Abstract
A mutant strain of Helicobacter pylori with weak urease activity was created by using N-methyl-N'-nitro-N-nitrosoguanidine. The urease activity of the mutant (0.036 +/- 0.009 nmol of urea per micrograms of bacterial protein per min) was 0.4% of that of the parental strain (8.20 +/- 2.30 nmol of urea per micrograms of bacterial protein per min). The mutant was otherwise indistinguishable from the parental strain. Both demonstrated prominent catalase and oxidase activities, and both produced vacuolating cytotoxin. Restriction endonuclease and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) patterns and ultrastructure were identical for the two strains. The mutant was fully motile, as evaluated by spreading in soft agar and by direct microscopic examination. Growth rate and colony size and morphology were identical for the mutant and parental strains. Seventeen gnotobiotic piglets were challenged with either the mutant or the parental strain and sacrificed 3 or 21 days after challenge. Gastric tissue was examined histologically and cultured for H. pylori. Of seven piglets challenged with the parental strain, all became infected. H. pylori was not recovered from any of 10 piglets challenged with the urease-negative strain. Lymphofollicular gastritis was present in all seven piglets challenged with the parental strain but in none of the piglets challenged with the urease-negative strain. These results suggest that prominent urease activity is essential for colonization by H. pylori.
Full text
PDF

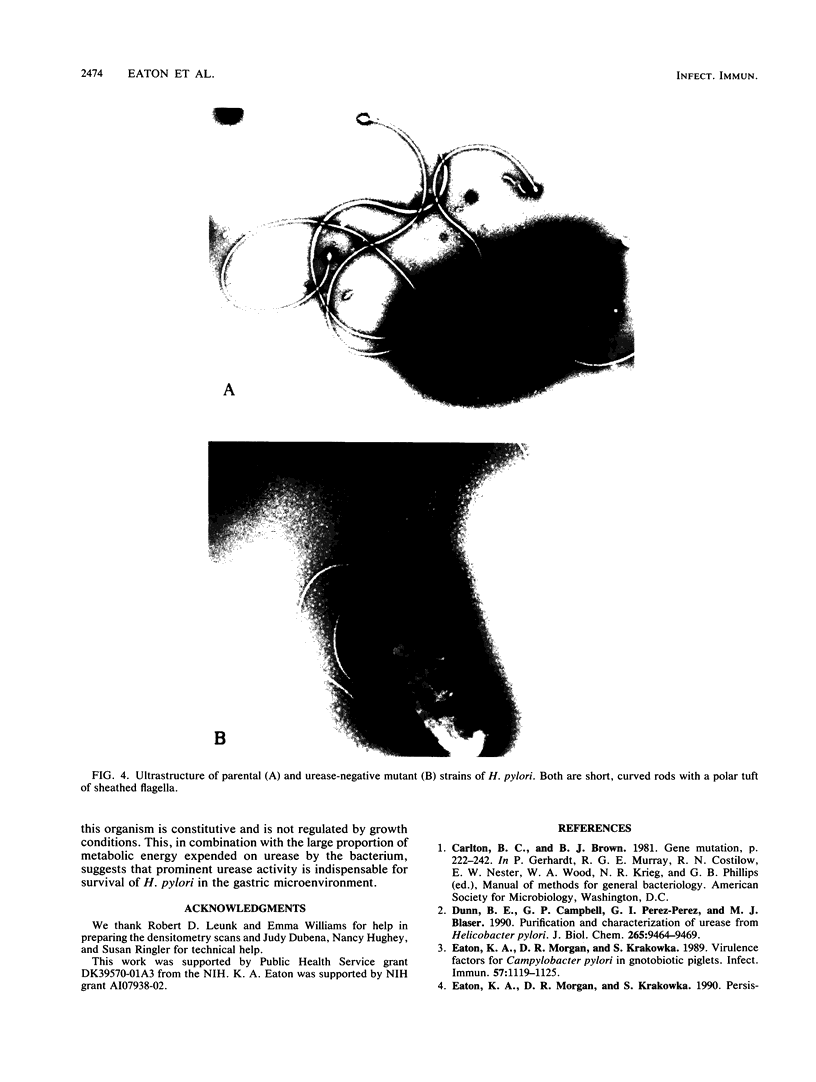

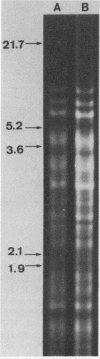
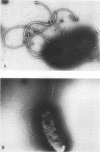
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dunn B. E., Campbell G. P., Perez-Perez G. I., Blaser M. J. Purification and characterization of urease from Helicobacter pylori. J Biol Chem. 1990 Jun 5;265(16):9464–9469. [PubMed] [Google Scholar]
- Eaton K. A., Morgan D. R., Krakowka S. Campylobacter pylori virulence factors in gnotobiotic piglets. Infect Immun. 1989 Apr;57(4):1119–1125. doi: 10.1128/iai.57.4.1119-1125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton K. A., Morgan D. R., Krakowka S. Persistence of Helicobacter pylori in conventionalized piglets. J Infect Dis. 1990 Jun;161(6):1299–1301. doi: 10.1093/infdis/161.6.1299. [DOI] [PubMed] [Google Scholar]
- Ferrero R. L., Hazell S. L., Lee A. The urease enzymes of Campylobacter pylori and a related bacterium. J Med Microbiol. 1988 Sep;27(1):33–40. doi: 10.1099/00222615-27-1-33. [DOI] [PubMed] [Google Scholar]
- Fox J. G., Lee A. Gastric campylobacter-like organisms: their role in gastric disease of laboratory animals. Lab Anim Sci. 1989 Nov;39(6):543–553. [PubMed] [Google Scholar]
- Hazell S. L., Lee A. Campylobacter pyloridis, urease, hydrogen ion back diffusion, and gastric ulcers. Lancet. 1986 Jul 5;2(8497):15–17. doi: 10.1016/s0140-6736(86)92561-4. [DOI] [PubMed] [Google Scholar]
- Kaltwasser H., Schlegel H. G. NADH-Dependent coupled enzyme assay for urease and other ammonia-producing systems. Anal Biochem. 1966 Jul;16(1):132–138. doi: 10.1016/0003-2697(66)90088-1. [DOI] [PubMed] [Google Scholar]
- Krakowka S., Morgan D. R., Kraft W. G., Leunk R. D. Establishment of gastric Campylobacter pylori infection in the neonatal gnotobiotic piglet. Infect Immun. 1987 Nov;55(11):2789–2796. doi: 10.1128/iai.55.11.2789-2796.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leunk R. D., Johnson P. T., David B. C., Kraft W. G., Morgan D. R. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J Med Microbiol. 1988 Jun;26(2):93–99. doi: 10.1099/00222615-26-2-93. [DOI] [PubMed] [Google Scholar]
- Mobley H. L., Cortesia M. J., Rosenthal L. E., Jones B. D. Characterization of urease from Campylobacter pylori. J Clin Microbiol. 1988 May;26(5):831–836. doi: 10.1128/jcm.26.5.831-836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. R., Freedman R., Depew C. E., Kraft W. G. Growth of Campylobacter pylori in liquid media. J Clin Microbiol. 1987 Nov;25(11):2123–2125. doi: 10.1128/jcm.25.11.2123-2125.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neman-Simha V., Mégraud F. In vitro model for Campylobacter pylori adherence properties. Infect Immun. 1988 Dec;56(12):3329–3333. doi: 10.1128/iai.56.12.3329-3333.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. J., Costas M., Morgan D. D., On S. L., Hill L. R., Pearson A. D., Morgan D. R. Strain variation in Campylobacter pylori detected by numerical analysis of one-dimensional electrophoretic protein patterns. Antonie Van Leeuwenhoek. 1989 Mar;55(3):253–267. doi: 10.1007/BF00393854. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Sarosiek J., Slomiany A., Slomiany B. L. Evidence for weakening of gastric mucus integrity by Campylobacter pylori. Scand J Gastroenterol. 1988 Jun;23(5):585–590. doi: 10.3109/00365528809093916. [DOI] [PubMed] [Google Scholar]