Abstract
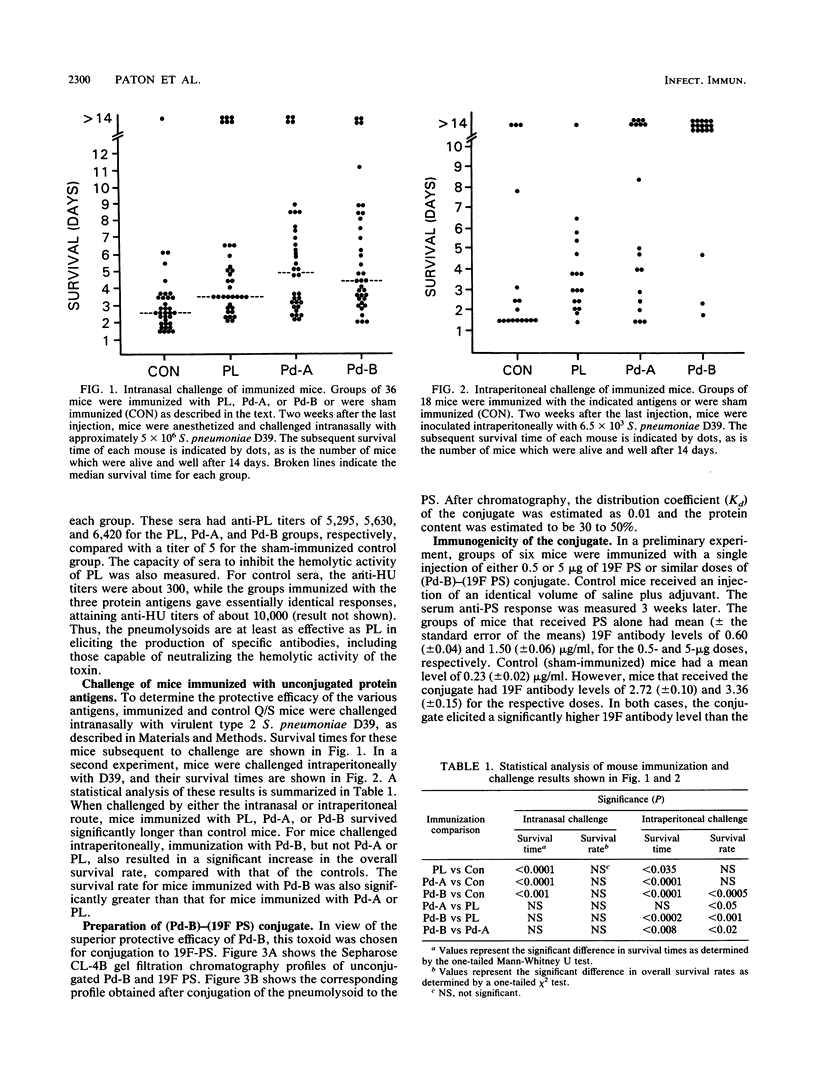
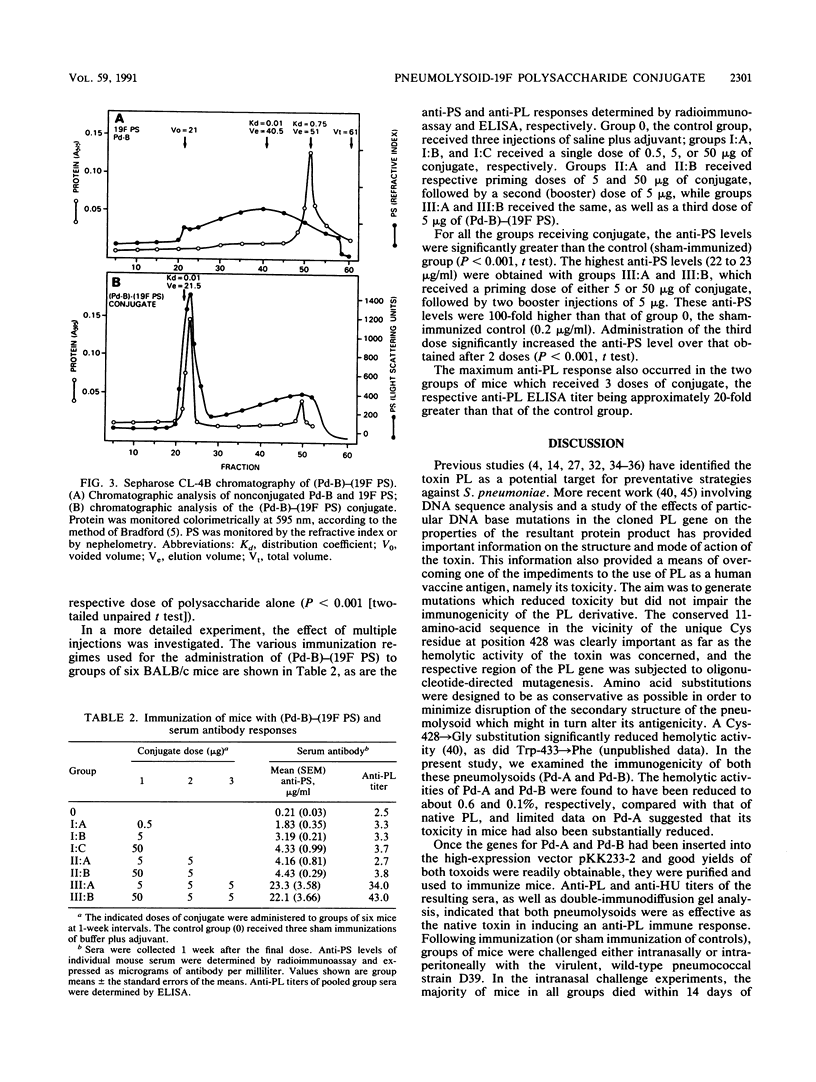
As part of an ongoing study concerned with improving human vaccines against Streptococcus pneumoniae, the genes for two defined pneumolysin (PL) toxoids (pneumolysoids), Pd-A (PL with a Cys----Gly substitution at amino acid 428) and Pd-B (PL with a Trp----Phe substitution at position 433), were inserted into the high-expression vector pKK233-2 in Escherichia coli and the pneumolysoids were purified. Groups of mice which had been immunized with either Pd-A, Pd-B, or native PL purified from S. pneumoniae were then challenged either intranasally or intraperitoneally with virulent pneumococci. Mice in all immunized groups survived significantly longer than sham-immunized controls. Both pneumolysoids were more effective than PL as protective immunogens. Pneumolysoid Pd-B was conjugated covalently with pneumococcal type 19F capsular polysaccharide (19F PS), and the immunogenicities of both the protein and the PS moieties of the conjugate in mice were determined. Significant anti-PL titers were obtained, and the immunogenicity of the 19F PS moiety was markedly enhanced compared with that of unconjugated PS. Conjugation also appears to have converted the 19F PS into an antigen capable of inducing a booster effect. These results support the notion that the efficacy of human, PS-based antipneumococcal vaccines might be improved by supplementation with pneumolysoid in the form of a covalent pneumolysoid-PS conjugate.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann E., Brosius J. "ATG vectors' for regulated high-level expression of cloned genes in Escherichia coli. Gene. 1985;40(2-3):183–190. doi: 10.1016/0378-1119(85)90041-1. [DOI] [PubMed] [Google Scholar]
- Berry A. M., Lock R. A., Hansman D., Paton J. C. Contribution of autolysin to virulence of Streptococcus pneumoniae. Infect Immun. 1989 Aug;57(8):2324–2330. doi: 10.1128/iai.57.8.2324-2330.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry A. M., Yother J., Briles D. E., Hansman D., Paton J. C. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect Immun. 1989 Jul;57(7):2037–2042. doi: 10.1128/iai.57.7.2037-2042.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Broome C. V. Efficacy of pneumococcal polysaccharide vaccines. Rev Infect Dis. 1981 Mar-Apr;3 (Suppl):S82–S96. doi: 10.1093/clinids/3.supplement_1.s82. [DOI] [PubMed] [Google Scholar]
- Cadoz M., Armand J., Arminjon F., Michel J. P., Michel M., Denis F., Schiffman G. A new 23 valent pneumococcal vaccine: immunogenicity and reactogenicity in adults. J Biol Stand. 1985 Jul;13(3):261–265. doi: 10.1016/s0092-1157(85)80010-x. [DOI] [PubMed] [Google Scholar]
- Chu C., Schneerson R., Robbins J. B., Rastogi S. C. Further studies on the immunogenicity of Haemophilus influenzae type b and pneumococcal type 6A polysaccharide-protein conjugates. Infect Immun. 1983 Apr;40(1):245–256. doi: 10.1128/iai.40.1.245-256.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coonrod J. D., Rytel M. W. Detection of type-specific pneumococcal antigens by counterimmunoelectrophoresis. I. Methodology and immunologic properties of pneumococcal antigens. J Lab Clin Med. 1973 May;81(5):770–777. [PubMed] [Google Scholar]
- Douglas R. M., Miles H. B. Vaccination against Streptococcus pneumoniae in childhood: lack of demonstrable benefit in young Australian children. J Infect Dis. 1984 Jun;149(6):861–869. doi: 10.1093/infdis/149.6.861. [DOI] [PubMed] [Google Scholar]
- Douglas R. M., Paton J. C., Duncan S. J., Hansman D. J. Antibody response to pneumococcal vaccination in children younger than five years of age. J Infect Dis. 1983 Jul;148(1):131–137. doi: 10.1093/infdis/148.1.131. [DOI] [PubMed] [Google Scholar]
- Eskola J., Käyhty H., Takala A. K., Peltola H., Rönnberg P. R., Kela E., Pekkanen E., McVerry P. H., Mäkelä P. H. A randomized, prospective field trial of a conjugate vaccine in the protection of infants and young children against invasive Haemophilus influenzae type b disease. N Engl J Med. 1990 Nov 15;323(20):1381–1387. doi: 10.1056/NEJM199011153232004. [DOI] [PubMed] [Google Scholar]
- Fattom A., Lue C., Szu S. C., Mestecky J., Schiffman G., Bryla D., Vann W. F., Watson D., Kimzey L. M., Robbins J. B. Serum antibody response in adult volunteers elicited by injection of Streptococcus pneumoniae type 12F polysaccharide alone or conjugated to diphtheria toxoid. Infect Immun. 1990 Jul;58(7):2309–2312. doi: 10.1128/iai.58.7.2309-2312.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante A., Rowan-Kelly B., Paton J. C. Inhibition of in vitro human lymphocyte response by the pneumococcal toxin pneumolysin. Infect Immun. 1984 Nov;46(2):585–589. doi: 10.1128/iai.46.2.585-589.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester H. L., Jahnigen D. W., LaForce F. M. Inefficacy of pneumococcal vaccine in a high-risk population. Am J Med. 1987 Sep;83(3):425–430. doi: 10.1016/0002-9343(87)90751-0. [DOI] [PubMed] [Google Scholar]
- Jalonen E., Paton J. C., Koskela M., Kerttula Y., Leinonen M. Measurement of antibody responses to pneumolysin--a promising method for the presumptive aetiological diagnosis of pneumococcal pneumonia. J Infect. 1989 Sep;19(2):127–134. doi: 10.1016/s0163-4453(89)91864-1. [DOI] [PubMed] [Google Scholar]
- Kehoe M. A., Miller L., Walker J. A., Boulnois G. J. Nucleotide sequence of the streptolysin O (SLO) gene: structural homologies between SLO and other membrane-damaging, thiol-activated toxins. Infect Immun. 1987 Dec;55(12):3228–3232. doi: 10.1128/iai.55.12.3228-3232.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer W., Drutsa V., Jansen H. W., Kramer B., Pflugfelder M., Fritz H. J. The gapped duplex DNA approach to oligonucleotide-directed mutation construction. Nucleic Acids Res. 1984 Dec 21;12(24):9441–9456. doi: 10.1093/nar/12.24.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C. J. Bacterial capsular polysaccharides--biochemistry, immunity and vaccine. Mol Immunol. 1987 Oct;24(10):1005–1019. doi: 10.1016/0161-5890(87)90067-8. [DOI] [PubMed] [Google Scholar]
- Lee C. J., Koizumi K. Immunochemical relations between pneumococcal group 19 and Klebsiella capsular polysaccharides. J Immunol. 1981 Oct;127(4):1619–1623. [PubMed] [Google Scholar]
- Lee C. J., Lin K. T. Studies on vaccine control and immunogenicity of polysaccharides of Streptococcus pneumoniae. Rev Infect Dis. 1981 Mar-Apr;3 (Suppl):S51–S60. doi: 10.1093/clinids/3.supplement_1.s51. [DOI] [PubMed] [Google Scholar]
- Lee C. J. The quantitative immunochemical determination of pneumococcal and meningococcal capsular polysaccharides by light scattering rate nephelometry. J Biol Stand. 1983 Jan;11(1):55–64. doi: 10.1016/s0092-1157(83)80046-8. [DOI] [PubMed] [Google Scholar]
- Leinonen M., Säkkinen A., Kalliokoski R., Luotonen J., Timonen M., Mäkelä P. H. Antibody response to 14-valent pneumococcal capsular polysaccharide vaccine in pre-school age children. Pediatr Infect Dis. 1986 Jan-Feb;5(1):39–44. doi: 10.1097/00006454-198601000-00008. [DOI] [PubMed] [Google Scholar]
- Lin K. T., Lee C. J. Immune response of neonates to pneumococcal polysaccharide-protein conjugate. Immunology. 1982 Jun;46(2):333–342. [PMC free article] [PubMed] [Google Scholar]
- Lock R. A., Paton J. C., Hansman D. Comparative efficacy of pneumococcal neuraminidase and pneumolysin as immunogens protective against Streptococcus pneumoniae. Microb Pathog. 1988 Dec;5(6):461–467. doi: 10.1016/0882-4010(88)90007-1. [DOI] [PubMed] [Google Scholar]
- Mengaud J., Chenevert J., Geoffroy C., Gaillard J. L., Cossart P. Identification of the structural gene encoding the SH-activated hemolysin of Listeria monocytogenes: listeriolysin O is homologous to streptolysin O and pneumolysin. Infect Immun. 1987 Dec;55(12):3225–3227. doi: 10.1128/iai.55.12.3225-3227.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell T. J., Mendez F., Paton J. C., Andrew P. W., Boulnois G. J. Comparison of pneumolysin genes and proteins from Streptococcus pneumoniae types 1 and 2. Nucleic Acids Res. 1990 Jul 11;18(13):4010–4010. doi: 10.1093/nar/18.13.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson M. A., Krause H. E., Schiffman G. Long-term persistence of antibody following immunization with pneumococcal polysaccharide vaccine. Proc Soc Exp Biol Med. 1983 Jun;173(2):270–275. doi: 10.3181/00379727-173-41643. [DOI] [PubMed] [Google Scholar]
- Mäkelä P. H., Leinonen M., Pukander J., Karma P. A study of the pneumococcal vaccine in prevention of clinically acute atttacks of recurrent otitis media. Rev Infect Dis. 1981 Mar-Apr;3 (Suppl):S124–S132. doi: 10.1093/clinids/3.supplement_1.s124. [DOI] [PubMed] [Google Scholar]
- Nandoskar M., Ferrante A., Bates E. J., Hurst N., Paton J. C. Inhibition of human monocyte respiratory burst, degranulation, phospholipid methylation and bactericidal activity by pneumolysin. Immunology. 1986 Dec;59(4):515–520. [PMC free article] [PubMed] [Google Scholar]
- Paton J. C., Berry A. M., Lock R. A., Hansman D., Manning P. A. Cloning and expression in Escherichia coli of the Streptococcus pneumoniae gene encoding pneumolysin. Infect Immun. 1986 Oct;54(1):50–55. doi: 10.1128/iai.54.1.50-55.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton J. C., Ferrante A. Inhibition of human polymorphonuclear leukocyte respiratory burst, bactericidal activity, and migration by pneumolysin. Infect Immun. 1983 Sep;41(3):1212–1216. doi: 10.1128/iai.41.3.1212-1216.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton J. C., Lock R. A., Hansman D. J. Effect of immunization with pneumolysin on survival time of mice challenged with Streptococcus pneumoniae. Infect Immun. 1983 May;40(2):548–552. doi: 10.1128/iai.40.2.548-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton J. C., Rowan-Kelly B., Ferrante A. Activation of human complement by the pneumococcal toxin pneumolysin. Infect Immun. 1984 Mar;43(3):1085–1087. doi: 10.1128/iai.43.3.1085-1087.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley I. D., Douglas R. M. An epidemiologic approach to pneumococcal disease. Rev Infect Dis. 1981 Mar-Apr;3(2):233–245. doi: 10.1093/clinids/3.2.233. [DOI] [PubMed] [Google Scholar]
- Robbins J. B., Austrian R., Lee C. J., Rastogi S. C., Schiffman G., Henrichsen J., Mäkelä P. H., Broome C. V., Facklam R. R., Tiesjema R. H. Considerations for formulating the second-generation pneumococcal capsular polysaccharide vaccine with emphasis on the cross-reactive types within groups. J Infect Dis. 1983 Dec;148(6):1136–1159. doi: 10.1093/infdis/148.6.1136. [DOI] [PubMed] [Google Scholar]
- Sarnaik S., Kaplan J., Schiffman G., Bryla D., Robbins J. B., Schneerson R. Studies on Pneumococcus vaccine alone or mixed with DTP and on Pneumococcus type 6B and Haemophilus influenzae type b capsular polysaccharide-tetanus toxoid conjugates in two- to five-year-old children with sickle cell anemia. Pediatr Infect Dis J. 1990 Mar;9(3):181–186. doi: 10.1097/00006454-199003000-00007. [DOI] [PubMed] [Google Scholar]
- Saunders F. K., Mitchell T. J., Walker J. A., Andrew P. W., Boulnois G. J. Pneumolysin, the thiol-activated toxin of Streptococcus pneumoniae, does not require a thiol group for in vitro activity. Infect Immun. 1989 Aug;57(8):2547–2552. doi: 10.1128/iai.57.8.2547-2552.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman G., Douglas R. M., Bonner M. J., Robbins M., Austrian R. A radioimmunoassay for immunologic phenomena in pneumococcal disease and for the antibody response to pneumococcal vaccines. I. Method for the radioimmunoassay of anticapsular antibodies and comparison with other techniques. J Immunol Methods. 1980;33(2):133–144. doi: 10.1016/s0022-1759(80)80004-4. [DOI] [PubMed] [Google Scholar]
- Schneerson R., Robbins J. B., Parke J. C., Jr, Bell C., Schlesselman J. J., Sutton A., Wang Z., Schiffman G., Karpas A., Shiloach J. Quantitative and qualitative analyses of serum antibodies elicited in adults by Haemophilus influenzae type b and pneumococcus type 6A capsular polysaccharide-tetanus toxoid conjugates. Infect Immun. 1986 May;52(2):519–528. doi: 10.1128/iai.52.2.519-528.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippe H., van Houte A. J., van Dam J. E., De Reuver M. J., Jansze M., Willers J. M. Immunogenic properties in mice of hexasaccharide from the capsular polysaccharide of Streptococcus pneumoniae type 3. Infect Immun. 1983 Jun;40(3):856–861. doi: 10.1128/iai.40.3.856-861.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweten R. K. Nucleotide sequence of the gene for perfringolysin O (theta-toxin) from Clostridium perfringens: significant homology with the genes for streptolysin O and pneumolysin. Infect Immun. 1988 Dec;56(12):3235–3240. doi: 10.1128/iai.56.12.3235-3240.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. A., Allen R. L., Falmagne P., Johnson M. K., Boulnois G. J. Molecular cloning, characterization, and complete nucleotide sequence of the gene for pneumolysin, the sulfhydryl-activated toxin of Streptococcus pneumoniae. Infect Immun. 1987 May;55(5):1184–1189. doi: 10.1128/iai.55.5.1184-1189.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P. F., Sell S. H., Vaughn W. K., Andrews C., McConnell K. B., Schiffman G. Clinical studies of pneumococcal vaccines in infants. II. Efficacy and effect on nasopharyngeal carriage. Rev Infect Dis. 1981 Mar-Apr;3 (Suppl):S108–S112. doi: 10.1093/clinids/3.supplement_1.s108. [DOI] [PubMed] [Google Scholar]


