Abstract
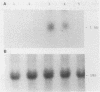
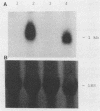
Treatment of monocytes with recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF) was shown to enhance their antimycobacterial activity in an in vitro assay. Furthermore, Mycobacterium avium-M. intracellulare was found to induce the production of this hemopoietic growth factor. Human peripheral blood mononuclear cells were fractionated by plastic adherence and Percoll density centrifugation, and each population of cells was stimulated with mycobacteria. GM-CSF was produced by both monocytes and large granular lymphocytes (LGL) but not T lymphocytes. The phenotype of the GM-CSF-producing LGL was found to be CD2+, CD16+, and HLA-DR+ but negative for T-cell and monocyte markers. Kinetic studies demonstrated that GM-CSF appeared in the supernatant fluids within 2 days of culture of either monocytes or LGL and continued to be produced up to 7 days of incubation. Northern (RNA) blot analysis of RNA from both cell types demonstrated the expression of GM-CSF message within 24 h of stimulation. From these studies, LGL and monocytes are capable of responding to M. avium-M. intracellulare by producing factors that augment normal immune functions, including the antibacterial capability of monocytes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avanzi G. C., Lista P., Giovinazzo B., Miniero R., Saglio G., Benetton G., Coda R., Cattoretti G., Pegoraro L. Selective growth response to IL-3 of a human leukaemic cell line with megakaryoblastic features. Br J Haematol. 1988 Jul;69(3):359–366. doi: 10.1111/j.1365-2141.1988.tb02374.x. [DOI] [PubMed] [Google Scholar]
- Begley C. G., Lopez A. F., Nicola N. A., Warren D. J., Vadas M. A., Sanderson C. J., Metcalf D. Purified colony-stimulating factors enhance the survival of human neutrophils and eosinophils in vitro: a rapid and sensitive microassay for colony-stimulating factors. Blood. 1986 Jul;68(1):162–166. [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Recombinant granulocyte-macrophage colony-stimulating factor activates human macrophages to inhibit growth or kill Mycobacterium avium complex. J Leukoc Biol. 1990 Jul;48(1):67–73. doi: 10.1002/jlb.48.1.67. [DOI] [PubMed] [Google Scholar]
- Blanchard D. K., Michelini-Norris M. B., Djeu J. Y. A rapid [3H]glycerol radioassay for determination of monocyte-mediated growth inhibition of Mycobacterium avium. J Immunol Methods. 1990 Oct 19;133(2):285–290. doi: 10.1016/0022-1759(90)90370-b. [DOI] [PubMed] [Google Scholar]
- Cannistra S. A., Vellenga E., Groshek P., Rambaldi A., Griffin J. D. Human granulocyte-monocyte colony-stimulating factor and interleukin 3 stimulate monocyte cytotoxicity through a tumor necrosis factor-dependent mechanism. Blood. 1988 Mar;71(3):672–676. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collins F. M. Mycobacterium avium-complex infections and development of the acquired immunodeficiency syndrome: casual opportunist or causal cofactor? Int J Lepr Other Mycobact Dis. 1986 Sep;54(3):458–474. [PubMed] [Google Scholar]
- Cuturi M. C., Anegón I., Sherman F., Loudon R., Clark S. C., Perussia B., Trinchieri G. Production of hematopoietic colony-stimulating factors by human natural killer cells. J Exp Med. 1989 Feb 1;169(2):569–583. doi: 10.1084/jem.169.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djeu J. Y., Blanchard D. K., Richards A. L., Friedman H. Tumor necrosis factor induction by Candida albicans from human natural killer cells and monocytes. J Immunol. 1988 Dec 1;141(11):4047–4052. [PubMed] [Google Scholar]
- Fleischmann J., Golde D. W., Weisbart R. H., Gasson J. C. Granulocyte-macrophage colony-stimulating factor enhances phagocytosis of bacteria by human neutrophils. Blood. 1986 Sep;68(3):708–711. [PubMed] [Google Scholar]
- Gasson J. C., Weisbart R. H., Kaufman S. E., Clark S. C., Hewick R. M., Wong G. G., Golde D. W. Purified human granulocyte-macrophage colony-stimulating factor: direct action on neutrophils. Science. 1984 Dec 14;226(4680):1339–1342. doi: 10.1126/science.6390681. [DOI] [PubMed] [Google Scholar]
- Grabstein K. H., Urdal D. L., Tushinski R. J., Mochizuki D. Y., Price V. L., Cantrell M. A., Gillis S., Conlon P. J. Induction of macrophage tumoricidal activity by granulocyte-macrophage colony-stimulating factor. Science. 1986 Apr 25;232(4749):506–508. doi: 10.1126/science.3083507. [DOI] [PubMed] [Google Scholar]
- Handman E., Burgess A. W. Stimulation by granulocyte-macrophage colony-stimulating factor of Leishmania tropica killing by macrophages. J Immunol. 1979 Mar;122(3):1134–1137. [PubMed] [Google Scholar]
- Hassan N. F., Campbell D. E., Douglas S. D. Purification of human monocytes on gelatin-coated surfaces. J Immunol Methods. 1986 Dec 24;95(2):273–276. doi: 10.1016/0022-1759(86)90415-1. [DOI] [PubMed] [Google Scholar]
- Iseman M. D., Corpe R. F., O'Brien R. J., Rosenzwieg D. Y., Wolinsky E. Disease due to Mycobacterium avium-intracellulare. Chest. 1985 Feb;87(2 Suppl):139S–149S. doi: 10.1378/chest.87.2.139s. [DOI] [PubMed] [Google Scholar]
- Karbassi A., Becker J. M., Foster J. S., Moore R. N. Enhanced killing of Candida albicans by murine macrophages treated with macrophage colony-stimulating factor: evidence for augmented expression of mannose receptors. J Immunol. 1987 Jul 15;139(2):417–421. [PubMed] [Google Scholar]
- Kasahara T., Djeu J. Y., Dougherty S. F., Oppenheim J. J. Capacity of human large granular lymphocytes (LGL) to produce multiple lymphokines: interleukin 2, interferon, and colony stimulating factor. J Immunol. 1983 Nov;131(5):2379–2385. [PubMed] [Google Scholar]
- Metcalf D. The molecular biology and functions of the granulocyte-macrophage colony-stimulating factors. Blood. 1986 Feb;67(2):257–267. [PubMed] [Google Scholar]
- Moore R. N., Oppenheim J. J., Farrar J. J., Carter C. S., Jr, Waheed A., Shadduck R. K. Production of lymphocyte-activating factor (Interleukin 1) by macrophages activated with colony-stimulating factors. J Immunol. 1980 Sep;125(3):1302–1305. [PubMed] [Google Scholar]
- Morrissey P. J., Bressler L., Park L. S., Alpert A., Gillis S. Granulocyte-macrophage colony-stimulating factor augments the primary antibody response by enhancing the function of antigen-presenting cells. J Immunol. 1987 Aug 15;139(4):1113–1119. [PubMed] [Google Scholar]
- Nagamine Y., Sudol M., Reich E. Hormonal regulation of plasminogen activator mRNA production in porcine kidney cells. Cell. 1983 Apr;32(4):1181–1190. doi: 10.1016/0092-8674(83)90301-x. [DOI] [PubMed] [Google Scholar]
- Pistoia V., Zupo S., Corcione A., Roncella S., Matera L., Ghio R., Ferrarini M. Production of colony-stimulating activity by human natural killer cells: analysis of the conditions that influence the release and detection of colony-stimulating activity. Blood. 1989 Jul;74(1):156–164. [PubMed] [Google Scholar]
- Scala G., Allavena P., Djeu J. Y., Kasahara T., Ortaldo J. R., Herberman R. B., Oppenheim J. J. Human large granular lymphocytes are potent producers of interleukin-1. Nature. 1984 May 3;309(5963):56–59. doi: 10.1038/309056a0. [DOI] [PubMed] [Google Scholar]
- Timonen T., Saksela E. Isolation of human NK cells by density gradient centrifugation. J Immunol Methods. 1980;36(3-4):285–291. doi: 10.1016/0022-1759(80)90133-7. [DOI] [PubMed] [Google Scholar]
- Wang M., Friedman H., Djeu J. Y. Enhancement of human monocyte function against Candida albicans by the colony-stimulating factors (CSF): IL-3, granulocyte-macrophage-CSF, and macrophage-CSF. J Immunol. 1989 Jul 15;143(2):671–677. [PubMed] [Google Scholar]
- Weisbart R. H., Golde D. W., Clark S. C., Wong G. G., Gasson J. C. Human granulocyte-macrophage colony-stimulating factor is a neutrophil activator. 1985 Mar 28-Apr 3Nature. 314(6009):361–363. doi: 10.1038/314361a0. [DOI] [PubMed] [Google Scholar]
- Woods G. L., Washington J. A., 2nd Mycobacteria other than Mycobacterium tuberculosis: review of microbiologic and clinical aspects. Rev Infect Dis. 1987 Mar-Apr;9(2):275–294. doi: 10.1093/clinids/9.2.275. [DOI] [PubMed] [Google Scholar]