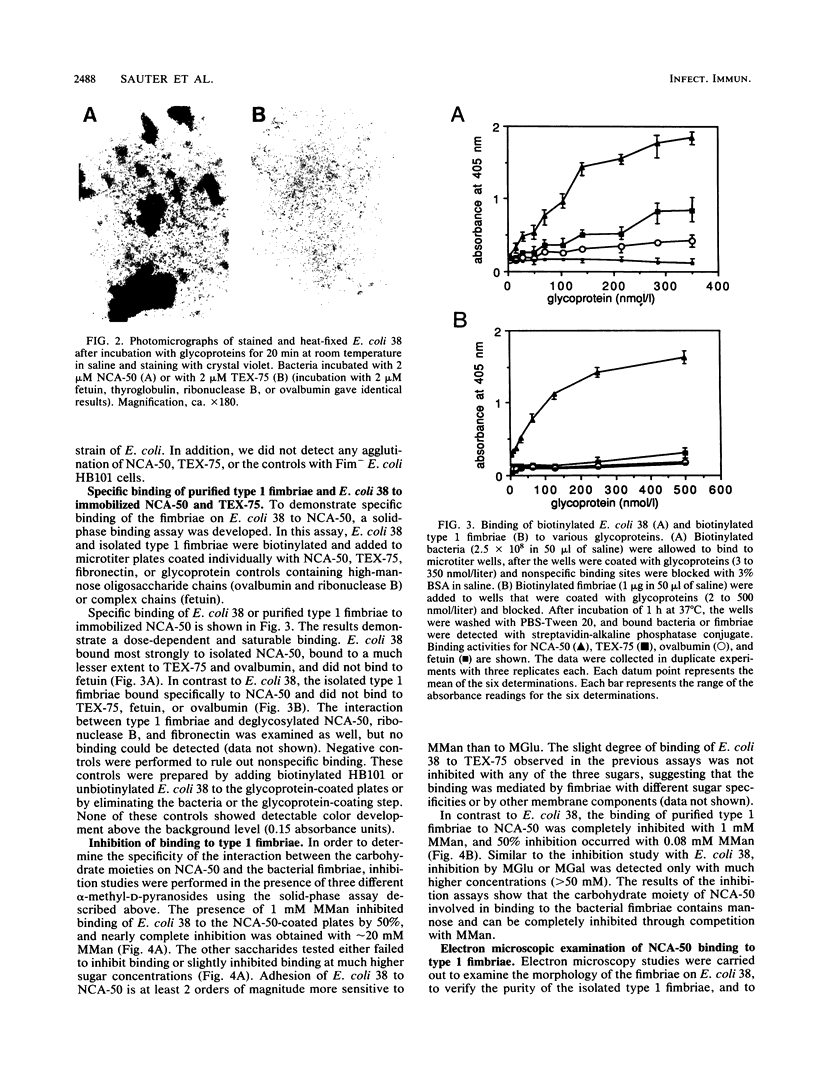
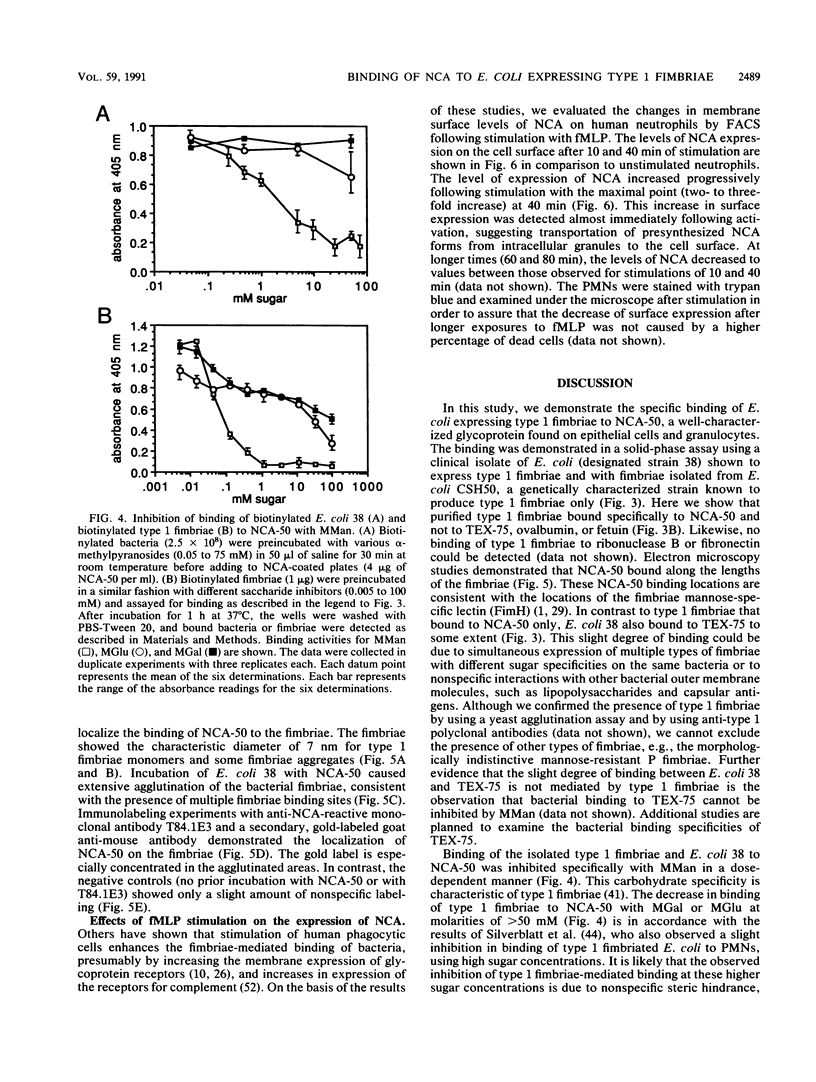
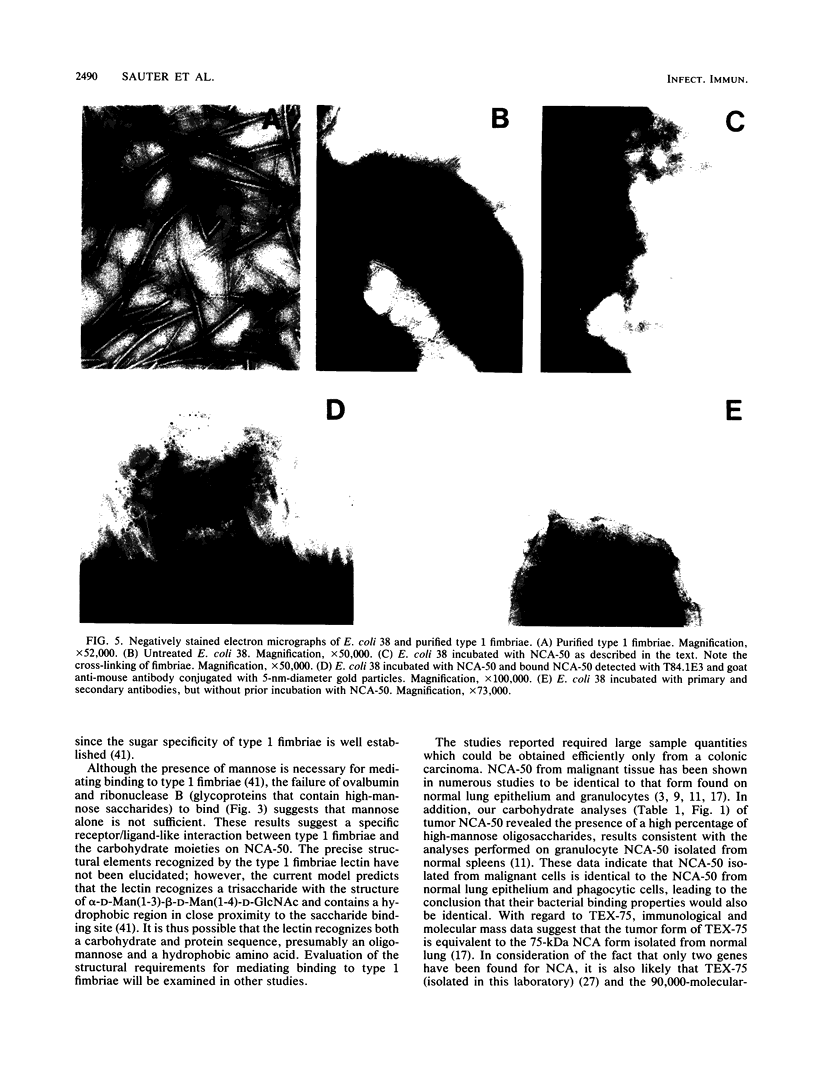
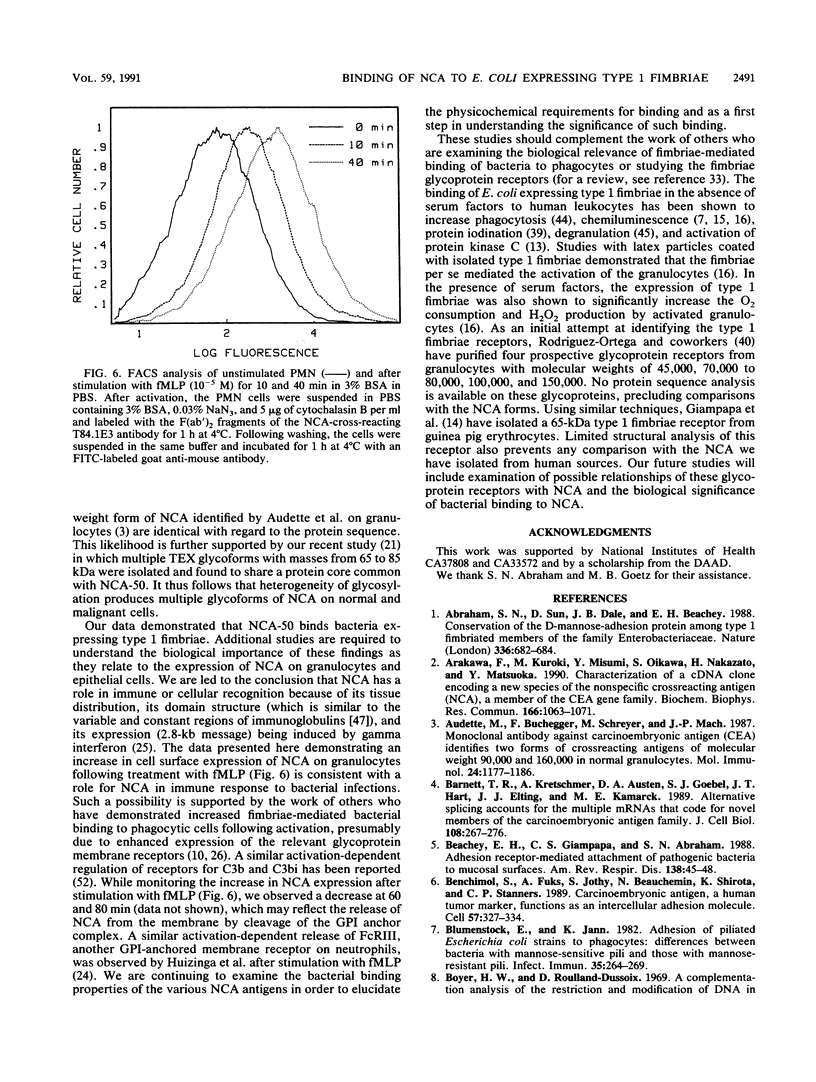
Abstract
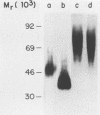
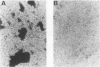
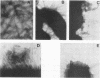
Nonspecific cross-reacting antigen (NCA) is a well-characterized membrane glycoprotein on granulocytes, macrophages, and lung epithelium. Structural studies at the protein and genomic levels have revealed that NCA is a member of the immunoglobulin supergene family, and hybridization studies showed that the transcript level of NCA is induced by treatment with gamma interferon. These studies, as well as the expression of NCA on granulocytes, suggest a role for NCA in immune response. For a first step in studying this possible role, we have examined the binding of two glycoforms of NCA designated NCA-50 (Mr, 50,000) and TEX-75 (Mr, 75,000). Here we report the results from binding assays which demonstrate carbohydrate-mediated binding of Escherichia coli expressing type 1 fimbriae and of isolated type 1 fimbriae to NCA-50. TEX-75 did not bind to the purified fimbriae but bound slightly to the bacterial strain. Inhibition studies showed that the binding to NCA-50 involved interaction of mannose moieties on NCA-50 and lectins on the fimbriae. The binding of NCA-50 to bacterial fimbriae was confirmed by electron microscopy studies, using immunolabeling techniques. In addition, we show that the surface expression of NCA-50 (and presumably of other NCA species) on isolated polymorphonuclear leukocytes is increased following activation with the bacterial peptide formylmethionyl-leucyl-phenylalanine, consistent with a role for NCA in immune response.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham S. N., Sun D., Dale J. B., Beachey E. H. Conservation of the D-mannose-adhesion protein among type 1 fimbriated members of the family Enterobacteriaceae. Nature. 1988 Dec 15;336(6200):682–684. doi: 10.1038/336682a0. [DOI] [PubMed] [Google Scholar]
- Arakawa F., Kuroki M., Misumi Y., Oikawa S., Nakazato H., Matsuoka Y. Characterization of a cDNA clone encoding a new species of the nonspecific cross-reacting antigen (NCA), a member of the CEA gene family. Biochem Biophys Res Commun. 1990 Feb 14;166(3):1063–1071. doi: 10.1016/0006-291x(90)90975-s. [DOI] [PubMed] [Google Scholar]
- Audette M., Buchegger F., Schreyer M., Mach J. P. Monoclonal antibody against carcinoembryonic antigen (CEA) identifies two new forms of crossreacting antigens of molecular weight 90,000 and 160,000 in normal granulocytes. Mol Immunol. 1987 Nov;24(11):1177–1186. doi: 10.1016/0161-5890(87)90164-7. [DOI] [PubMed] [Google Scholar]
- Barnett T. R., Kretschmer A., Austen D. A., Goebel S. J., Hart J. T., Elting J. J., Kamarck M. E. Carcinoembryonic antigens: alternative splicing accounts for the multiple mRNAs that code for novel members of the carcinoembryonic antigen family. J Cell Biol. 1989 Feb;108(2):267–276. doi: 10.1083/jcb.108.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchimol S., Fuks A., Jothy S., Beauchemin N., Shirota K., Stanners C. P. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell. 1989 Apr 21;57(2):327–334. doi: 10.1016/0092-8674(89)90970-7. [DOI] [PubMed] [Google Scholar]
- Blumenstock E., Jann K. Adhesion of piliated Escherichia coli strains to phagocytes: differences between bacteria with mannose-sensitive pili and those with mannose-resistant pili. Infect Immun. 1982 Jan;35(1):264–269. doi: 10.1128/iai.35.1.264-269.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchegger F., Schreyer M., Carrel S., Mach J. P. Monoclonal antibodies identify a CEA crossreacting antigen of 95 kD (NCA-95) distinct in antigenicity and tissue distribution from the previously described NCA of 55 kD. Int J Cancer. 1984 May 15;33(5):643–649. doi: 10.1002/ijc.2910330515. [DOI] [PubMed] [Google Scholar]
- Catanzaro A., Wright S. D. Binding of Mycobacterium avium-Mycobacterium intracellulare to human leukocytes. Infect Immun. 1990 Sep;58(9):2951–2956. doi: 10.1128/iai.58.9.2951-2956.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Shively J. E., Wrann M. Isolation and characterization of the normal crossreacting antigen: homology of its NH2-terminal amino acid sequence with that of carcinoembryonic antigen. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1670–1674. doi: 10.1073/pnas.75.4.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshdat Y., Silverblatt F. J., Sharon N. Dissociation and reassembly of Escherichia coli type 1 pili. J Bacteriol. 1981 Oct;148(1):308–314. doi: 10.1128/jb.148.1.308-314.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gbarah A., Mhashilkar A. M., Boner G., Sharon N. Involvement of protein kinase C in activation of human granulocytes and peritoneal macrophages by type 1 fimbriated (mannose specific) Escherichia coli. Biochem Biophys Res Commun. 1989 Dec 29;165(3):1243–1249. doi: 10.1016/0006-291x(89)92735-6. [DOI] [PubMed] [Google Scholar]
- Giampapa C. S., Abraham S. N., Chiang T. M., Beachey E. H. Isolation and characterization of a receptor for type 1 fimbriae of Escherichia coli from guinea pig erythrocytes. J Biol Chem. 1988 Apr 15;263(11):5362–5367. [PubMed] [Google Scholar]
- Goetz M. B. Priming of polymorphonuclear neutrophilic leukocyte oxidative activity by type 1 pili from Escherichia coli. J Infect Dis. 1989 Mar;159(3):533–542. doi: 10.1093/infdis/159.3.533. [DOI] [PubMed] [Google Scholar]
- Goetz M. B., Silverblatt F. J. Stimulation of human polymorphonuclear leukocyte oxidative metabolism by type 1 pili from Escherichia coli. Infect Immun. 1987 Mar;55(3):534–540. doi: 10.1128/iai.55.3.534-540.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunert F., AbuHarfeil N., Schwarz K., von Kleist S. Two CEA and three NCA species, although distinguishable by monoclonal antibodies, have nearly identical peptide patterns. Int J Cancer. 1985 Sep 15;36(3):357–362. [PubMed] [Google Scholar]
- Grunert F., Kolbinger F., Schwarz K., Schwaibold H., von Kleist S. Protein analysis of NCA-50 shows identity to NCA cDNA deduced sequences and indicates posttranslational modifications. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1105–1115. doi: 10.1016/s0006-291x(88)81342-1. [DOI] [PubMed] [Google Scholar]
- Harbeck R. J., Hoffman A. A., Redecker S., Biundo T., Kurnick J. The isolation and functional activity of polymorphonuclear leukocytes and lymphocytes separated from whole blood on a single percoll density gradient. Clin Immunol Immunopathol. 1982 Jun;23(3):682–690. doi: 10.1016/0090-1229(82)90331-2. [DOI] [PubMed] [Google Scholar]
- Hardy M. R., Townsend R. R., Lee Y. C. Monosaccharide analysis of glycoconjugates by anion exchange chromatography with pulsed amperometric detection. Anal Biochem. 1988 Apr;170(1):54–62. doi: 10.1016/0003-2697(88)90089-9. [DOI] [PubMed] [Google Scholar]
- Hefta S. A., Paxton R. J., Shively J. E. Sequence and glycosylation site identity of two distinct glycoforms of nonspecific cross-reacting antigen as demonstrated by sequence analysis and fast atom bombardment mass spectrometry. J Biol Chem. 1990 May 25;265(15):8618–8626. [PubMed] [Google Scholar]
- Hinoda Y., Neumaier M., Hefta S. A., Drzeniek Z., Wagener C., Shively L., Hefta L. J., Shively J. E., Paxton R. J. Molecular cloning of a cDNA coding biliary glycoprotein I: primary structure of a glycoprotein immunologically crossreactive with carcinoembryonic antigen. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6959–6963. doi: 10.1073/pnas.85.18.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga T. W., van der Schoot C. E., Jost C., Klaassen R., Kleijer M., von dem Borne A. E., Roos D., Tetteroo P. A. The PI-linked receptor FcRIII is released on stimulation of neutrophils. Nature. 1988 Jun 16;333(6174):667–669. doi: 10.1038/333667a0. [DOI] [PubMed] [Google Scholar]
- Kantor J., Tran R., Greiner J., Pestka S., Fisher P. B., Shively J. E., Schlom J. Modulation of carcinoembryonic antigen messenger RNA levels in human colon carcinoma cells by recombinant human gamma-interferon. Cancer Res. 1989 May 15;49(10):2651–2655. [PubMed] [Google Scholar]
- Kelly N. M., Kluftinger J. L., Pasloske B. L., Paranchych W., Hancock R. E. Pseudomonas aeruginosa pili as ligands for nonopsonic phagocytosis by fibronectin-stimulated macrophages. Infect Immun. 1989 Dec;57(12):3841–3845. doi: 10.1128/iai.57.12.3841-3845.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M. J., Shively J. E., Pritchard D. G., Todd C. W. Isolation, immunological characterization, and structural studies of a tumor antigen related to carcinoembryonic antigen. Cancer Res. 1978 Apr;38(4):1041–1048. [PubMed] [Google Scholar]
- Klemm P., Jørgensen B. J., van Die I., de Ree H., Bergmans H. The fim genes responsible for synthesis of type 1 fimbriae in Escherichia coli, cloning and genetic organization. Mol Gen Genet. 1985;199(3):410–414. doi: 10.1007/BF00330751. [DOI] [PubMed] [Google Scholar]
- Krogfelt K. A., Bergmans H., Klemm P. Direct evidence that the FimH protein is the mannose-specific adhesin of Escherichia coli type 1 fimbriae. Infect Immun. 1990 Jun;58(6):1995–1998. doi: 10.1128/iai.58.6.1995-1998.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leusch H. G., Hefta S. A., Drzeniek Z., Hummel K., Markos-Pusztai Z., Wagener C. Escherichia coli of human origin binds to carcinoembryonic antigen (CEA) and non-specific crossreacting antigen (NCA). FEBS Lett. 1990 Feb 26;261(2):405–409. doi: 10.1016/0014-5793(90)80603-g. [DOI] [PubMed] [Google Scholar]
- Mach J. P., Pusztaszeri G. Carcinoembryonic antigen (CEA): demonstration of a partial identity between CEA and a normal glycoprotein. Immunochemistry. 1972 Oct;9(10):1031–1034. doi: 10.1016/0019-2791(72)90113-9. [DOI] [PubMed] [Google Scholar]
- Neumaier M., Zimmermann W., Shively L., Hinoda Y., Riggs A. D., Shively J. E. Characterization of a cDNA clone for the nonspecific cross-reacting antigen (NCA) and a comparison of NCA and carcinoembryonic antigen. J Biol Chem. 1988 Mar 5;263(7):3202–3207. [PubMed] [Google Scholar]
- Ofek I., Sharon N. Lectinophagocytosis: a molecular mechanism of recognition between cell surface sugars and lectins in the phagocytosis of bacteria. Infect Immun. 1988 Mar;56(3):539–547. doi: 10.1128/iai.56.3.539-547.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa S., Inuzuka C., Kuroki M., Matsuoka Y., Kosaki G., Nakazato H. Cell adhesion activity of non-specific cross-reacting antigen (NCA) and carcinoembryonic antigen (CEA) expressed on CHO cell surface: homophilic and heterophilic adhesion. Biochem Biophys Res Commun. 1989 Oct 16;164(1):39–45. doi: 10.1016/0006-291x(89)91679-3. [DOI] [PubMed] [Google Scholar]
- Old D. C., Duguid J. P. Selective outgrowth of fimbriate bacteria in static liquid medium. J Bacteriol. 1970 Aug;103(2):447–456. doi: 10.1128/jb.103.2.447-456.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxton R. J., Mooser G., Pande H., Lee T. D., Shively J. E. Sequence analysis of carcinoembryonic antigen: identification of glycosylation sites and homology with the immunoglobulin supergene family. Proc Natl Acad Sci U S A. 1987 Feb;84(4):920–924. doi: 10.1073/pnas.84.4.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry A., Ofek I., Silverblatt F. J. Enhancement of mannose-mediated stimulation of human granulocytes by type 1 fimbriae aggregated with antibodies on Escherichia coli surfaces. Infect Immun. 1983 Mar;39(3):1334–1345. doi: 10.1128/iai.39.3.1334-1345.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Ortega M., Ofek I., Sharon N. Membrane glycoproteins of human polymorphonuclear leukocytes that act as receptors for mannose-specific Escherichia coli. Infect Immun. 1987 Apr;55(4):968–973. doi: 10.1128/iai.55.4.968-973.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon N. Bacterial lectins, cell-cell recognition and infectious disease. FEBS Lett. 1987 Jun 15;217(2):145–157. doi: 10.1016/0014-5793(87)80654-3. [DOI] [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins as cell recognition molecules. Science. 1989 Oct 13;246(4927):227–234. doi: 10.1126/science.2552581. [DOI] [PubMed] [Google Scholar]
- Shively J. E., Beatty J. D. CEA-related antigens: molecular biology and clinical significance. Crit Rev Oncol Hematol. 1985;2(4):355–399. doi: 10.1016/s1040-8428(85)80008-1. [DOI] [PubMed] [Google Scholar]
- Steadman R., Topley N., Jenner D. E., Davies M., Williams J. D. Type 1 fimbriate Escherichia coli stimulates a unique pattern of degranulation by human polymorphonuclear leukocytes. Infect Immun. 1988 Apr;56(4):815–822. doi: 10.1128/iai.56.4.815-822.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawaragi Y., Oikawa S., Matsuoka Y., Kosaki G., Nakazato H. Primary structure of nonspecific crossreacting antigen (NCA), a member of carcinoembryonic antigen (CEA) gene family, deduced from cDNA sequence. Biochem Biophys Res Commun. 1988 Jan 15;150(1):89–96. doi: 10.1016/0006-291x(88)90490-1. [DOI] [PubMed] [Google Scholar]
- Tawaragi Y., Oikawa S., Matsuoka Y., Kosaki G., Nakazato H. Primary structure of nonspecific crossreacting antigen (NCA), a member of carcinoembryonic antigen (CEA) gene family, deduced from cDNA sequence. Biochem Biophys Res Commun. 1988 Jan 15;150(1):89–96. doi: 10.1016/0006-291x(88)90490-1. [DOI] [PubMed] [Google Scholar]
- Thompson J. A., Pande H., Paxton R. J., Shively L., Padma A., Simmer R. L., Todd C. W., Riggs A. D., Shively J. E. Molecular cloning of a gene belonging to the carcinoembryonic antigen gene family and discussion of a domain model. Proc Natl Acad Sci U S A. 1987 May;84(9):2965–2969. doi: 10.1073/pnas.84.9.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Zimmermann W. The carcinoembryonic antigen gene family: structure, expression and evolution. Tumour Biol. 1988;9(2-3):63–83. doi: 10.1159/000217547. [DOI] [PubMed] [Google Scholar]
- Wagener C., Yang Y. H., Crawford F. G., Shively J. E. Monoclonal antibodies for carcinoembryonic antigen and related antigens as a model system: a systematic approach for the determination of epitope specificities of monoclonal antibodies. J Immunol. 1983 May;130(5):2308–2315. [PubMed] [Google Scholar]
- Weis W., Brown J. H., Cusack S., Paulson J. C., Skehel J. J., Wiley D. C. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature. 1988 Jun 2;333(6172):426–431. doi: 10.1038/333426a0. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Meyer B. C. Phorbol esters cause sequential activation and deactivation of complement receptors on polymorphonuclear leukocytes. J Immunol. 1986 Mar 1;136(5):1759–1764. [PubMed] [Google Scholar]
- von Kleist S., Chavanel G., Burtin P. Identification of an antigen from normal human tissue that crossreacts with the carcinoembryonic antigen. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2492–2494. doi: 10.1073/pnas.69.9.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]