Abstract
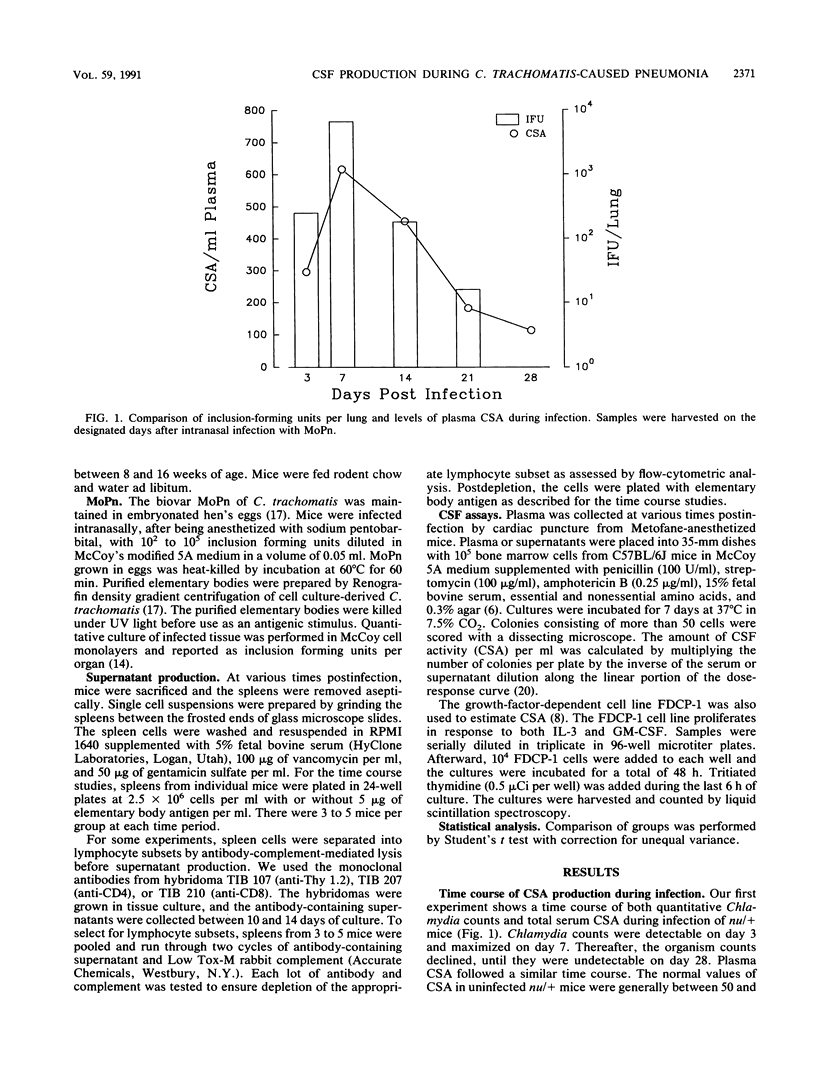
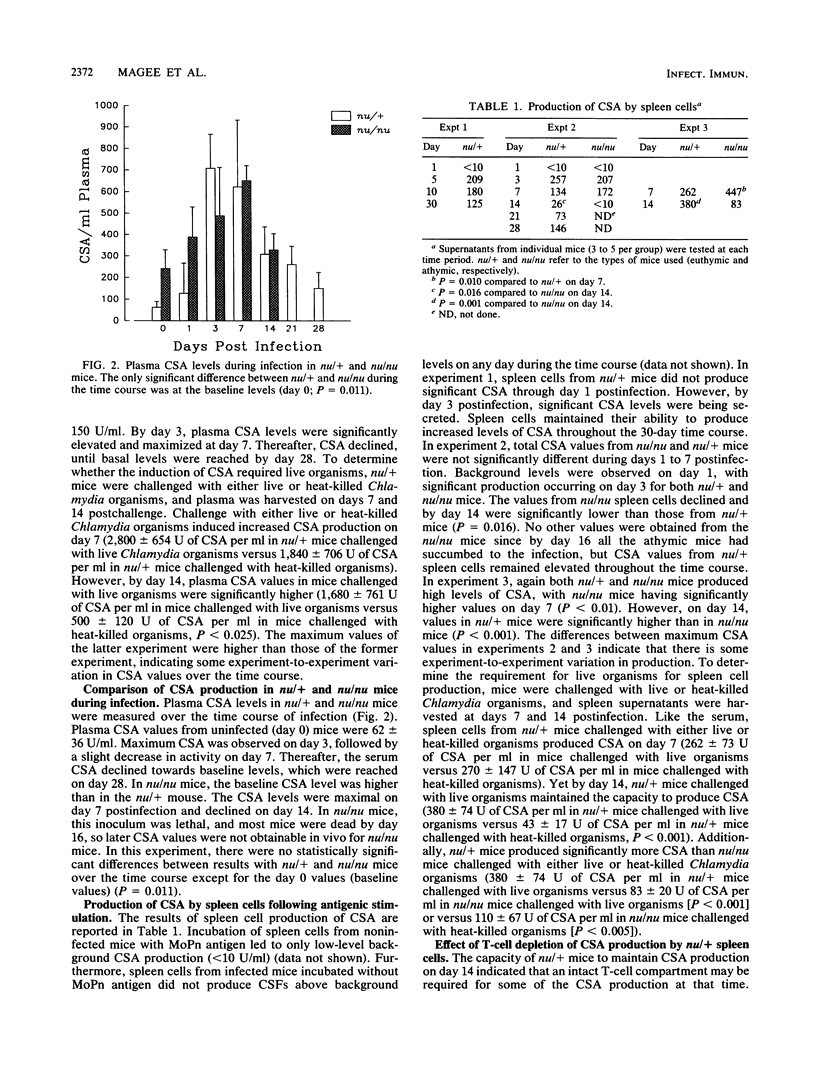
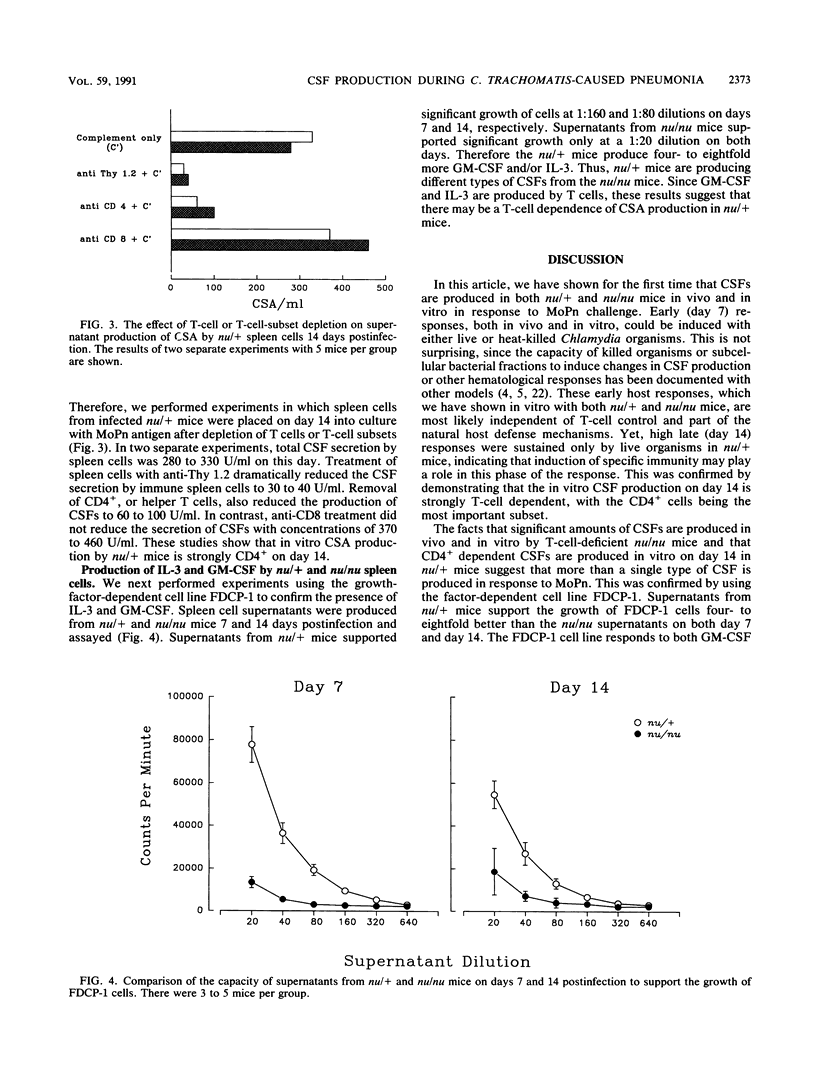
The colony-stimulating factors (CSFs) are cytokines involved in the production, differentiation, and activation of host phagocytes. During murine infection with Chlamydia trachomatis (MoPn), plasma CSF levels increased in euthymic (nu/+) and athymic (nu/nu) BALB/c mice. Levels declined later in infection, with the nu/+ mice resolving the infection but the nu/nu mice succumbing by day 16. Either live or heat-killed Chlamydia organisms could induce CSF increases on day 7 postchallenge in nu/+ mice; however, by day 14, only mice challenged with live organisms maintained high plasma levels. CSFs were also produced by spleen cells of nu/+ and nu/nu mice in response to Chlamydia antigen. Spleen cell CSF production was detectable by days 3 to 5 postinfection. In nu/+ mice, spleen cell CSF production was elevated throughout the rest of the time course but in nu/nu mice fell significantly at day 14. Like the plasma CSF activity (CSA) production, spleen cell CSA production on day 7 was seen in mice challenged with either live or heat-killed Chlamydia organisms, but on day 14 only nu/+ mice challenged with live organisms maintained significant CSA production. To further characterize the T-cell dependence of CSA production, spleen cells of nu/+ mice were depleted of T cells or T-cell subsets before producing supernatants. On day 14 postinfection, the CD4+ lymphocyte was the major producer of CSFs. Additionally, there were different types of CSFs secreted by nu/+ and nu/nu mice as determined by the ability of spleen cell supernatants to support the granulocyte-macrophage CSF/interleukin 3-dependent cell line FDCP-1. Supernatants from nu/+ mice had 4 to 8 times the level of FDCP-1 CSF activity of the supernatants from nu/nu mice. These results support the evidence that nu/+ mice were producing some CSFs by T-cell-dependent mechanisms. This is the first report of CSF production in vivo during Chlamydia infection. Furthermore, we show that CSFs are produced by both T-cell-dependent and T-cell-independent mechanisms. The capacity of the CSFs to increase the production and effector function of phagocytes may be important to host defenses.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byrne G. I., Grubbs B., Dickey T. J., Schachter J., Williams D. M. Interferon in recovery from pneumonia due to Chlamydia trachomatis in the mouse. J Infect Dis. 1987 Dec;156(6):993–996. doi: 10.1093/infdis/156.6.993. [DOI] [PubMed] [Google Scholar]
- Cheers C., Young A. M. Serum colony stimulating activity and colony forming cells in murine brucellosis: relationship to immunopathology. Microb Pathog. 1987 Sep;3(3):185–194. doi: 10.1016/0882-4010(87)90095-7. [DOI] [PubMed] [Google Scholar]
- Coalson J. J., Winter V. T., Bass L. B., Schachter J., Grubbs B. G., Williams D. M. Chlamydia trachomatis pneumonia in the immune, athymic and normal BALB mouse. Br J Exp Pathol. 1987 Jun;68(3):399–411. [PMC free article] [PubMed] [Google Scholar]
- Galelli A., Lefrancier P., Chedid L. Colony-stimulating activity induced by synthetic muramyl peptides: variation with chemical structure and association with anti-infectious activity. Infect Immun. 1984 Nov;46(2):495–500. doi: 10.1128/iai.46.2.495-500.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S., Masuno T., Hosoe S., Kawase I., Sakatani M., Ogura T., Kishimoto S., Yamamura Y. Augmented production of colony-stimulating factor in C3H/HeN mice immunized with Nocardia rubra cell wall skeleton. Infect Immun. 1986 Apr;52(1):128–133. doi: 10.1128/iai.52.1.128-133.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee D. M., Wing E. J. Antigen-specific production of colony-stimulating factors by Listeria monocytogenes-immune, L3T4-positive cells. J Infect Dis. 1988 May;157(5):941–949. doi: 10.1093/infdis/157.5.941. [DOI] [PubMed] [Google Scholar]
- Magee D. M., Wing E. J. Cloned L3T4+ T lymphocytes protect mice against Listeria monocytogenes by secreting IFN-gamma. J Immunol. 1988 Nov 1;141(9):3203–3207. [PubMed] [Google Scholar]
- Magee D. M., Wing E. J. Secretion of colony-stimulating factors by T cell clones. Role in adoptive protection against Listeria monocytogenes. J Immunol. 1989 Oct 1;143(7):2336–2341. [PubMed] [Google Scholar]
- Metcalf D. Haemopoietic growth factors 1. Lancet. 1989 Apr 15;1(8642):825–827. doi: 10.1016/s0140-6736(89)92280-0. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The molecular biology and functions of the granulocyte-macrophage colony-stimulating factors. Blood. 1986 Feb;67(2):257–267. [PubMed] [Google Scholar]
- Resnick M., Fibach E., Lebastard M., Levy L., Bercovier H. Response of the murine hematopoietic system to chronic infection with Mycobacterium lepraemurium. Infect Immun. 1988 Dec;56(12):3145–3151. doi: 10.1128/iai.56.12.3145-3151.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemer-Avni Y., Wallach D., Sarov I. Inhibition of Chlamydia trachomatis growth by recombinant tumor necrosis factor. Infect Immun. 1988 Sep;56(9):2503–2506. doi: 10.1128/iai.56.9.2503-2506.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudgett A., McNeill T. A., Killen M. Granulocyte-macrophage precursor cell and colony-stimulating factor responses of mice infected with Salmonella typhimurium. Infect Immun. 1973 Sep;8(3):450–455. doi: 10.1128/iai.8.3.450-455.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. M., Schachter J., Coalson J. J., Grubbs B. Cellular immunity to the mouse pneumonitis agent. J Infect Dis. 1984 Apr;149(4):630–639. doi: 10.1093/infdis/149.4.630. [DOI] [PubMed] [Google Scholar]
- Williams D. M., Schachter J., Drutz D. J., Sumaya C. V. Pneumonia due to Chlamydia trachomatis in the immunocompromised (nude) mouse. J Infect Dis. 1981 Feb;143(2):238–241. doi: 10.1093/infdis/143.2.238. [DOI] [PubMed] [Google Scholar]
- Williams D. M., Schachter J., Grubbs B. Role of natural killer cells in infection with the mouse pneumonitis agent (murine Chlamydia trachomatis). Infect Immun. 1987 Jan;55(1):223–226. doi: 10.1128/iai.55.1.223-226.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. M., Schachter J. Role of cell-mediated immunity in chlamydial infection: implications for ocular immunity. Rev Infect Dis. 1985 Nov-Dec;7(6):754–759. doi: 10.1093/clinids/7.6.754. [DOI] [PubMed] [Google Scholar]
- Wing E. J., Magee D. M., Barczynski L. K. Analysis of colony-stimulating factors and macrophage progenitor cells in mice immunized against Listeria monocytogenes by adoptive transfer. Infect Immun. 1987 Aug;55(8):1843–1847. doi: 10.1128/iai.55.8.1843-1847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing E. J., Waheed A., Shadduck R. K. Changes in serum colony-stimulating factor and monocytic progenitor cells during Listeria monocytogenes infection in mice. Infect Immun. 1984 Jul;45(1):180–184. doi: 10.1128/iai.45.1.180-184.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokokura T., Nomoto K., Shimizu T., Nomoto K. Enhancement of hematopoietic response of mice by subcutaneous administration of Lactobacillus casei. Infect Immun. 1986 Apr;52(1):156–160. doi: 10.1128/iai.52.1.156-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G. M., Peterson E. M., Czarniecki C. W., Schreiber R. D., de la Maza L. M. Role of endogenous gamma interferon in host defense against Chlamydia trachomatis infections. Infect Immun. 1989 Jan;57(1):152–157. doi: 10.1128/iai.57.1.152-157.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G. M., Peterson E. M., Czarniecki C. W., de la Maza L. M. Recombinant murine gamma interferon inhibits Chlamydia trachomatis serovar L1 in vivo. Infect Immun. 1988 Jan;56(1):283–286. doi: 10.1128/iai.56.1.283-286.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


