. Am Rev Respir Dis. 1983;127:221–6. doi: 10.1164/arrd.1983.127.2.221. [DOI] [PubMed] [Google Scholar]
- 35.Rehder K. The function of each lung of anesthetized and paralysed man during mechanical ventilation. Anesthesiology. 1972;37:16–26. doi: 10.1097/00000542-197207000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Marini JJ. Influence of head-dependent positions on lung volume and oxygen saturation in chronic air-flow obstruction. Am Rev Respir Dis. 1984;129:101–5. doi: 10.1164/arrd.1984.129.1.101. [DOI] [PubMed] [Google Scholar]
- 37.Washko GR. Volume-related and volume-independent effects of posture on esophageal and transpulmonary pressures in healthy subjects. J Appl Physiol. 2006;100:753–8. doi: 10.1152/japplphysiol.00697.2005. [DOI] [PubMed] [Google Scholar]
- 38.Hoffstein V. Lung volume dependence of pharyngeal cross-sectional area in patients with obstructive sleep apnea. Am Rev Respir Dis. 1984;130:175–8. doi: 10.1164/arrd.1984.130.2.175. [DOI] [PubMed] [Google Scholar]
- 39.Van de Graaff WB. Thoracic influence on upper airway patency. J Appl Physiol. 1988;65:2124–31. doi: 10.1152/jappl.1988.65.5.2124. [DOI] [PubMed] [Google Scholar]
- 40.Thut DC. Tracheal and neck position influence upper airway airflow dynamics by altering airway length. J Appl Physiol. 1993;75:2084–90. doi: 10.1152/jappl.1993.75.5.2084. [DOI] [PubMed] [Google Scholar]
- 41.Tagaito Y. Lung volume and collapsibility of the passive pharynx in patients with sleep-disordered breathing. J Appl Physiol. 2007;103:1379–85. doi: 10.1152/japplphysiol.00026.2007. [DOI] [PubMed] [Google Scholar]
- 42.Sauerland EK. Electromyographic activity of intrinsic and extrinsic muscles of the human tongue. Tex Rep Biol Med. 1975;33:444–55. [PubMed] [Google Scholar]
- 43.Douglas NJ. Effect of posture and breathing route on genioglossal electromyogram activity in normal subjects and in patients with the sleep apnea/hypopnea syndrome. Am Rev Respir Dis. 1993;148:1341–5. doi: 10.1164/ajrccm/148.5.1341. [DOI] [PubMed] [Google Scholar]
- 44.Otsuka R. Respiratory-related genioglossus electromyographic activity in response to head rotation and changes in body position. Angle Orthod. 2000;70:63–9. doi: 10.1043/0003-3219(2000)070<0063:RRGEAI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 45.Malhotra A, et al. Postural effects on pharyngeal protective reflex mechanisms. Sleep. 2004;27:1105–12. doi: 10.1093/sleep/27.6.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ormeno G, et al. Body position effects on EMG activity of the temporal and suprahyoid muscles in healthy subjects and in patients with myogenic cranio-cervical-mandibular dysfunction. Cranio. 1999;17:132–42. doi: 10.1080/08869634.1999.11746087. [DOI] [PubMed] [Google Scholar]
- 47.De Mayo T, et al. Breathing type and body position effects on sternocleidomastoid and suprahyoid EMG activity. J Oral Rehabil. 2005;32:487–94. doi: 10.1111/j.1365-2842.2005.01453.x. [DOI] [PubMed] [Google Scholar]