Abstract
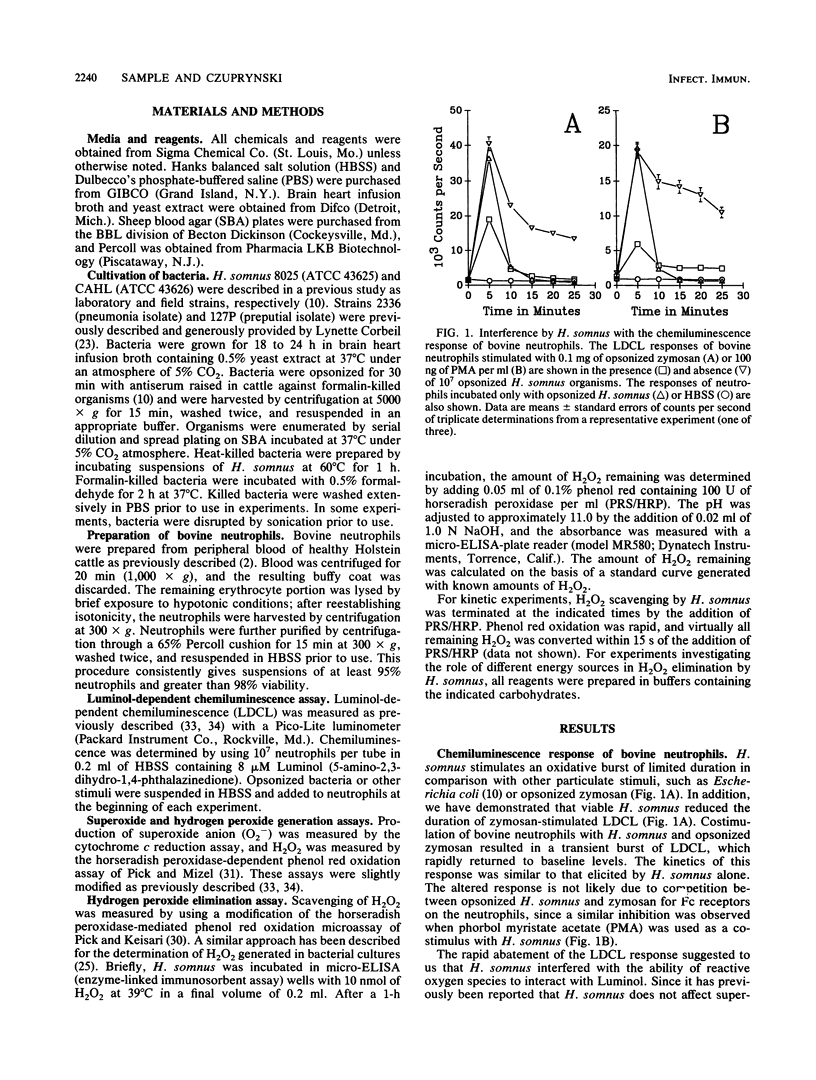
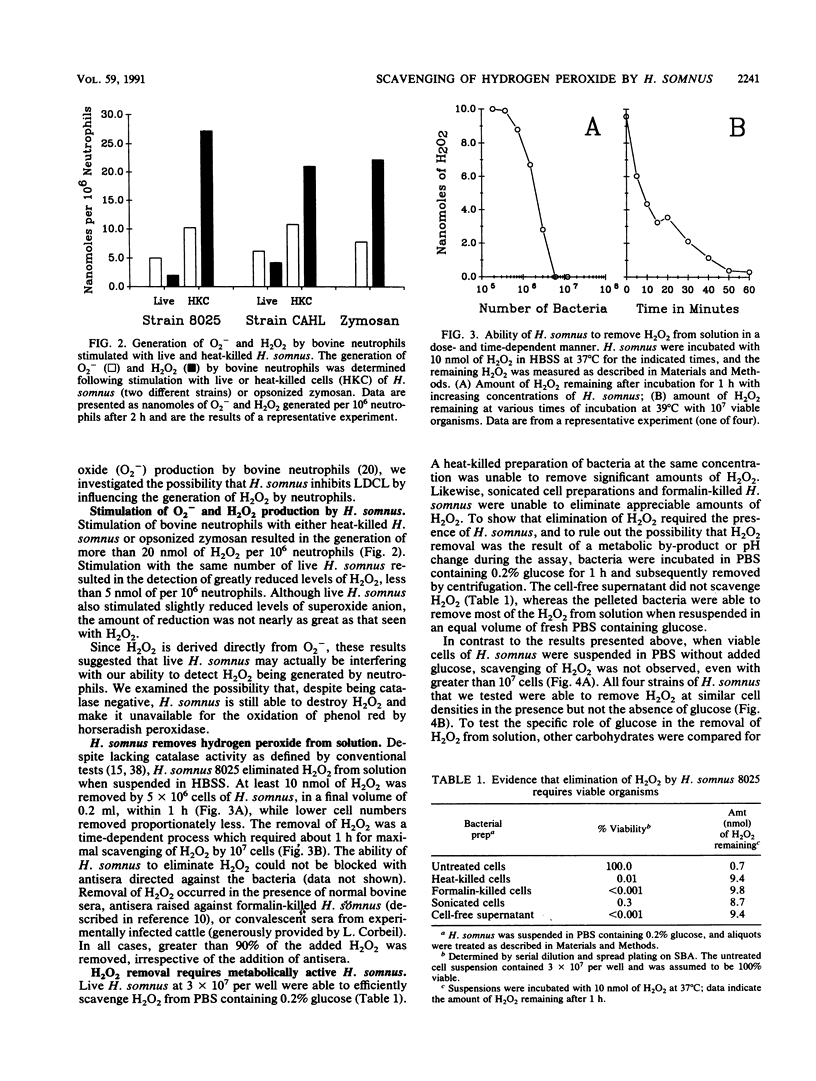
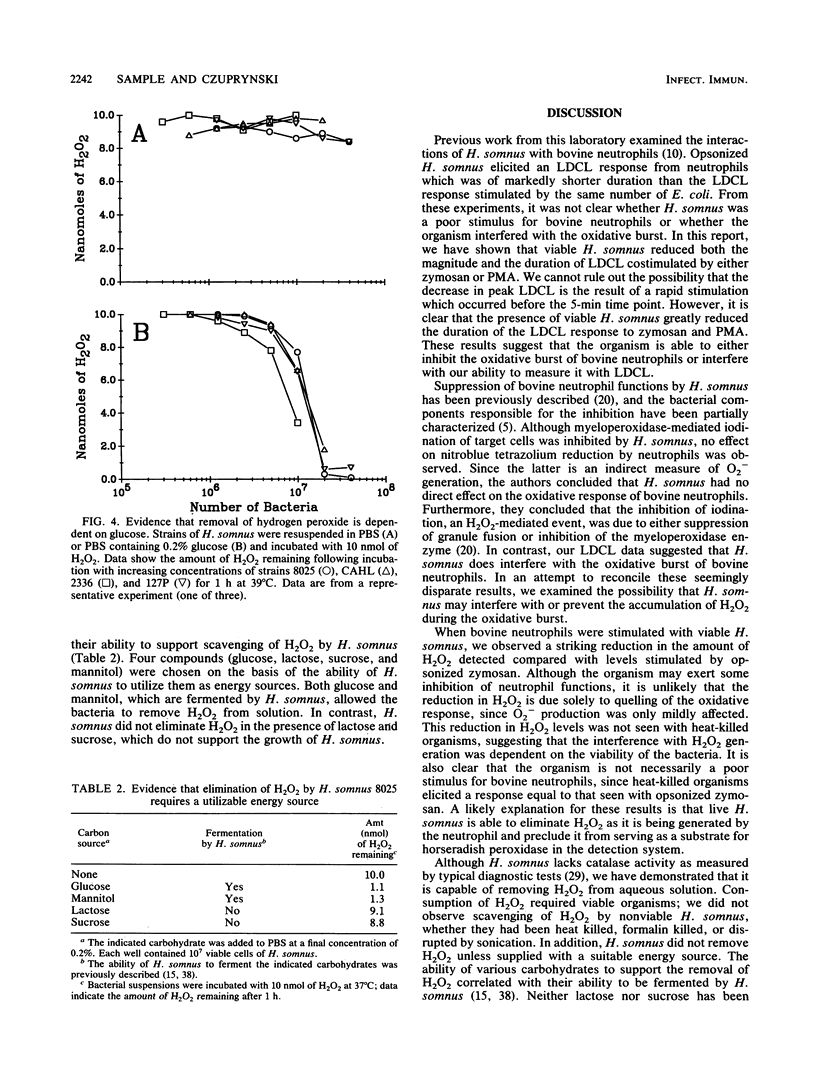
Haemophilus somnus is a catalase-negative, gram-negative pathogen of cattle which is refractory to killing by bovine neutrophils. In this report, we showed that H. somnus rapidly inhibited Luminol-dependent chemiluminescence of bovine neutrophils costimulated with opsonized zymosan or phorbol myristate acetate. We have postulated that this inhibition resulted in part from H. somnus preventing the accumulation of hydrogen peroxide (H2O2) during the oxidative burst. In support of this hypothesis, we have demonstrated that when stimulated with viable H. somnus, bovine neutrophils accumulate lower levels of H2O2 than did neutrophils stimulated with heat-killed H. somnus or opsonized zymosan. We have presented evidence that four separate strains of H. somnus, despite being catalase negative by conventional criteria, removed H2O2 from solution. Viable cells of H. somnus were required for the removal of H2O2 from solution; little or no activity was observed when suspensions of heat-killed, formalin-killed, or sonicated cells of H. somnus were incubated with H2O2. In addition, the elimination of H2O2 occurred only in the presence of carbon sources that could be utilized by H. somnus, indicating that elimination of H2O2 was an energy-dependent process. The amount of H2O2 that could be eliminated by 10(7) cells of H. somnus was greater than 10 nmol, an amount comparable to that produced by a similar number of stimulated bovine neutrophils. Thus, we suggest that the ability of H. somnus to remove H2O2 from solution may be an important virulence mechanism that contributes to the survival of the organism following ingestion by bovine neutrophils.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews J. J., Anderson T. D., Slife L. N., Stevenson G. W. Microscopic lesions associated with the isolation of Haemophilus somnus from pneumonic bovine lungs. Vet Pathol. 1985 Mar;22(2):131–136. doi: 10.1177/030098588502200206. [DOI] [PubMed] [Google Scholar]
- Bailie W. E., Anthony H. D., Weide K. D. Infectious thromboembolic meningoencephalomyelitis (sleeper syndrome) in feedlot cattle. J Am Vet Med Assoc. 1966 Jan 15;148(2):162–166. [PubMed] [Google Scholar]
- Beaman L., Beaman B. L. The role of oxygen and its derivatives in microbial pathogenesis and host defense. Annu Rev Microbiol. 1984;38:27–48. doi: 10.1146/annurev.mi.38.100184.000331. [DOI] [PubMed] [Google Scholar]
- Chiang Y. W., Kaeberle M. L., Roth J. A. Identification of suppressive components in "Haemophilus somnus" fractions which inhibit bovine polymorphonuclear leukocyte function. Infect Immun. 1986 Jun;52(3):792–797. doi: 10.1128/iai.52.3.792-797.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chladek D. W. Bovine abortion associated with Haemophilus somnus. Am J Vet Res. 1975 Jul;36(7):1041–1041. [PubMed] [Google Scholar]
- Corbeil L. B., Blau K., Prieur D. J., Ward A. C. Serum susceptibility of Haemophilus somnus from bovine clinical cases and carriers. J Clin Microbiol. 1985 Aug;22(2):192–198. doi: 10.1128/jcm.22.2.192-198.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbeil L. B., Widders P. R., Gogolewski R., Arthur J., Inzana T. J., Ward A. C. Haemophilus somnus: Bovine Reproductive and Respiratory Disease. Can Vet J. 1986 Feb;27(2):90–93. [PMC free article] [PubMed] [Google Scholar]
- Czuprynski C. J., Hamilton H. L. Bovine neutrophils ingest but do not kill Haemophilus somnus in vitro. Infect Immun. 1985 Nov;50(2):431–436. doi: 10.1128/iai.50.2.431-436.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLIN M. I. The oxidation and per-oxidation of DPNH2 in extracts of Streptococcus faecalis, 10C1. Arch Biochem Biophys. 1953 Oct;46(2):483–485. doi: 10.1016/0003-9861(53)90221-5. [DOI] [PubMed] [Google Scholar]
- Densen P., Mandell G. L. Phagocyte strategy vs. microbial tactics. Rev Infect Dis. 1980 Sep-Oct;2(5):817–838. doi: 10.1093/clinids/2.5.817. [DOI] [PubMed] [Google Scholar]
- FIREHAMMER B. D. Bovine abortion due to Haemophilus species. J Am Vet Med Assoc. 1959 Oct 15;135:421–422. [PubMed] [Google Scholar]
- Garcia-Delgado G. A., Little P. B., Barnum D. A. A comparison of various Haemophilus somnus strains. Can J Comp Med. 1977 Oct;41(4):380–388. [PMC free article] [PubMed] [Google Scholar]
- Hazlett M. J., Little P. B., Barnum D. A., Maxie M. G., Leslie K. E., Miller R. B. Haemophilus somnus: investigations of its potential role in bovine mastitis. Am J Vet Res. 1985 Nov;46(11):2229–2234. [PubMed] [Google Scholar]
- Hoblet K. H., Haibel G. K., Kowalski J. J., Rojko J. L. Culture-positive persistence and serum agglutinating antibody response after intrauterine inoculation of Haemophilus somnus in virgin heifers. Am J Vet Res. 1989 Jul;50(7):1008–1014. [PubMed] [Google Scholar]
- Hubbard R. D., Kaeberle M. L., Roth J. A., Chiang Y. W. Haemophilus somnus-induced interference with bovine neutrophil functions. Vet Microbiol. 1986 Jun;12(1):77–85. doi: 10.1016/0378-1135(86)90043-x. [DOI] [PubMed] [Google Scholar]
- Humphrey J. D., Little P. B., Stephens L. R., Barnum D. A., Doig P. A., Thorsen J. Prevalence and distribution of Haemophilus somnus in the male bovine reproductive tract. Am J Vet Res. 1982 May;43(5):791–795. [PubMed] [Google Scholar]
- KENNEDY P. C., BIBERSTEIN E. L., HOWARTH J. A., FRAZIER L. M., DUNGWORTH D. L. Infectious meningo-encephalitis in cattle, caused by a haemophilus-like organism. Am J Vet Res. 1960 Mar;21:403–409. [PubMed] [Google Scholar]
- Kania S. A., Gogolewski R. P., Corbeil L. B. Characterization of a 78-kilodalton outer membrane protein of Haemophilus somnus. Infect Immun. 1990 Jan;58(1):237–244. doi: 10.1128/iai.58.1.237-244.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell G. L. Catalase, superoxide dismutase, and virulence of Staphylococcus aureus. In vitro and in vivo studies with emphasis on staphylococcal--leukocyte interaction. J Clin Invest. 1975 Mar;55(3):561–566. doi: 10.1172/JCI107963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messersmith R. E., Anderson S. W., Brown L. N., Hussey F. J. Respiratory disease in recently-shipped Minnesota steers (a clinical study). Vet Med Small Anim Clin. 1972 Sep;67(9):1011–1016. [PubMed] [Google Scholar]
- Miller R. B., Barnum D. A., McEntee K. E. Hemophilus somnus in the reproductive tracts of slaughtered cows: location and frequency of isolations and lesions. Vet Pathol. 1983 Sep;20(5):515–521. doi: 10.1177/030098588302000502. [DOI] [PubMed] [Google Scholar]
- Pick E., Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38(1-2):161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- Pick E., Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Methods. 1981;46(2):211–226. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- Poole L. B., Claiborne A. Interactions of pyridine nucleotides with redox forms of the flavin-containing NADH peroxidase from Streptococcus faecalis. J Biol Chem. 1986 Nov 5;261(31):14525–14533. [PubMed] [Google Scholar]
- SEELEY H. W., DEL RIO-ESTRADA C. The role of riboflavin in the formation and disposal of hydrogen peroxide by streptococcus faecalis. J Bacteriol. 1951 Nov;62(5):649–656. doi: 10.1128/jb.62.5.649-656.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEELEY H. W., VANDEMARK P. J. An adaptive peroxidation by Streptococcus faecalis. J Bacteriol. 1951 Jan;61(1):27–35. doi: 10.1128/jb.61.1.27-35.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample A. K., Czuprynski C. J. Priming and stimulation of bovine neutrophils by recombinant human interleukin-1 alpha and tumor necrosis factor alpha. J Leukoc Biol. 1991 Feb;49(2):107–115. doi: 10.1002/jlb.49.2.107. [DOI] [PubMed] [Google Scholar]
- Sample A. K., Czuprynski C. J. Recombinant bovine interferon-gamma, but not interferon-alpha, potentiates bovine neutrophil oxidative responses in vitro. Vet Immunol Immunopathol. 1990 May;25(1):23–35. doi: 10.1016/0165-2427(90)90107-4. [DOI] [PubMed] [Google Scholar]
- Sibley L. D., Lawson R., Weidner E. Superoxide dismutase and catalase in Toxoplasma gondii. Mol Biochem Parasitol. 1986 Apr;19(1):83–87. doi: 10.1016/0166-6851(86)90069-1. [DOI] [PubMed] [Google Scholar]
- Stephens L. R., Humphrey J. D., Little P. B., Barnum D. A. Morphological, biochemical, antigenic, and cytochemical relationships among Haemophilus somnus, Haemophilus agni, Haemophilus haemoglobinophilus, Histophilus ovis, and Actinobacillus seminis. J Clin Microbiol. 1983 May;17(5):728–737. doi: 10.1128/jcm.17.5.728-737.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldhalm D. G., Hall R. F., Meinershagen W. A., Card C. S., Frank F. W. Haemophilus somnus infection in the cow as a possible contributing factor to weak calf syndrome: isolation and animal inoculation studies. Am J Vet Res. 1974 Nov;35(11):1401–1403. [PubMed] [Google Scholar]
- Weiss S. J. Tissue destruction by neutrophils. N Engl J Med. 1989 Feb 9;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- van Dreumel A. A., Kierstead M. Abortion associated with Hemophilus somnus infection in a bovine fetus. Can Vet J. 1975 Dec;16(12):367–370. [PMC free article] [PubMed] [Google Scholar]


