Abstract
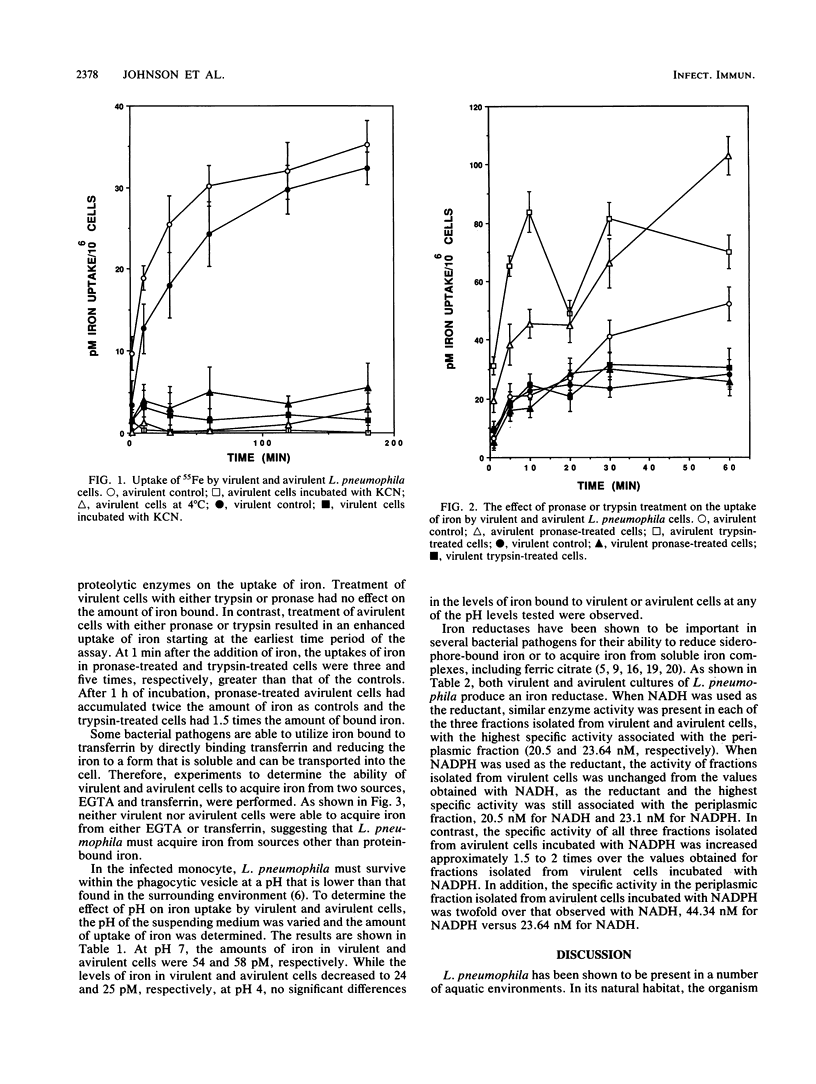
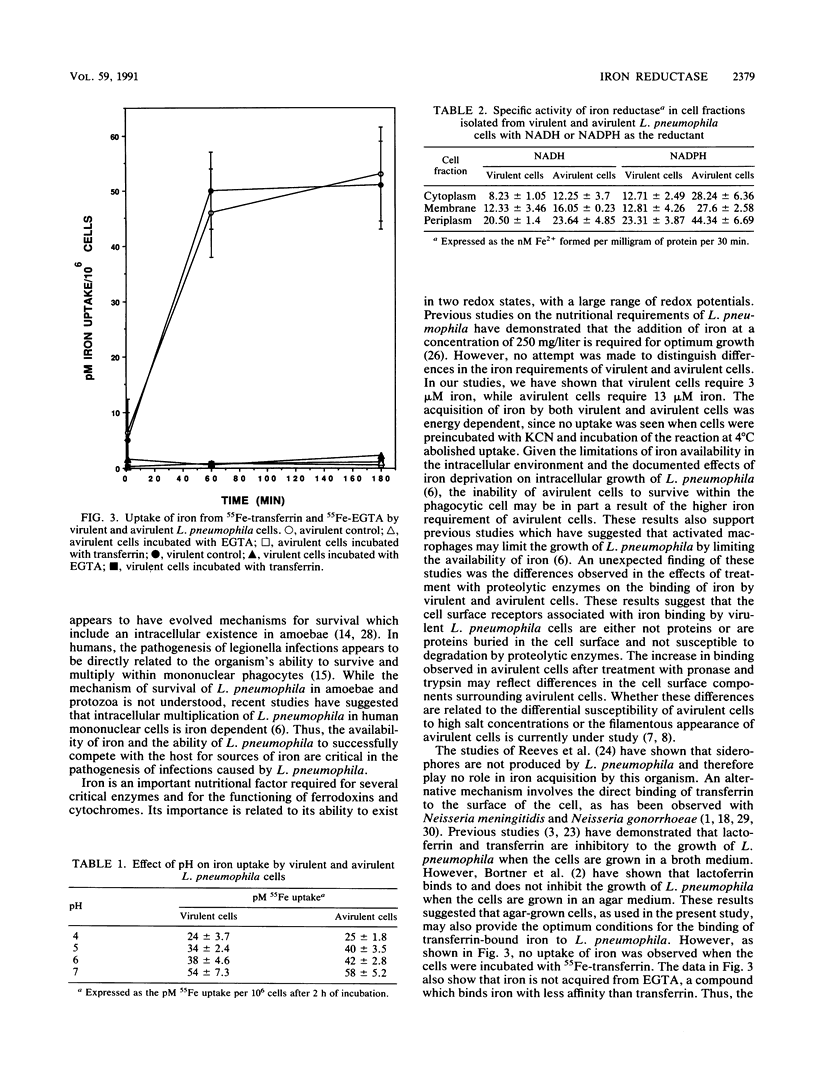
Legionella pneumophila has been shown to survive and multiply in a variety of intracellular environments, including protozoa and human mononuclear phagocytes. However, the mechanism by which this organism acquires iron in the intracellular environment has not been studied. Since L. pneumophila does not produce siderophores, alternative methods of iron acquisition were investigated. Virulent strains of L. pneumophila were able to grow in media containing as little as 3 microM iron, whereas avirulent cells required a minimum of 13 microM iron for growth. Neither virulent nor avirulent cells were able to utilize 55Fe bound to transferrin. When incubated in the presence of 55Fe in the form of ferric chloride, both virulent and avirulent cells accumulated equal amounts of iron. The uptake of iron was energy dependent as indicated by inhibition of 55Fe uptake at 4 degrees C and preincubation of the cells with KCN. Treatment of virulent cells with pronase or trypsin had no effect on iron uptake. In contrast, pronase or trypsin treatment of avirulent cells resulted in increased uptake of iron. Iron reductase activity in both virulent and avirulent cells was demonstrated, with the highest specific activity associated with the periplasmic fraction. Maximum reductase activity of virulent cells occurred with NADH as the reductant. In contrast, avirulent cells showed a twofold increase in enzyme activity when NADPH was used as the reductant. These results suggest that an iron reductase is important in iron acquisition by L. pneumophila.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanton K. J., Biswas G. D., Tsai J., Adams J., Dyer D. W., Davis S. M., Koch G. G., Sen P. K., Sparling P. F. Genetic evidence that Neisseria gonorrhoeae produces specific receptors for transferrin and lactoferrin. J Bacteriol. 1990 Sep;172(9):5225–5235. doi: 10.1128/jb.172.9.5225-5235.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortner C. A., Arnold R. R., Miller R. D. Bactericidal effect of lactoferrin on Legionella pneumophila: effect of the physiological state of the organism. Can J Microbiol. 1989 Nov;35(11):1048–1051. doi: 10.1139/m89-174. [DOI] [PubMed] [Google Scholar]
- Bortner C. A., Miller R. D., Arnold R. R. Bactericidal effect of lactoferrin on Legionella pneumophila. Infect Immun. 1986 Feb;51(2):373–377. doi: 10.1128/iai.51.2.373-377.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome C. V., Fraser D. W. Epidemiologic aspects of legionellosis. Epidemiol Rev. 1979;1:1–16. doi: 10.1093/oxfordjournals.epirev.a036204. [DOI] [PubMed] [Google Scholar]
- Brown K. A., Ratledge C. Iron transport in Mycobacterium smegmatis: ferrimycobactin reductase (nad(p)h:ferrimycobactin oxidoreductase), the enzyme releasing iron from its carrier. FEBS Lett. 1975 May 1;53(2):262–266. doi: 10.1016/0014-5793(75)80033-0. [DOI] [PubMed] [Google Scholar]
- Byrd T. F., Horwitz M. A. Interferon gamma-activated human monocytes downregulate transferrin receptors and inhibit the intracellular multiplication of Legionella pneumophila by limiting the availability of iron. J Clin Invest. 1989 May;83(5):1457–1465. doi: 10.1172/JCI114038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catrenich C. E., Johnson W. Characterization of the selective inhibition of growth of virulent Legionella pneumophila by supplemented Mueller-Hinton medium. Infect Immun. 1989 Jun;57(6):1862–1864. doi: 10.1128/iai.57.6.1862-1864.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catrenich C. E., Johnson W. Virulence conversion of Legionella pneumophila: a one-way phenomenon. Infect Immun. 1988 Dec;56(12):3121–3125. doi: 10.1128/iai.56.12.3121-3125.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D. Iron reductases from Pseudomonas aeruginosa. J Bacteriol. 1980 Jan;141(1):199–204. doi: 10.1128/jb.141.1.199-204.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D. Iron uptake with ferripyochelin and ferric citrate by Pseudomonas aeruginosa. J Bacteriol. 1980 May;142(2):581–587. doi: 10.1128/jb.142.2.581-587.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey H. A., Jr, Lascelles J. Reduction of iron and synthesis of protoheme by Spirillum itersonii and other organisms. J Bacteriol. 1977 Feb;129(2):815–820. doi: 10.1128/jb.129.2.815-820.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondero T. J., Jr, Rendtorff R. C., Mallison G. F., Weeks R. M., Levy J. S., Wong E. W., Schaffner W. An outbreak of Legionnaires' disease associated with a contaminated air-conditioning cooling tower. N Engl J Med. 1980 Feb 14;302(7):365–370. doi: 10.1056/NEJM198002143020703. [DOI] [PubMed] [Google Scholar]
- Feeley J. C., Gibson R. J., Gorman G. W., Langford N. C., Rasheed J. K., Mackel D. C., Baine W. B. Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J Clin Microbiol. 1979 Oct;10(4):437–441. doi: 10.1128/jcm.10.4.437-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields B. S., Shotts E. B., Jr, Feeley J. C., Gorman G. W., Martin W. T. Proliferation of Legionella pneumophila as an intracellular parasite of the ciliated protozoan Tetrahymena pyriformis. Appl Environ Microbiol. 1984 Mar;47(3):467–471. doi: 10.1128/aem.47.3.467-471.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Legionnaires' disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J Clin Invest. 1980 Sep;66(3):441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyer M., Page W. J. Ferric reductase activity in Azotobacter vinelandii and its inhibition by Zn2+. J Bacteriol. 1989 Jul;171(7):4031–4037. doi: 10.1128/jb.171.7.4031-4037.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi A., Suzuki Y., Ono S., Sato H., Sato Y. Heptakis(2,6-O-dimethyl)beta-cyclodextrin: a novel growth stimulant for Bordetella pertussis phase I. J Clin Microbiol. 1983 May;17(5):781–786. doi: 10.1128/jcm.17.5.781-786.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. C., Schryvers A. B. Specificity of the lactoferrin and transferrin receptors in Neisseria gonorrhoeae. Mol Microbiol. 1988 Nov;2(6):827–829. doi: 10.1111/j.1365-2958.1988.tb00095.x. [DOI] [PubMed] [Google Scholar]
- Lodge J. S., Gaines C. G., Arceneaux J. E., Byers B. R. Ferrisiderophore reductase activity in Agrobacterium tumefaciens. J Bacteriol. 1982 Feb;149(2):771–774. doi: 10.1128/jb.149.2.771-774.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G. K., Patton C. M., Feeley J. C., Johnson S. E., Gorman G., Martin W. T., Skaliy P., Mallison G. F., Politi B. D., Mackel D. C. Isolation of the Legionnaires' disease bacterium from environmental samples. Ann Intern Med. 1979 Apr;90(4):664–666. doi: 10.7326/0003-4819-90-4-664. [DOI] [PubMed] [Google Scholar]
- Pine L., George J. R., Reeves M. W., Harrell W. K. Development of a chemically defined liquid medium for growth of Legionella pneumophila. J Clin Microbiol. 1979 May;9(5):615–626. doi: 10.1128/jcm.9.5.615-626.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves M. W., Pine L., Neilands J. B., Balows A. Absence of siderophore activity in Legionella species grown in iron-deficient media. J Bacteriol. 1983 Apr;154(1):324–329. doi: 10.1128/jb.154.1.324-329.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristroph J. D., Hedlund K. W., Allen R. G. Liquid medium for growth of Legionella pneumophila. J Clin Microbiol. 1980 Jan;11(1):19–21. doi: 10.1128/jcm.11.1.19-21.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristroph J. D., Hedlund K. W., Gowda S. Chemically defined medium for Legionella pneumophila growth. J Clin Microbiol. 1981 Jan;13(1):115–119. doi: 10.1128/jcm.13.1.115-119.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi F., Romeo D., Patriarca P. Mechanism of phagocytosis-associated oxidative metabolism in polymorphonuclear leucocytes and macrophages. J Reticuloendothel Soc. 1972 Aug;12(2):127–149. [PubMed] [Google Scholar]
- Rowbotham T. J. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J Clin Pathol. 1980 Dec;33(12):1179–1183. doi: 10.1136/jcp.33.12.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schryvers A. B., Morris L. J. Identification and characterization of the transferrin receptor from Neisseria meningitidis. Mol Microbiol. 1988 Mar;2(2):281–288. doi: 10.1111/j.1365-2958.1988.tb00029.x. [DOI] [PubMed] [Google Scholar]
- Simonson C., Brener D., DeVoe I. W. Expression of a high-affinity mechanism for acquisition of transferrin iron by Neisseria meningitidis. Infect Immun. 1982 Apr;36(1):107–113. doi: 10.1128/iai.36.1.107-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


